Abstract
We report here that ZIP, a selective inhibitor of the atypical protein kinase C isoform PKMζ, abolishes very long-term conditioned taste aversion (CTA) associations in the insular cortex of the behaving rat, at least 3 mo after encoding. The effect of ZIP is not replicated by a general serine/threonine protein kinase inhibitor that is relatively ineffective toward PKMζ, is independent of the intensity of training and the perceptual quality of the taste saccharin (conditioned stimulus, CS), and does not affect the ability of the insular cortex to re-encode the same specific CTA association again. The memory trace is, however, insensitive to ZIP during or immediately after training. This implies that the experience-dependent cellular plasticity mechanism targeted by ZIP is established following a brief time window after encoding, consistent with the standard period of cellular consolidation, but then, once established, does not consolidate further to gain immunity to the amnesic agent. Hence, we conclude that PKMζ is not involved in short-term CTA memory, but is a critical component of the cortical machinery that stores long- and very long-term CTA memories.
That protein kinases may serve as molecular devices of memory storage was proposed long ago (Crick 1984; Lisman 1985; Saitoh and Schwartz 1985; Buxbaum and Dudai 1989). But experimental evidence that a protein kinase is indeed critical for maintaining long-term memory in brain became available only recently (Pastalkova et al. 2006). Specifically, persistent phosphorylation by the atypical protein kinase C isoform PKMζ was shown to be required for maintaining long-term potentiation (LTP) in hippocampus and for sustaining hippocampus-dependent spatial memory (Pastalkova et al. 2006). Following this finding, we have demonstrated that microinfusion of the selective PKMζ pseudosubstrate inhibitory peptide, ZIP, into the insular cortex (IC) of the behaving rat, erases long-term memory of conditioned taste aversion (CTA) (Shema et al. 2007). This indicates that PKMζ also plays an obligatory role in the persistence of memory in neocortex, which is considered the ultimate repository of multiple types of long-term memory (Squire and Kandel 2000; Dudai 2002; Ross and Eichenbaum 2006).
The objective of this study was to further unveil boundary conditions of the effect of ZIP in the IC on CTA. We have previously shown that memory associations lasting from a few days to a few weeks can be quickly abolished by ZIP; but are short-term memory on the one hand, and very long-term memory on the other? We deemed additional attributes of the ZIP effect of interest for further elucidation of the memory mechanisms that are disrupted by the inhibitor. These include the potential relevance of training intensity, previously shown to affect the stability and fate of CTA memory in the IC (Eisenberg et al. 2003); the effect of ZIP in the IC on repetitive conditioning of the same taste–malaise association; the ability of ZIP to erase multiple taste associations involving different taste qualities; and the possibility that more general protein kinase inhibitors that are relatively insensitive toward PKMζ might have an effect similar to ZIP in cortex. Our findings demonstrate that on the one hand, ZIP exerts a rather sweeping effect on long- and very long-term memory associations in cortex, which is not mimicked by a more general inhibitor of serine/threonine protein kinases, but on the other, that the effect is delineated in time: The PKMζ inhibitor is ineffective during conditioning and immediately afterward. This suggests that the cellular mechanism targeted by ZIP consolidates within hours to a few days, but once this happens, the memory trace does not seem to consolidate further to lose this sensitivity to the amnesic agent. In other words, at least up to a few months after encoding, PKMζ remains a critical component of the machinery that keeps memory going in cortex.
