Abstract
One of the main features of Alzheimer’s disease (AD) is the severe reduction of the cerebral metabolic rate for glucose (CMRglc). In vivo imaging using positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose (FDG–PET) demonstrates consistent and progressive CMRglc reductions in AD patients, the extent and topography of which correlate with symptom severity. Increasing evidence suggests that CMRglc reductions occur at the preclinical stages of AD. CMRglc reductions were observed on FDG–PET before the onset of disease in several groups of at-risk individuals, including patients with mild cognitive impairment (MCI), often a prodrome to AD; presymptomatic individuals carrying mutations responsible for early-onset familial AD; cognitively normal elderly individuals followed for several years until they declined to MCI and eventually to AD; normal, middle-aged individuals who expressed subjective memory complaints and were carriers of the apolipoprotein E epsilon-4 allele, a strong genetic risk factor for late-onset AD. However, the causes of the early metabolic dysfunction forerunning the onset of AD are not known. An increasing body of evidence indicates a deficient or altered energy metabolism that could change the overall oxidative microenvironment for neurons during the pathogenesis and progression of AD, leading to alterations in mitochondrial enzymes and in glucose metabolism in AD brain tissue. The present paper reviews findings that implicate hypometabolism and oxidative stress as crucial players in the initiation and progression of synaptic pathology in AD.
Keywords: FDG, PET, Alzheimer’s disease, hypometabolism, oxidative stress, preclinical, early diagnosis
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly, accounting for up to 70% of dementia cases, and is the fourth leading cause of death in developed nations after heart disease, cancer, and stroke. AD is an age-related progressive neurodegenerative disorder with an insidious onset and deadly outcome. Currently, a diagnostic test for AD is not available, and the clinical diagnosis of AD remains a diagnosis of exclusion of other causes; only postmortem examinations can demonstrate whether a patient with a clinical diagnosis of AD-type dementia really suffered from AD pathology or a similar neurodegenerative disease. The definitive diagnosis of AD is based on the postmortem observation of specific pathological lesions: intracellular neurofibrillary tangles (NFT), amyloid beta (Aβ) deposition in the form of extracellular senile plaques and blood vessel deposits, neuronal and synaptic loss, and brain atrophy in specific brain areas.1,2
Several studies have shown that the pathological lesions associated with AD develop many years before the clinical manifestations of the disease become evident.3–6 However, the presence of plaques and tangles alone does not imply that they lead to the clinical aspects of the disease, or that they cause the other cellular changes. In fact, typical amyloid and NFT lesions are found in both demented and non-demented individuals.6–8 A series of papers provided intriguing evidence that, although increased deposition of Aβ plaques and NFT correlate with clinical status at autopsy, neuronal loss is the real turning point for developing clinical symptoms of dementia in life.5,9 Plaques and tangles may therefore be necessary, though not sufficient, to develop the clinical signs of AD. In AD, specific brain circuits are structurally disrupted through synapse loss and neuronal death in keeping with the principle of selective neuronal vulnerability, according to which specific neuronal populations die and others are resistant to neurodegeneration (see10 for review).
The most vulnerable brain areas in AD are the medial temporal lobes (MTL, i.e., the hippocampus, transentorhinal and entorhinal cortices, and parahippocampal gyrus [PHG]).3,4,6 In the neocortex, the pyramidal cells anatomically connected to the EC and the CA1 and subiculum regions of the hippocampus are particularly prone to NFT formation and degeneration, whereas primary sensorimotor and occipital areas and cerebellum exhibit minimal neuronal loss.3,4,6 Disruption of the pyramidal neurons in the perforant path is thought to disconnect the hippocampus from the rest of the cortex, strongly contributing to the decline in memory observed in early AD.11 Despite a predilection for the neocortex, Aβ depositions are also found in the MTL at later stages of the disease.12–14
The neocortex and hippocampus are both affected in AD, but the pathology is not uniform, nor does it affect all cell types. The physical and chemical characteristics, functional properties, and location of neurons seem to impact their likelihood of being affected. In particular, large-projection neurons with relatively long axons are most damaged in AD. These neurons: (1) have high-energy requirements (i.e., high metabolic rates) and, because the brain relies almost exclusively on glucose as a substrate for energy production, their function is directly dependent on glucose availability and use; (2) rely on axonal transport (retrograde and anterograde) for functional support—the axons of cortico-cortical projection neurons travel long distances, which makes these neurons more prone to receiving multiple insults and more sensitive to cytoskeletal dysfunction; and (3) have a large cell surface area, which may increase exposure to toxic environmental conditions.10,15
The causes of neuronal dysfunction in neurodegenerative disorders have been the subject of extensive investigations. Normal synapse function requires a multitude of coordinated mechanisms, including the generation of gene products responsible for formation and maintenance of membrane complexes; the synthesis and delivery of mRNAs, proteins, and transmitters; the regulation of vesicle trafficking, release, and reuptake; and many more.16 For all of these actions to be performed efficiently, sufficient energetic substrates must be supplied. In the brain, the free energy necessary to drive most cellular reactions is derived from phosphorylation of adenosine 5′-triphosphate, which is mostly produced in the mitochondria from the oxidation of glucose under aerobic conditions. Therefore, a disruption in glucose metabolism may be a very direct determinant of synaptic dysfunction.