Results
ZIP abolishes very long-term memory
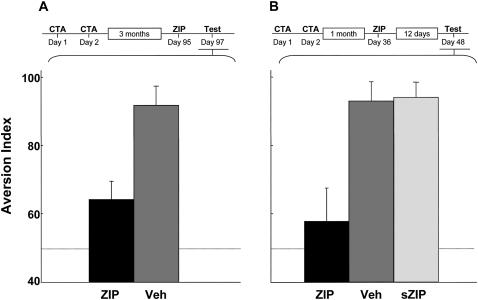
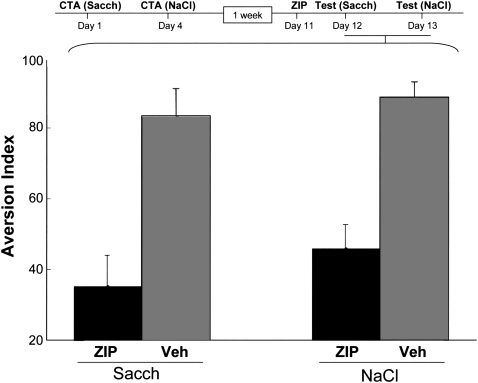
Application of ZIP into the IC up to a month after CTA training results in markedly reduced memory performance (Shema et al. 2007). Memories that depend on cortico-hippocampal circuits in their acquisition are known to undergo a systems consolidation process, which renders the memory practically independent of the hippocampus (Dudai and Morris 2000). In the rat this process takes about a month (Kim and Fanselow 1992; Anagnostaras et al. 1999; Bontempi et al. 1999). Although CTA does not require an intact hippocampus for acquisition (Shema et al. 2007), the possibility still exists that systems consolidation can also take place in nonhippocampal systems (Dudai 2004), in which case one could claim that a month-old memory is not yet consolidated and older memories might still become resistant to the effect of ZIP. We now report that this is not the case, as ZIP abolishes CTA memory even 3 mo after encoding (one-way ANOVA, F (1,12) = 12.96, P < 0.005, ZIP n = 9, vehicle [Veh] n = 5; Fig. 1A).
Figure 1.
Effect of ZIP on very long-term CTA memory in the insular cortex. (A) ZIP/vehicle were administered 3 mo after training, and memory was tested 2 d later. The dashed line indicates equal preference for the CS and water, i.e., AI = 50. (B) ZIP/vehicle/scrambled ZIP were administered 1 mo after training, and memory was tested 12 d later. Saccharin was the CS in both A and B.
The effect of ZIP is long lasting even when the inhibitor is applied long after encoding
The effect of ZIP on memory is long lasting when the peptide is microinfused into the IC a few days after training (Shema et al. 2007). One could postulate, however, that although ZIP is effective in abolishing CTA memory even when the inhibitor is applied months after training (Fig. 1A), the persistence of the effect of the inhibitor over time could itself be time dependent. To test this possibility, we trained rats, microinfused ZIP into the IC over a month later, and tested memory performance close to two weeks afterward. The results show that the effect of ZIP on CTA memory is long lasting even when the inhibitor is applied long after encoding (one-way ANOVA, F (2,19) = 8.19, P < 0.005; post hoc comparisons show that whereas both the scrambled ZIP [sZIP] [n = 8] and Veh groups [n = 6] differ from the ZIP group [n = 8, P < 0.05], they do not differ from each other [P = 0.99]; Fig. 1B). Hence, we detect no evidence for closure of the ZIP-sensitivity window, as judged by the efficacy of ZIP application over time (Fig. 1A) and by the persistence of the effect on memory over time once ZIP is administered (Fig. 1B).
Intensive training and robust memory do not confer immunity to ZIP effect
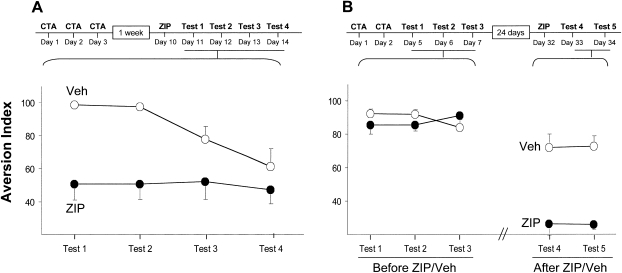
Could intensive training and robustness of memory rescue the trace from the effect of ZIP? To address this possibility, we performed two complementary experiments. In the first, we trained the rats intensively, by subjecting them to three successive CTA training sessions, on three successive days, using 0.1% saccharin as CS. By day 3 of training, the rats became so aversive, they hardly approached the pipettes and the small amount of saccharin they drank probably served only as a reinforcing reminder (10.2 ± 0.5, 2.5 ± 0.5, and 0.9 ± 0.2 mL in the first, second, and third conditioning day, respectively). When injected with vehicle or ZIP a week later and tested after an extra day, CTA in the control rats was prominent and robust, yet essentially nonexistent in the ZIP group (Fig. 2A) (repeated-measures ANOVA shows significant group effect F (1,12) = 9.35, P < 0.01, and time effect F (3,36) = 3.34, P < 0.05, ZIP n = 9, Veh n = 5). In a second experiment, we subjected rats to successive CTA training sessions, on two subsequent days, using 0.45% NaCl as CS, and then 3 d later subjected them to an extinction protocol, which unveiled no extinction. Almost a month later, we applied ZIP, which abolished the memory (repeated-measures ANOVA shows no significant difference between the groups for the first three tests F (1,12) < 1, however, a significant difference for the fourth and fifth tests; F (1,12) = 71.61, P < 0.001, n = 7 each; Fig. 2B). All in all, we conclude that even a highly robust CTA trace is sensitive to fast disruption by a single application of ZIP into the IC.
Figure 2.
Robust memory is not immune to the ZIP effect in cortex. (A) Three consecutive CTA training sessions (CS = saccharin) generated robust memory. ZIP was administrated to the IC 1 wk after training and significantly disrupted the memory, as shown in successive tests, 1 d apart. (B) Two successive CTA trainings (CS = NaCl) generated a strong memory, which is resistant to extinction by three consecutive tests (Tests 1–3; open and filled circles represent the groups of rats which were subsequently used as control and ZIP injected, respectively). Microinfusion of ZIP into the IC 24 d later abolished memory performance, while the control rats still showed marked preference to water (Tests 4 and 5).
Once erased and reacquired, CTA can be erased again by ZIP in the IC
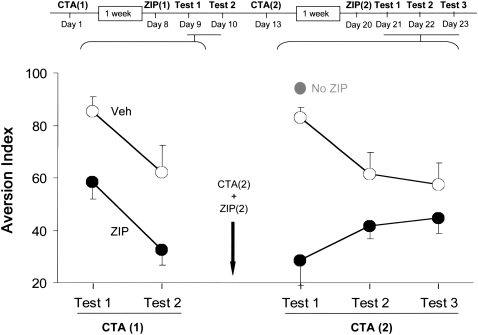
Is the effect of ZIP on CTA memory in the IC detrimental for subsequent encoding of the same association? We already know that application of ZIP before training does not prevent subsequent encoding and CTA memory, and that erasing CTA to one tastant by ZIP does not affect the ability to encode new CTA for another tastant (Shema et al. 2007). The question, however, remains whether the circuits that subserve the memory of a specific tastant–malaise association (Accolla et al. 2007; Grossman et al. 2008) are damaged by ZIP application, and that as a result, new circuits may subserve the re-encoding of CTA. We have therefore trained to CTA to saccharin, demonstrated that the rats have indeed acquired the association, and a week later microinfused ZIP into the IC. As expected, ZIP disrupted the memory (repeated-measures ANOVA for the first two tests, F (1,15) = 9.24, P < 0.01, ZIP n = 9, Veh n = 8; Fig. 3). Three days after the last nonreinforced test, the rats were subjected to another CTA training session, using the same CS, after which the control and ZIP groups were microinfused again into the IC with ZIP or vehicle, leaving some rats from the ZIP group untreated, to control for the retraining effect. When tested a day later, rats that were not microinfused with ZIP a second time into the IC had reacquired the memory, while the ZIP group had lost the reacquired memory (one-way ANOVA for the third test, F (1,12) = 26.62, P < 0.001; Fig. 3). We hence conclude that ZIP-induced erasure of a specific taste–malaise association neither damages the ability of the IC to subsequently encode the same taste–malaise association, nor, because a second infusion of ZIP into the insular cortex erased the new memory, is a new region recruited.