In keeping with this observation, recent evidence suggests that altered glucose metabolism is a very early change in AD17–21 and is an excellent correlate of the clinical disabilities in dementia.22 Clinical AD symptoms essentially never occur without metabolic decreases, the extent of which is related to the severity of cognitive impairment both in vivo23–25 and in vitro.26 At the molecular level, metabolic changes are intimately linked to glucose consumption and oxidative phosphorylation. Dysregulation of brain metabolism results in the overproduction of reactive oxygen species (ROS) and changes in cellular calcium regulation, as was recently reviewed in.27 Production of ROS is a normal part of the electron transport chain in the mitochondria, and impairment of electron transport promotes ROS production. Increased cytosolic free calcium influx upon interruption of metabolism alters intracellular calcium dynamics, leading to excitotoxicity. Thus, brain metabolism, production of ROS, and calcium homeostasis are directly related and are all altered in AD brains, which are generally under severe oxidative stress.27 An increasing body of evidence has implicated oxidative damage as a critical mechanism in synapse disruption in AD, including evidence for increased lipid peroxidation, protein oxidation, oxidative damage to both nuclear and mitochondrial DNA, and decreased brain glucose metabolism, strongly associated with the known topographical and neuronal distribution of pathology observed in AD (see28–31 for a review). Oxidative stress is associated with plaques and tangles and can plausibly be connected to the clinical aspects of the disease, mainly via disruption of synaptic activity.32,33
Functional neuroimaging offers the unique capability to both visualize the direct effects of neuronal activity and quantitate the rates of specific biological processes at the tissue level in vivo. Positron emission tomography (PET) imaging with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) as the tracer has long been used to track AD-related brain changes by providing qualitative and quantitative estimates of the cerebral metabolic rate of glucose (CMRglc). CMRglc, as measured with FDG–PET, is a direct index of synaptic functioning and density.34–37 The present review will provide an overview of FDG–PET results at the preclinical stages of AD and discuss possible relationships between the hypometabolism observed with neuroimaging and current theories of oxidative stress as a driving force in AD.
Brain Hypometabolism as a Preclinical Event in Alzheimer’s Disease
Alterations of Brain Glucose Metabolism in Alzheimer’s Disease
One of the striking features of AD is the drastic reduction of CMRglc in specific brain areas. FDG–PET studies in AD demonstrate consistent and progressive CMRglc reductions, the extent and topography of which correlate with symptom severity (see38 for a review). Virtually all FDG–PET studies report that, compared with age-matched healthy normal controls, AD patients show regional metabolic reductions involving the parieto-temporal39 and posterior cingulate cortices (PCCs)40 and the frontal areas in advanced disease;41 these reductions are present upon a background of widespread global metabolic impairment42 and are prominent with respect to primary motor and visual areas, cerebellum, thalamus, and basal ganglia nuclei, which are relatively spared.43 These findings have been largely replicated since the early 1980s, and this pattern of hypometabolism is now largely accepted as a reliable in vivo hallmark of AD because of its high sensitivity in distinguishing AD from normal aging as well as from other diseases that affect the brain regionally and globally.44 Interestingly, although innumerable pathological, histological, in vitro, animal model–based, and in vivo magnetic resonance imaging (MRI) studies provided converging evidence for significant MTL damage in patients with AD, for several years FDG–PET studies failed to report MTL abnormalities. This led to the paradoxical conclusion that the MTL either was not hypometabolic or that it maintained high metabolic rates as a compensatory mechanism against advancing disease (see38 for discussion). Only recently, owing to technical achievements leading to higher spatial resolution and improved detector sensitivity of PET instrumentation as well as the use of anatomically precise brain sampling with MRI guidance, reports increasingly appeared indicating MTL CMRglc abnormalities in AD17,18,45–51 along with the characteristic cortical hypometabolism; this put an end to the uncertainty.
Importantly, these CMRglc reductions have been observed on FDG–PET before the onset of the disease in several groups of at-risk individuals, including presymptomatic individuals carrying autosomal dominant mutations responsible for early-onset familial AD; patients with mild cognitive impairment (MCI), which is in many cases a prodrome to AD;52 normal elderly subjects who declined to MCI and AD several years after PET;17,18 normal individuals who were carriers of the apolipoprotein E (APOE) E4 allele, the strongest genetic risk factor for late-onset AD;53 and in subjects with subjective memory complaints.54 A common feature of these studies is the detection of pre-clinical CMRglc abnormalities in the same regions as clinical AD patients; this suggests a metabolic continuum between aging and dementia. The main FDG–PET findings from these studies are reviewed in what follows.
Presymptomatic Early-Onset Familial AD
Autosomal dominant mutations have been identified in three genes—amyloid precursor protein (APP, on chromosome 21), pre-senilin 1 (PS1, on chromosome 14), and pre-senilin 2 (PS2, on chromosome 1)—that are associated with early-onset familial AD (FAD). FAD accounts for a minority of AD cases in the general population and is characterized by autosomal dominant inheritance with 100% penetrance and a specific age at symptom onset for a given pedigree (see55 for review). Therefore, study of presymptomatic mutation carriers close to the expected age of dementia onset provides unique information about preclinical AD-related brain changes.
A few FDG–PET studies have been performed with FAD patients, and unfortunately only in cross-section. These studies showed parieto-temporal, posterior cingulate, and frontal cortex hypometabolism in most FAD cases compared with age-matched controls.56,57 A study by Kennedy et al. (1997)57 showed that presymptomatic FAD individuals have whole-brain CMRglc levels intermediate between controls and symptomatic FAD patients, which suggests a progression of global CMRglc impairment along with the course of the disease. However, these early studies examined only the neocortex, which precludes examination of the MTL, and did not perform partial volume correction of the FDG–PET values. Because their subjects also showed significant volume losses (atrophy) in the same regions on MRI, it remained to be established whether the CMRglc reductions were an effect of increasing the cerebrospinal fluid (CSF) pool. The presence of brain atrophy artificially lowers the FDG–PET measures because of the partial volume effects of CSF, which are not resolved by the PET camera, and the resulting CMRglc measures reflect the combined effects of hypometabolism and atrophy.
We recently addressed these questions in an FDG–PET and MRI study of presymptomatic PS1 carriers from families with early-onset FAD, examined an average of 13 years prior to the estimated age at disease onset.21 Our data showed that the MTL is also hypometabolic in presymptomatic FAD, and that CMRglc reductions exceed tissue loss in these individuals. Specifically, we compared CMRglc and volumes in several brain regions, including the hippocampus, EC, PCC, parietal and temporal cortices, and the whole brain, between patients with FAD and age-matched noncarriers from the same families. Volume reductions in patients with FAD compared with controls were restricted to the parietal cortex. Conversely, CMRglc reductions on FDG–PET were observed in all regions examined, and remained significant after partial volume correction from MRI. Partial volume–corrected CMRglc reductions ranged from 13% (whole brain) to 21% (PCC), reflecting true reductions of brain glucose use per unit brain volume. With respect to the MTL, CMRglc was reduced 12% in the hippocampus and 20% in the EC. Overall, presymptomatic FAD patients showed generalized and widespread CMRglc reductions in the brain regions typically hypometabolic in clinical dementia patients in the relative absence of structural brain atrophy21 (Fig. 1). These results provide definitive evidence that MTL and cortical CMRglc reductions are implicated in the preclinical stages of AD.