Figure 3.
Memory erased by ZIP can be reacquired and re-erased in cortex. ZIP/vehicle were administrated a week after CTA training (CS = saccharin), followed by two tests. ZIP blocked the memory. Three days later, rats underwent CTA training again to the same CS, after which the control and ZIP groups were reinjected with vehicle and ZIP, respectively; two rats from the ZIP group were not reinjected (denoted as No ZIP). When tested a day later, the Vehicle and No ZIP groups had reacquired the memory, while the ZIP group had lost the reacquired memory, as shown in consecutive tests.
ZIP is ineffective during conditioning and immediately afterward
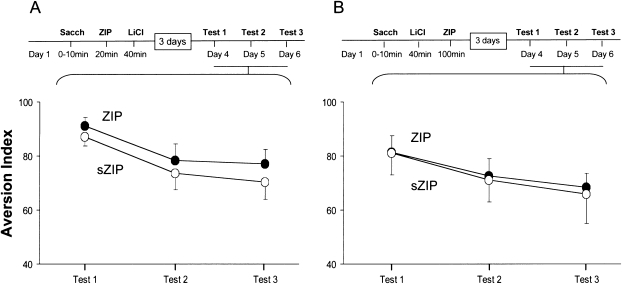
We have set out to determine at which stage of the formation of the CTA trace, ZIP becomes effective. First, we tested whether ZIP blocks CTA memory when applied in the course of training. Toward that end, we administered ZIP to the IC 20 min after exposure to the taste CS (saccharin) and 20 min before the administration of the unconditioned stimulus (US) in the training session. No effect of ZIP on subsequent long-term memory performance and stability was detected (repeated-measures ANOVA F (1,15) = 1.5, P = 0.24, ZIP n = 8, sZIP n = 9; Fig, 4A). This implies that ZIP has no effect on the short-term memory of taste and possibly also not on the encoding of the US per se. The data could also be construed, however, as indicating that ZIP has no effect on memory before the CS–US association is formed. This latter conclusion is in line with our previous finding that ZIP disrupts taste–memory associations, but not taste recognition (Shema et al. 2007).
Figure 4.
Lack of effect on long-term memory of ZIP application within or immediately after CTA training. (A) ZIP/sZIP was administrated 20 min after exposure to the CS (saccharin), followed 20 min later by LiCl i.p. injection. Three days later, three successive tests were conducted, 1 d apart. No effect of ZIP on memory was observed. (B) ZIP/sZIP was administrated 1 h after the LiCl injection in CTA training. Three days later, three successive tests were conducted, one day apart. Again, no effect of ZIP on memory was observed.
We then set out to determine whether ZIP affects long-term memory immediately once the CTA association is formed. Toward that end, we applied ZIP 1 h after LiCl injection in CTA training, and tested long-term CTA memory 3 d later. No ZIP effect was observed, implying that the susceptibility of long-term memory to ZIP develops >1 h after training (repeated-measures ANOVA, F (1,17) < 1, ZIP n = 10, sZIP n = 9; Fig. 4B).
Having established that ZIP does not impair long-term CTA memory when applied immediately after training, we proceeded to determine whether it could nonetheless impair short-term memory at that stage. Attempts to investigate this question are inherently challenging. First, the thirsty rats are trained to satiate their thirst by consuming their daily liquid ration in the training session. Therefore, they are unlikely to drink a significant volume of liquid in the first few hours after conditioning, which renders tests of short-term memory difficult. Second, providing too little fluid during training, to maintain thirst, impedes encoding, as the rats must drink a certain amount of the CS to allow effective conditioning (Rosenblum et al. 1997). And third, after administration of the US, overt behavioral symptoms of malaise, which are detected within a few minutes, last for up to about 2 h, and some lingering effects are detected even up to 5 h (Lamprecht et al. 1997). This implies that measures of behavior up to a few hours after training are contaminated with online malaise symptoms, including avoidance of drinking. Having said all that, we followed the following procedure: Rats were presented with 4 mL of saccharin 0.1% for 5 min only, to balance between the need to avoid satiation and the need to allow sufficient consumption of the taste CS. Thirty-five min later, they were injected i.p. with the LiCl solution. This was followed 1 h later by microinfusion of ZIP (or vehicle) into the IC. Test was performed 3 h after the ZIP administration, by presenting the rats with six pipettes, three containing 3 mL saccharin each and three containing 3 mL water each.
Of the nine rats in each group, five in the ZIP group and seven in the vehicle group drank over 2 mL within 30 min. The other rats were left with the pipettes until they consumed liquid as above. The aversion index calculated for this period (test 1) was 93.0 ± 1.1 in the ZIP group and 95.7 ± 0.8 in the vehicle group. After a day break with water only, the rats were tested again, once a day on three subsequent days. The aversion indices were 75.6 ± 10.0 (test 2), 70.2 ± 4.7 (test 3), and 59.9 ± 7.1 (test 4) in the ZIP group, and 76.9 ± 8.8, 65.5 ± 10.2, and 57.3 ± 7.2, respectively, in the vehicle group. Despite the expected suppression of immediate post-training drinking and the resulting high variability in the aversion indices, one can conclude that ZIP has no effect on short-term memory of CTA. In contrast, we repeatedly find that ZIP prevents subsequent memory when applied already 72 h after encoding (in a representative experiment, ZIP, 40.6 ± 8.8 [n = 7], Veh, 86.5 ± 4.4 [n = 5], P < 0.005, t-test). We therefore conclude that the molecular target of ZIP becomes critical for memory at some time between 1 h and 72 h after encoding.
A single application of ZIP abolishes long-term memory of multiple associations involving different taste qualities
We have previously reported that ZIP in the IC erases CTA to both saccharin and glycine (Shema et al. 2007). Although these tastant solutions are discriminated by the rat, they both share a sweet quality (Tapper and Halpern 1968; du Villard et al. 1981). We have therefore replicated the protocol, but this time using tastants with different taste qualities: saccharin and NaCl. As can be seen in Figure 5, a single microinfusion of ZIP into the IC abolishes long-term memory of both the saccharin–malaise and the NaCl–malaise associations, reinforcing the conclusion that a single treatment with ZIP erases multiple taste associations in the IC regardless of their taste qualities (one-way ANOVA for the first saccharin test, F (1,17) = 16.83, P < 0.001, and for the first NaCl test, F (1,17) = 27.89, P < 0.001, ZIP n = 10, Veh n = 9).
Figure 5.
A single application of ZIP can abolish multiple associations of different taste qualities. Rats were trained on CTA using saccharin as CS, and 3 d later to CTA using NaCl as CS. One week later, the groups were microinfused into the IC with either ZIP or vehicle. Rats were tested on saccharin and NaCl, 1 and 2 d later, respectively.
The effect of ZIP on memory in the IC is dose dependent
The concentration of ZIP microinfused into the cortex of the behaving animal was set in line with the expected effectiveness of the peptide in vivo (Pastalkova et al. 2006). To determine whether a lower concentration is still effective, we compared the effect on CTA memory of 10 nmol/μL ZIP, 3.3 nmol/μL ZIP, and 10 nmol/μL sZIP. The microinfusion into the IC was performed one week after a single CTA training and memory tested 24 h later. As can be seen in Figure 6D, whereas the higher dose of ZIP was highly effective in blocking memory, the lower dose displayed only a trend in that direction. Hence repeated-measures ANOVA show a significant group effect F (2,19) = 39.5, P < 0.001; post hoc comparisons reveal that the 10 nmol/μL ZIP group (n = 8) is significantly different than both the 3.3 nmol/μL (n = 7) and sZIP (n = 7, P < 0.001), whereas the 3.3 nmol/μL and sZIP groups are not significantly different (P = 0.16). Thus, doses higher than 3.3 nmol/μL are required to obtain a robust effect on long-term memory in cortex in vivo.