Figure 1.

CMRglc reductions (red arrows) on FDG–PET in the parietal regions (left) and MTL (right) in the absence of atrophy on MRI in a 35-year-old woman, a PS1 carrier examined 35 years prior to the expected AD onset age.21
Mild Cognitive Impairment
The preferred strategy by which to examine the preclinical stages of sporadic AD has been the investigation of MCI patients. MCI is recognized by many as a transitional state between healthy aging and dementia, during which individuals are able to perform the usual activities of daily living but suffer isolated memory and or other difficulties exceeding those expected on the basis of normal aging; this puts these individuals at higher risk for developing future AD.58,59 In particular, patients with severe isolated memory deficits, such as amnestic MCI, decline to AD with an estimated conversion rate of 10%–30% per year,58,59 at least in controlled research settings.
Although parieto-temporal and PCC hypometabolism is consensually recognized as the metabolic feature of AD, and reports of MTL deficits are increasing, currently no specific pattern of hypometabolism is considered to be a hallmark for MCI (see38,60 for recent reviews). In keeping with the concept of MCI as an intermediate stage along the hypothesized continuum from normal aging to AD, MCI patients present with mild global and regional hypometabolism within the same brain regions typically affected in clinical AD, compared with controls.17,40,47–50,61–66 However, regional patterns of CMRglc reductions in patients with MCI are more variable and correspond to patterns of cognitive and behavioral abnormalities in individual patients.24,66 FDG–PET studies showed that hippocampal hypometabolism is evident in MCI patients regardless of the neuropsychological profile,17,18,47,50 whereas the cortical involvement is more diversified. Studies in nonamnestic MCI patients (i.e., patients with selective deficits in attention and language58) showed that they had either an absence of cortical hypometabolism or hypometabolism in brain regions including, but not restricted to, the parieto-temporal cortices,17,47,50,67,68 whereas amnestic MCI patients more consistently showed pronounced abnormalities in the PCC and parieto-temporal cortices.40,48,63,64,69 Given the higher rate of decline to AD in patients with amnestic than nonamnestic MCI,59 these data suggest that the more severe and spatially extended CMRglc reductions in AD-specific regions may predispose these patients to develop AD in the near future.
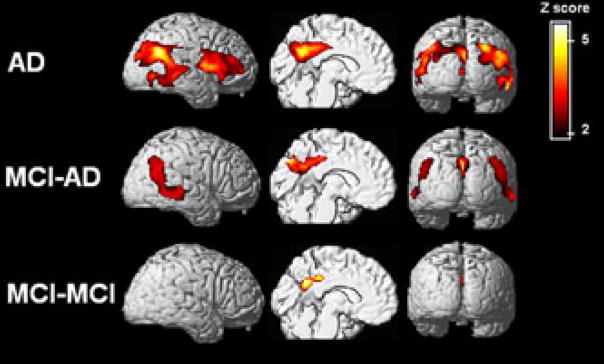
Once it was established that FDG–PET measures are useful in the differentiation of MCI from normal aging, a growing body of longitudinal FDG–PET examinations have been carried out to examine the predictive value of these measures in the decline from MCI to AD. The major findings are summarized in Table 1. These studies were restricted to amnestic MCI patients, and showed that CMRglc reductions are retrospectively more pronounced in those patients with MCI who eventually developed AD than in those who remained stable (Fig. 2), with prediction accuracies ranging from 75% to 100%.40,61–63,65,66 Moreover, evidence suggests that the metabolic changes in the declining MCI patients are progressive, indicating that CMRglc measures both predict and correlate with the decline to AD.64 The main limitation to the above studies was the short time to follow-up, which ranged from 1 to 3 years. Longitudinal studies with longer follow-ups are needed to ascertain whether some of the stable MCI patients also go on to develop AD, and whether FDG–PET is predictive of decline over longer time periods.
TABLE 1.
Prediction of Decline from MCI to AD Using FDG–PET
| Reference | Decliners/Nondecliners | Follow-up (years) | Regional CMRglc reductions | Prediction accuracy |
|---|---|---|---|---|
| 40 | 8/23 | 2 | Parieto-temporal and PCC | Not reported |
| 61 | 19/52 | 2 | Global association cortex | 78% |
| 62 | 9/20 | 3 | Parieto-temporal cortex | 75% |
| 63 | 7/17 | 1.5 | Parieto-temporal and PCC | 94–100% |
| 64 | 8/22 | 1 | Parieto-temporal and PCC and frontal cortex | Not reported |
| 69 | 8/37 | 1 | Parietal cortex | 89% |
| 65 | 12/18 | 1.5 | Parieto-temporal and PCC and/or frontal cortex | 90% |
| 66 | 14/67 | 1 | Parieto-temporal and PCC | 92% |
Figure 2.
Regional CMRglc reductions in AD patients (top row), individuals with MCI who declined to AD after 2 years (MCI–AD, middle row), and stable MCI patients (MCI–MCI, bottom row) compared with age-matched controls.65 Statistical parametric maps of reduced CMRglc are shown on a red-to-yellow color-coded scale reflecting Z scores between 2 and 6, and are displayed (from left to right) on the left lateral, left medial and posterior views of spatially standardized, volume-rendered MRI.
The above studies were able to imply CMR-glc reductions in the transition to AD; however, to validate CMRglc measures as predictors of decline for routine diagnostic evaluations, the risk for future AD must be examined prospectively and on an individual basis. A few studies have undertaken this approach—first in mild AD patients44,70 and more recently in patients with MCI65—and showed that the CMRglc reductions have high predictive value in forecasting patients’ subsequent clinical course. In these studies, the FDG–PET diagnosis at the time of the first evaluation correctly predicted cognitive deterioration in mild AD and MCI patients 3 to 5 years later, with accuracies higher than 80%.44,65,70
Normal and “Abnormal” Aging
Age itself is the single most important risk factor for sporadic AD, which is the most common form of age-related dementia in the general population, affecting approximately 2% of individuals 65 years of age, with the incidence doubling every 5 years up to age 90, at which point the incidence is over 50%.71 FDG–PET studies in normal aging showed mild age-related CMRglc declines, mainly involving the frontal regions.72,17,18
Very little work has been done with FDG–PET to monitor the progression from normal aging to sporadic AD because of the intrinsic difficulty in carrying out such studies for the low incidence and slow progression of normal elderly to AD (1–3% per year).52 Such studies call for very large subject samples, long follow-up, and great expenses to follow cognitively normal persons over time until they develop dementia.
Only three FDG–PET studies have been published that monitored decline from normal to MCI17 or from normal to MCI and dementia.18,73 The first study of this kind showed that reduced baseline CMRglc in the EC predicts an MCI diagnosis 3 years later with 83% sensitivity and 85% specificity.17 Moreover, longitudinal CMRglc reductions were found in the EC, hippocampus, and lateral temporal cortex during the progression to MCI. Importantly, these effects remained significant after correcting the CMRglc values for partial volume effects from MRI, suggesting that these early CMRglc reductions in MCI are independent of tissue loss and represent a real reduction of glucose consumption per gram of brain tissue. However, as not all MCI patients go on to develop AD, it remained to be established whether the observed CMRglc reductions were in fact related to future AD.
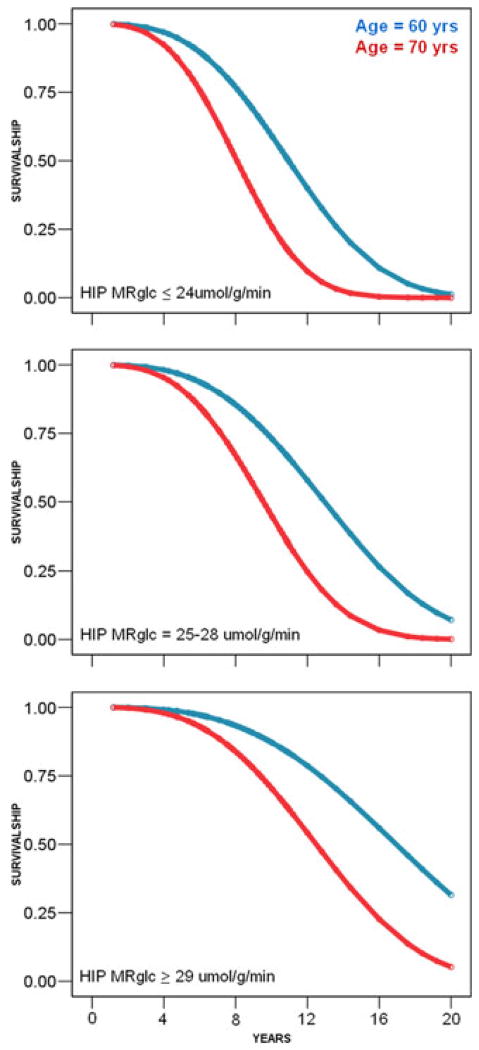
We addressed this question in a recent longitudinal FDG–PET study in normal elderly subjects that were followed over 6–14 years (mean 7 years). This study expanded on our prior work17 by increasing the sample size from 23 to 77 longitudinally followed subjects and by increasing the study duration and the number of follow-ups. This allowed us to follow subjects long enough that 11 baseline normal subjects developed dementia, 6 of whom were diagnosed with AD, and 19 declined to MCI. Decline occurred, on average, 8 years after the baseline exam. CMRglc in the hippocampus and cortical regions was examined as predictors and correlates of change in clinical status. The baseline hippocampal CMRglc was the only regional predictor of future cognitive decline; this measure predicted decline from normal to AD with 81% accuracy, including two postmortem confirmed AD cases, from normal to another dementia with 77% accuracy, and from normal to MCI with 71% accuracy. Hippocampal hypometabolism was also a significant predictor of the time to decline. For every unit decrease in baseline hippocampal CMRglc, the time to decline to AD was decreased by 8.7% (95% confidence interval [CI]: 3.0%–14.1%), which corresponded to a time ratio (TR) of 1.1 (95% CI: 1.0–1.4) years; the time to decline to another dementia was decreased by 4.7% (95% CI: 0.3%–8.9%), for a TR of 1.0 (95% CI: 0.8–1.2) years; and the time to decline to MCI was decreased by 7.2% (95% CI: 2.8%–11.5%), for a TR of 1.08 years (95% CI: 1.03–1.11) (Fig. 3).
Figure 3.
Weibull survival prediction models showing the association between hippocampal (HIP) CMRglc during normal aging and the time to decline to AD.18 In cognitively normal 70-year-old subjects, for HIP CMRglc ≤ 24 μmol/g/min, the predicted median time to decline is 7 years; for CMRglc = 25–28 μmol/g/min, the predicted time to decline is 9.5 years; and for CMRglc ≥ 29 μmol/g/min, the predicted time to decline is 12 years. In comparison, in cognitively normal 60-year-old subjects, HIP CMR-glc ≤ 24 μmol/g/min predicts a median time to decline of 11 years; for CMRglc = 25–28 μmol/g/min, the predicted time to decline is 13 years; and for CMRglc ≥ 29 μmol/g/min, the predicted time to decline is 18 years.
Greater rates of hippocampal, PCC, and temporal cortex CMRglc reductions were found in the declining compared with the non-declining normal subjects. In addition, these FDG–PET data provided direct evidence for a topographical progression of CMRglc abnormalities, which appear to originate in the MTL during the normal stages of cognition, extend to the PCC at the MCI stage of AD, and finally spread to the parieto-temporal cortices in full-blown dementia,18 in keeping with NFT pathology findings.6 Overall, our results showed an association between reduced hippocampal CMRglc during normal aging, shorter times until onset of dementia, and increased risk for cognitive decline years in advance of the clinical diagnosis.