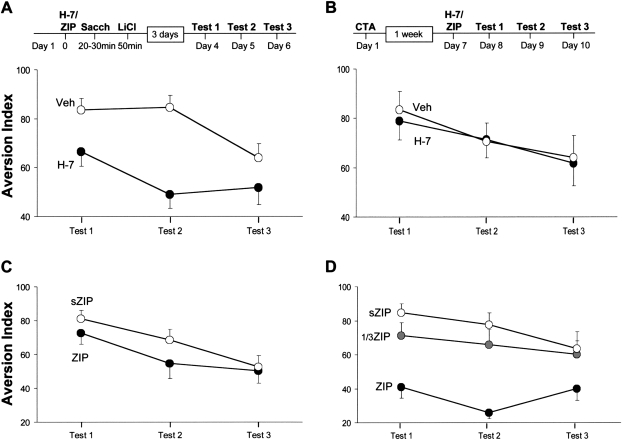
Figure 6.
Double-dissociation of the effect of ZIP and of a general serine/threonine kinase inhibitor relatively ineffective toward PKMζ on learning and memory of CTA. (A) Microinfusion of the serine/threonine protein kinase inhibitor H-7 into the IC 20 min before exposure to taste in CTA training. Memory was tested 3 d later. (B) Microinfusion of H-7 1 wk after CTA training. Memory was tested a day later. (C) Microinfusion of ZIP/sZIP 20 min before learning, under the same conditions in which H-7 was administrated in C. (D) Microinfusion into the IC of different concentrations of ZIP (3.3 or 10 nmol/μL, denoted 1/3ZIP and ZIP, respectively) and of sZIP (10 nmol/μL), a week after CTA training, under the same conditions in which H-7 was administrated in B. As can be seen, whereas H-7 impaired learning but not memory, ZIP impaired memory but not learning.
Unlike ZIP, the serine/threonine protein kinase inhibitor H-7 disrupts learning but has no effect on long-term memory once established
Although ZIP is a selective inhibitor of PKMζ (Laudanna et al. 1998; Ling et al. 2002), the selectivity of its effects on CTA memory in the IC has yet to be established. Toward that end, we have replaced ZIP with H-7, a water-soluble ligand that inhibits serine/threonine protein kinases, including conventional PKCs (Hidaka et al. 1984), but has a higher IC50 toward atypical PKCs, such as ζ (Mizuno et al. 1995). H-7 injection into the IC 20 min before the beginning of CTA training inhibited CTA memory tested 72 h later (Fig. 6A) (repeated-measures ANOVA shows significant group effect F (1,15) = 12.85, P < 0.005, H-7 n = 9, Veh n = 8). This is in line with earlier reports of the role of PKC in encoding of taste memories in the IC (Bahar and Dudai 1997; Nunez-Jaramillo et al. 2007). Under the same conditions, ZIP has no significant effect on memory (Fig. 6C; repeated-measures ANOVA, F (1,14) = 1.3, P = 0.27). However, microinfusion of H-7 into the IC a week after CTA training, in the same conditions in which ZIP disrupts memory, had no effect either on memory or on the extinction of that memory (Fig. 6B,D; repeated-measures ANOVA, F (1,16) < 1, n = 9 for each group). Hence H-7 prevented memory encoding or consolidation, but had no effect on long-term memory once established. This suggests that the effect of ZIP in the IC is not due to inhibition of serine/threonine protein kinases other than PKMζ.
Discussion
The present study extends our previous report concerning the ability of the selective PKMζ pseudosubstrate inhibitor, ZIP, to erase long-term memory associations in neocortex. Specifically, we have recently reported that a single application of ZIP bilaterally into the IC quickly abolishes CTA memory, but not taste familiarity, up to a month after encoding, and that there is no evidence that the effect is reversible (Shema et al. 2007). We now show that a single application of ZIP into the IC abolishes long-term memory even at much longer periods after encoding. We have selected the 3 mo time point to measure remote memory because this is long after the closure of cortico-hippocampal systems consolidation window reported in the rat (Anagnostaras et al. 1999). Although one cannot exclude the possibility that a very long, hippocampus-independent consolidation process takes place in the rat neocortex that lasts for longer than 3 mo, we take our finding as congruent with our working hypothesis that the cellular mechanism that ZIP jams remains critical for the storage of memory associations in cortex as long as these specific associations persist in memory. We further show that this putative memory-keeping machinery consolidates to become critical in memory maintenance only within <72 h after training.