Apolipoprotein E E4 Genotype
The APOE is encoded by three alleles—APOE E2, APOE E3, and APOE E4—of a gene on chromosome 19q13.2. The APOE E4 genotype is a major genetic risk factor for late-onset sporadic AD.53,74 The APOE E4 genotype is considered a risk factor because 40% of AD patients have at least one APOE E4 allele, and there is a negative association between the dose of APOE E4 allele and the mean age of onset of AD, which is approximately 70 years for individuals carrying two APOE E4 alleles, 76 for one allele, and over 85 for the noncarriers.53
The mechanisms by which the E4 allele is implicated in AD onset are not clear. Apolipoproteins constitute the protein portion of the lipoproteins that transport cholesterol, an essential constituent of all cell membranes that provides membrane fluidity. APOE is the major lipoprotein for lipid transport in the CSF and between cells in the brain tissue by acting as a binding site for low-density lipoproteins receptors, allowing for lipids or cholesterol to be assimilated into cells. APOE transport of cholesterol in the CNS is of great importance for synapse plasticity and repair of damaged neurons.75 The APOE E4 allele is thought to increase the risk for AD possibly because of less effective neural protection and repair mechanisms compared with the other allelic variants.75
FDG–PET studies examining the effects of the APOE E4 allele on CMRglc in nondemented individuals have reported that, compared with noncarriers, cognitively normal individuals who are APOE E4 carriers have mild but definite CMRglc reductions in the same regions as clinically affected AD patients.19,20,54,76–79 Evidence suggests that, in middle-aged E4 carriers, the metabolic reductions are progressive and correlate with reductions in cognitive performance.19,77 A study of cognitively normal persons aged 50–63 having two APOE E4 alleles showed a 25% decline in CMRglc over an interval of 2 years.77 Moreover, the same pattern of hypometabolism was observed in 20- to 40-year-old subjects, and these CMRglc reductions are considered the earliest brain abnormalities yet found in living persons at risk for AD.78
These studies also suggest a link between Aβ and CMRglc impairment because APOE E4 is associated with increased deposition of Aβ but has little or no effect on the rate of NFT accumulation.80 Moreover, compared with the other alleles, APOE E4 has the lowest antioxidant metal-binding capacity75 and gives the least protection against Aβ-generated covalent modification of proteins by 4-hydroxynonenal;81 this leads to lipid peroxidation and hydrogen peroxide production.82 APOE E2 and APOE E3 bind and remove Aβ, whereas APOE E4 apparently does not bind Aβ,75 except to promote Aβ sheet formation.83
Subjective Memory Complaints
Subjective memory complaints (SMC) are fairly widespread in the elderly community with a prevalence of 25%–50% and may represent a preclinical sign of incipient dementia,84 although their predictive value remains to be validated. We recently published the first FDG–PET study to examine CMRglc in cognitively normal individuals with and without SMC. The results showed that normal persons with SMC have significant CMRglc reductions in several brain regions, including the PHG, parieto-temporal and inferior frontal cortex, fusiform gyrus, and thalamus, compared with demographically matched individuals with no such complaints. Hypometabolism in the PHG region, which mainly included the entorhinal cortex, was the most significant predictor of SMC status; this measure distinguished subjects with and without SMC with 75% accuracy with an odds ratio of 2.4 (95% CI = 1.3–4.8; P < 0.001), which indicates that normal individuals with SMC have more than a two times greater chance of PHG deficits compared with those with no complaints.54 Moreover, we also explored the effects of the APOE genotype on CMRglc and showed a significant interaction between SMC and APOE status. Among subjects with SMC, carriers of the APOE E4 genotype had significantly lower CMRglc measures in the PHG, temporal and frontal cortices, and thalamus compared with all other possible subgroups. Again, the CMRglc reductions were most prominent in the PHG (18%).
Although hypometabolism reflects synapse dysfunction and is considered a sensitive indicator of neuronal damage, this measure does not provide information on the pathological hallmarks specific to AD. We therefore examined the relationship between hypometabolism and markers of AD pathology in these subjects by combining FDG–PET with CSF biomarkers of AD pathology. We measured some of the most widely used CSF analytes in AD, including markers for tau (i.e., total [T-Tau], a marker of neuronal degeneration, and hyperphosphorylated tau [P-Tau231], a marker for NFTs), and Aβ pathology (i.e., peptide fragments of Aβ40 and Aβ42 amino acid residues, the CSF levels of which are reflective of Aβ sequestration into neuritic plaques) and lipid membrane oxidative damage (F2-isoprostane, IsoP). These CSF markers have value in discriminating AD and MCI from controls and other dementias85,86 and in predicting the transition from MCI to AD,87 although more replication studies are needed.
Across all subjects, CMRglc in the MTL, parieto-temporal, and frontal cortices were significantly correlated with IsoP, T-Tau, and P-Tau231 levels (Ps < 0.02), whereas no significant relationships were found between Aβ40 or Aβ42 and CMRglc in any regions.54 Interestingly, the APOE E4 carriers with SMC showed the highest IsoP levels compared with all other subgroups, and these IsoP levels were strongly related to the PHG CMRglc reductions in these subjects (Ps ≤ 0.01). The combination of PHG hypometabolism and increased IsoP levels significantly discriminated APOE E4 carriers with SMC from the other groups with 79% accuracy and an odds ratio of 3.1 (95% CI = 1.4–9.1; P = 0.001). These data indicate that a relationship exists between PHG CMRglc reductions and lipid membrane peroxidation in normal individuals at risk for late-onset AD; the relationship between these biomarkers becomes tighter when subjects start to show memory deficits.
PHG hypometabolism in normal subjects with memory complaints, particularly those carrying an unfavorable APOE genotype, may have an impact on subjects’ awareness of memory decline. Similar to findings of MTL hypometabolism in normal individuals who progress to MCI and AD,17,18 these data suggest that CMRglc reductions related to the presence of SMC may confer increased risk for developing AD in these subjects. Although the evidence for CMRglc reductions in SMC individuals is compelling, this group classification is susceptible to error. SMC status is based on individual judgment of the subject’s memory deficits, which can vary with recent experiences and changes in mood. Longitudinal follow-up examinations of subjects with and without SMC are needed to determine whether the observed CMRglc reductions foreshadow clinical decline.
Brain CMRglc Reductions and AD Pathology
Very little work has been done to directly relate CMRglc measures to the pathological markers of AD, and the causes of the early CMRglc dysfunction in AD are largely unknown. Nonetheless, evidence suggests that CMRglc reductions correlate with regional densities of NFT, although only moderately, and not with senile plaque distribution.88 The MTL was metabolically affected the earliest and most severely, and contained the highest densities of NFT.88 A 99mTc-HMPAO single-photon emission computed tomography (SPECT) study also showed that changes in cerebral perfusion correlate with Braak’s stages of NFT distribution in AD, and the changes in both cerebral perfusion and NFT distribution originate in the MTL.89 Less direct evidence for a relationship between CMRglc reductions and AD pathology comes from studies that showed agreement between postmortem diagnosis of AD and the parieto-temporal hypometabolism detected in life with PET. In the largest series of cases available to date, the presence of cortical CMRglc abnormalities on antemortem FDG–PET correctly predicted postmortem AD diagnosis with 88% accuracy.44 However, postmortem studies do not clarify whether CMRglc reductions forerunning AD onset are a consequence of NFTs, Aβ, or other pathological lesions. Unfortunately, no studies have directly examined the relationship between CMRglc FDG–PET measures and synapse or neuronal loss, let alone the relationship with markers of oxidative stress.
Indirect evidence implicating impaired glucose regulation in AD, possibly related to synapse dysfunction, comes from kinetic FDG–PET studies showing downregulation of glucose transport and phosphorylation rates in hypometabolic regions in AD patients.90–92 Kinetic FDG–PET studies with dynamic imaging and arterial plasma sampling allow determination of the kinetic rates of forward and backward glucose transport (K1 and k2) and phosphorylation (k3), which are then used to derive absolute CMRglc.93,94 The few FDG–PET kinetic studies that have been performed showed reduced kinetic rates of glucose transport and phosphorylation in the neocortex of moderate-to-severe AD patients compared with normal controls (NL).90–92 We recently showed that the typical CMRglc reductions in the hippocampus and PCC in mild AD patients are also accompanied by reductions in kinetic rates of glucose transport (25%) and phosphorylation (23%–36%).51 We also found a trend toward reduced kinetic rates and CMRglc in the parieto-temporal areas and no effects in other regions, such as frontal and occipital cortices, thalamus, and cerebellum. Altogether, these data suggest that abnormalities in kinetic glucose rate constants may follow the topographical progression of AD, such that they are more pronounced in the hippocampus and PCC in mild dementia and extend to the parieto-temporal regions in moderate-to-severe AD.51,90–92
However, resting-state CMRglc reductions on FDG–PET do not indicate whether this dysfunction is attributable to reduced availability of glucose or to intrinsic brain changes resulting in reduced energy demand. Activation H2O15− and FDG–PET studies provided evidence that, during cognitive stimulation, cerebral blood flow (CBF) and CMRglc can increase to the same extent in mildly demented AD patients as in normal controls, even in the brain areas that show reduced CBF and CMRglc in the resting state.95,96 These results suggest that increasing synapse dysfunction may result in reduced energy demand and downregulation of the enzymes responsible for glucose delivery and consumption in mild AD, although low energy availability because of reduced CBF or CMRglc is not rate-limiting at the early stage of disease.95,96 Responses to stimulation declined with dementia severity and were markedly reduced in severely demented patients.95,96 As proposed by Rapoport and colleagues,30 the in vivo stages of brain responsiveness to stimulation probably reflect stages in synaptic loss and dysfunction in the brain.
Conclusions
The cellular and molecular changes naturally occurring during normal aging may render specific neurons vulnerable to degeneration. During the normal aging process, brain cells undergo changes in oxido-reduction (redox) reactions and experience increasing levels of oxidative stress, perturbed energy homeostasis, and accumulation of damaged proteins and lipid membranes as well as oxidative modified nucleid acid bases (for a review about age-related cellular changes and AD see15). Aging is associated with decreases in mitochondrial function and increased vulnerability of mitochondria to toxins.32 In addition to alterations in mitochondria, neurons also show impaired glucose uptake during normal aging,97 further compromising their ability to maintain ion homeostasis and other energy-dependent cellular processes. Many of the age-related deficits in energy metabolism might be a consequence of oxidative stress, and excessive oxidative stress may in turn provide an unfavorable cellular environment that puts individuals at increased risk for developing pathological lesions. The free radical theory of aging suggests that oxidative stress is the major player in the degeneration of cells,98 and cell cycle disruption is one of the earliest events in AD.99 As age is the primary risk factor for the majority of AD cases, oxidative stress has been implicated in the pathogenesis and progression of AD.100,101 A growing body of evidence indicates that a deficient or altered energy metabolism could change the overall oxidative microenvironment for neurons during the pathogenesis and progression of AD, leading to alterations in mitochondrial enzymes and in glucose metabolism in AD brain tissue.101
In recent years, several FDG–PET studies have shown that brain glucose hypometabolism within key brain regions accurately distinguishes AD from normal aging, precedes cognitive decline and the onset of dementia among normal elderly and presymptomatic FAD individuals, and can be found in cognitively normal subjects with subjective memory complaints and in carriers of the APOE E4 genotype. FDG–PET studies in dizygotic twins discordant for AD are also of great interest as a new strategy to tap preclinical CMRglc abnormalities in AD and examine the influence of genetics and the environment in the onset of dementia.102,103 FDG–PET neuroimaging is therefore considered a candidate modality for the detection of early AD brain changes prior to the onset of clinical symptoms of the disease. Additional work is required to better understand the relationships between hypometabolism at the tissue level, as measured with FDG–PET, and the molecular mechanisms implicated in glucose dysregulation and oxidative stress in AD. Although a relationship appears to exist between oxidative stress, neuronal dysfunction, and reduced glucose metabolism on FDG–PET in AD, several other and possibly co-occurring factors may also be involved. With improved understanding of the molecular basis for the clinical symptoms of dementia, it is hoped that the elucidation of the etiologic causes, particularly the positive feedback loops involving radical damage and a reduced glucose metabolism, will help to develop novel neuroprotective treatment strategies able to interrupt this vicious cycle of oxidative stress and energy shortage in AD.
Acknowledgments
We thank Ms. Rachel Mistur for her assistance in the preparation of the manuscript. This study was supported by NIH-NIA AG13616, AG12101, AG08051, AG022374, and the Alzheimer’s Association.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mirra SS, Heyman A, Mckeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurol. 1991:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 2.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathologica. 1996;92:197–201. doi: 10.1007/s004010050508. [DOI] [PubMed] [Google Scholar]
- 4.Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurol. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Storandt M, Mckeel DW, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurol. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Crystal HA, Dickson D, Davies P, et al. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonogenerians. Arch Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 8.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurol. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 9.Price JL, Ko AI, Wade MJ, et al. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 11.Hyman BT, van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 12.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurol. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 13.Giannakopoulos P, Hof PR, Mottier S, et al. Neuropathological changes in the cerebral cortex of 1258 cases from a geriatric hospital: Retrospective clinicopathological evaluation of a 10-year autopsy population. Acta Neuropathologica. 1994;87:456–468. doi: 10.1007/BF00294172. [DOI] [PubMed] [Google Scholar]
- 14.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanefeld M, Marx N, Pfutzner A, et al. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: The PIOSTAT Study. J Am College Cardiol. 2007;49:290–297. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 17.De Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273:942–947. [PubMed] [Google Scholar]
- 20.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the E4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 21.Mosconi L, Sorbi S, De Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset Familial Alzheimer’s disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 22.Blass JP. Alzheimer’s disease and Alzheimer’s dementia: Distinct but overlapping entities. Neurobiol Aging. 2002;23:1077–1084. doi: 10.1016/s0197-4580(02)00036-2. [DOI] [PubMed] [Google Scholar]
- 23.Grady CL, Haxby JV, Schlageter NL, et al. Stability of metabolic and neuropsychological asymmetries in dementia of the Alzheimer type. Neurol. 1986;36:1390–1392. doi: 10.1212/wnl.36.10.1390. [DOI] [PubMed] [Google Scholar]
- 24.Haxby JV, Grady CL, Koss E, et al. Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type. Arch Neurol. 1990;47:753–760. doi: 10.1001/archneur.1990.00530070043010. [DOI] [PubMed] [Google Scholar]
- 25.Desgranges B, Baron JC, De La Sayette V, et al. The neural substrates of memory systems impairment in Alzheimer’s Disease a PET study of resting brain glucose utilization. Brain. 1998;121:611–631. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- 26.Sheu RK, Mohs R, Haraoutunian V, Blass JP. Correlation of the clinical severity of Alzheimer’s disease with an aberration in mitochondrial DNA (mtDNA) J Mol Neurosci. 2001;16:41–48. doi: 10.1385/JMN:16:1:41. [DOI] [PubMed] [Google Scholar]
- 27.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer’s syndrome. Ann New York Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 29.Behl C, Behl C. Alzheimer’s disease and oxidative stress: Implications for novel therapeutic approaches. Progress Neurobiol. 1999;57:301–323. doi: 10.1016/s0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport SI. In vivo PET imaging and postmortem studies suggest potentially reversible and irreversible stages of brain metabolic failure in Alzheimer’s disease [abstract] Eur Arch Psych Clin Neurosci. 1999;249:46–55. doi: 10.1007/pl00014174. [DOI] [PubMed] [Google Scholar]
- 31.Gibson GE. Interactions of oxidative stress with cellular calcium dynamics and glucose metabolism in Alzheimer’s disease. Free Radical Biol Med. 2002;32:1061–1070. doi: 10.1016/s0891-5849(02)00802-x. [DOI] [PubMed] [Google Scholar]
- 32.Keller JN, Pang Z, Geddes JW, et al. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: Role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- 33.Keller JN, Mark RJ, Bruce AJ, et al. 4-hydroxynonenal, an aldehyde product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neurosci. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 34.Sokoloff L. Relation between physiological functions and energy metabolism in the central nervous system. J Neurochem. 1977;29:13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 35.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 36.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectrosopy: Implications for functional brain mapping. Science. 1999;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 37.Rocher AB, Chapon F, Blaizot X, et al. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: A study in baboons. Neuroimage. 2004;20:1894–1898. doi: 10.1016/j.neuroimage.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. Eur J Nucl Med. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 39.Friedland RP, Budinger TF, Ganz E, et al. Regional cerebral metabolic alterations in dementia of the Alzheimer type: Positron emission tomography with [18F] fluorodeoxyglucose. J Comp Assist Tomogr. 1983;7:590–598. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 41.Foster NL, Chase TN, Mansi L, et al. Cortical abnormalities in Alzheimer’s disease. Ann Neurol. 1984;16:649–654. doi: 10.1002/ana.410160605. [DOI] [PubMed] [Google Scholar]
- 42.Ferris SH, De Leon MJ, Wolf AP, et al. Positron emission tomography in the study of aging and senile dementia. Neurobiol Aging. 1980;1:127–131. doi: 10.1016/0197-4580(80)90005-6. [DOI] [PubMed] [Google Scholar]
- 43.Mazziotta JC, Phelps ME. Positron emission tomography studies of the brain. In: Phelps ME, Mazziotta JC, Schelbert H, editors. Positron Emission Tomography & Autoradiography: Principles & Applications for the Brain & Heart. Raven Press; New York, NY: 1986. pp. 493–579. [Google Scholar]
- 44.Silverman DHS, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 45.De Leon MJ, Mcrae T, Rusinek H, et al. Cortisol reduces hippocampal glucose metabolism in normal elderly but not in Alzheimer’s disease. J Clin Endocrinol Metab. 1997;82:3251–3259. doi: 10.1210/jcem.82.10.4305. [DOI] [PubMed] [Google Scholar]
- 46.Ouchi Y, Nobezawa S, Okada H, et al. Altered glucose metabolism in the hippocampal head in memory impairment. Neurol. 1998;51:136–142. doi: 10.1212/wnl.51.1.136. [DOI] [PubMed] [Google Scholar]
- 47.De Santi S, De Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 48.Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 49.Mosconi L, Tsui WH, De Santi S, et al. Reduced hippocampal metabolism in mild cognitive impairment and Alzheimer’s disease: Automated FDG-PET image analysis. Neurol. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- 50.Mosconi L, De Santi S, Li Y, et al. Visual rating of medial temporal lobe metabolism in mild cognitive impairment and Alzheimer’s disease using FDG-PET. Eur J Nucl Med Mol Imaging. 2006;33:210–221. doi: 10.1007/s00259-005-1956-z. [DOI] [PubMed] [Google Scholar]
- 51.Mosconi L, Tsui WH, Rusinek H, et al. Quantitation, regional vulnerability and kinetic modeling of brain glucose metabolism in mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2007;34:1467–1479. doi: 10.1007/s00259-007-0406-5. [DOI] [PubMed] [Google Scholar]
- 52.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 53.Farrer LA, Cuples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 54.Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered CSF markers in normal ApoE E4 carriers with subjective memory complaints. Biol Psychiatry. 2007;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32:181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy AM, Newman SK, Frackowiak RS, et al. Chromosome 14 linked familial Alzheimer’s disease. A clinico-pathological study of a single pedigree. Brain. 1995;118:185–205. doi: 10.1093/brain/118.1.185. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy AM, Frackowiak RSJ, Newman SK, et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- 58.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology Neurol. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 59.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 60.Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer’s disease. Nat Med. 2004;10:S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- 61.Herholz K, Nordberg A, Salmon E, et al. Impairment of neocortical metabolism predicts progression in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 1999;10:494–504. doi: 10.1159/000017196. [DOI] [PubMed] [Google Scholar]
- 62.Arnaiz E, Jelic V, Almkvist O, et al. Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. NeuroReport. 2001;12:851–855. doi: 10.1097/00001756-200103260-00045. [DOI] [PubMed] [Google Scholar]
- 63.Chetelat G, Desgranges B, De La Sayette V, et al. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurol. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 64.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: A PET follow-up study. Eur J Nucl Med Molec Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 65.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual outcome in MCI by means of genetic assessment and 18F-FDG PET. J Nucl Med. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 66.Anchisi D, Borroni B, Franceschi M, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurology. 2005;62:1728–1733. doi: 10.1001/archneur.62.11.1728. [DOI] [PubMed] [Google Scholar]
- 67.Reed BR, Jagust WJ, Seab JP, Ober BA. Memory and regional cerebral blood flow in mildly symptomatic Alzheimer’s disease. Neurol. 1989;39:1537–1539. doi: 10.1212/wnl.39.11.1537. [DOI] [PubMed] [Google Scholar]
- 68.Berent S, Giordani B, Foster N, et al. Neuropsychological function and cerebral glucose utilization in isolated memory impairment and Alzheimer’s disease. J Psychiat Res. 1999;33:7–16. doi: 10.1016/s0022-3956(98)90048-6. [DOI] [PubMed] [Google Scholar]
- 69.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurol. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 70.Silverman DHS, Truong CT, Kim SK, et al. Prognostic value of regional cerebral metabolism in patients undergoing dementia evaluation: Comparison to a quantifying parameter of subsequent cognitive performance and to prognostic assessment without PET. Mol Genet Metab. 2003;80:350–355. doi: 10.1016/S1096-7192(03)00139-2. [DOI] [PubMed] [Google Scholar]
- 71.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons: Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 72.Herholz K, Salmon E, Perani D, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 73.Jagust W, Gitcho A, Sun F, et al. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- 74.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 Allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 75.Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer’s disease: Possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 76.Small GW, Ercoli LM, Silverman DHS, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiman EM, Uecker A, Caselli RJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 80.Gomez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 81.Pedersen WA, Chan SL, Mattson MP. A mechanism for the neuroprotective effect of apolipoprotein E: Isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2000;74:1426–1433. doi: 10.1046/j.1471-4159.2000.0741426.x. [DOI] [PubMed] [Google Scholar]
- 82.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 83.Evans KC, Berger EP, Cho CG, et al. Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer disease. Proc Natl Acad Sci USA. 1995;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geerlings MI, Jonker C, Bouter LM, et al. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psychiat. 1999;156:531–537. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 85.Buerger K, Zinkowski R, Teipel SJ, et al. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 86.Pratico D, Clark CM, Lee VM, et al. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- 87.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 88.Decarli C, Atack JR, Ball MJ, et al. Post-mortem regional neurofibrillary tangle densities but not senile plaque densities are related to regional cerebral metabolic rates for glucose life in Alzheimer’s disease patients. Neurodegeneration. 1992;1:113–121. [Google Scholar]
- 89.Bradley KM, V, O’Sullivan T, Soper ND, et al. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain. 2002;125:1772–1781. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- 90.Friedland RP, Jagust WJ, Huesman RH, et al. Regional cerebral glucose transport and utilization in Alzheimer’s disease. Neurol. 1989;39:1427–1434. doi: 10.1212/wnl.39.11.1427. [DOI] [PubMed] [Google Scholar]
- 91.Jagust WJ, Seab JP, Huesman RH, et al. Diminished glucose transport in Alzheimer’s disease: Dynamic PET studies. J Cereb Blood Flow Metab. 1991;11:323–330. doi: 10.1038/jcbfm.1991.65. [DOI] [PubMed] [Google Scholar]
- 92.Piert M, Koeppe RA, Giordani B, et al. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med. 1996;37:201–208. [PubMed] [Google Scholar]
- 93.Phelps ME, Huang SC, Hoffman EJ, et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-flouro-2-deoxy-D-glucose: Validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 94.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 95.Rapoport SI, Grady CL. Parametric in vivo brain imaging during activation to examine pathological mechanisms of functional failure in Alzheimer’s disease. Int J Neurosci. 1993;70:36–59. doi: 10.3109/00207459309000559. [DOI] [PubMed] [Google Scholar]
- 96.Pietrini P, Furey ML, Alexander GE, et al. Association between brain functional failure and dementia severity in Alzheimer’s disease: Resting versus stimulation PET study. Am J Psychiat. 1999;156:470–473. doi: 10.1176/ajp.156.3.470. [DOI] [PubMed] [Google Scholar]
- 97.Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- 98.Harman D, Eddy DE, Noffsinger J. Free radical theory of aging: Inhibition of amyloidosis in mice by antioxidants; possible mechanisms. J Am Geriatr Soc. 1976;24:203–210. doi: 10.1111/j.1532-5415.1976.tb06780.x. [DOI] [PubMed] [Google Scholar]
- 99.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 102.Jarvenpaa T, Raiha I, Kaprio J, et al. A 90-year-old monozygotic female twin pair discordant for Alzheimer’s disease. Neurobiol Aging. 2003;24:941–945. doi: 10.1016/s0197-4580(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 103.Virta JJ, Karrasch M, Kaprio J, et al. Cerebral glucose metabolism in dizygotic twin pairs discordant for Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:9–16. doi: 10.1159/000111114. [DOI] [PubMed] [Google Scholar]