PKMζ, which ZIP blocks selectively, was shown to be necessary and sufficient for the maintenance of LTP in hippocampus (Ling et al. 2002). Our finding that a general serine/threonine protein kinase inhibitor has no effect on long-term CTA memory further reinforces the assumption that the effect we see on CTA memory in the IC is indeed a consequence of PKMζ inhibition. Cellular models of LTP, which guide our interpretation of the molecular basis of the ZIP effect in cortex (see Jones et al. [1999] and Escobar et al. [2002] for evidence of LTP in the IC), distinguish early from late phases. The late phase is itself a multiphase process, which involves concerted action of molecular machineries (Shi et al. 2001; Malenka and Bear 2004; Lynch et al. 2007; Reymann and Frey 2007). PKMζ specifically maintains the late phase (Ling et al. 2002; Serrano et al. 2005) through action on N-ethylmaleimide-sensitive factor (NSF)/glutamate receptor subunit 2 (GluR2)-dependent trafficking of AMPA receptors to the synapse (Ling et al. 2006; Yao et al. 2008). The substrates of PKMζ phosphorylation that mediate this mechanism could involve, among others, reorganization of cytoskeletal and scaffold elements and the AMPA receptor-trafficking proteins, themselves perhaps guided by “slot proteins” or molecular placeholders, as hypothesized for the early phase of LTP. All this culminates in persistent enhancement of synaptic efficacy. The consolidation window during which ZIP is yet ineffective in erasing memory in the IC might reflect the time required to attain the aforementioned specific reorganization, which then becomes critically dependent on persistent activity PKMζ, and collapses when this activity is jammed.
Our findings also indicate that ZIP has no effect on recognition memory of the CS or on the encoding of the US when the agent is injected 20 min after the presentation of the former and 20 min before administration of the latter. The lack of effect on the CS complements our previous finding that despite the involvement of the IC in perception and encoding of taste CS (Katz et al. 2002; Bahar et al. 2004; Koh and Bernstein 2005; Nunez-Jaramillo et al. 2008), ZIP in the IC does not block taste familiarity even in the long term (Shema et al. 2007). It is noteworthy that recognition in cortex is subserved by long-term depression (LTD) (Griffiths et al. 2008), which, in contrast with long-term potentiation (LTP), does not depend on PKMζ for its maintenance (Sajikumar et al. 2005).
Intensive training does not confer immunity to the ZIP effect on memory. But once abolished by ZIP, CTA can again be normally reacquired—and then rapidly erased again by application of ZIP into the same area. This indicates that the circuit that encodes the relevant association can be reset and encode the same information again. Further, multiple associations of CSs of different taste qualities are erased by a single ZIP application, all together suggesting that ZIP globally resets the circuits in the region regardless of the quality and intensity of the specific taste associations. A caveat is, however, appropriate. We cannot exclude the possibility that the storage capacity of individual neurons is affected in the long run and that reacquisition is done by different microcircuits within the cortex, in which case after additional acquisition-encoding cycles the system might lose its capacity to encode associations. These postulated different microcircuits, however, were not prevented from participating in long-term memory storage by prior exposure to ZIP. A complementary experimental approach could involve analysis of the effect of ZIP on individual neurons that subserve encoding of CTA associations in the IC. Indeed cellular physiology of the effect of ZIP on the neuronal signature of CTA in IC (Accolla et al. 2007; Grossman et al. 2008), as well as molecular analysis of the role of PKMζ in the IC in vivo (R. Shema, T.C. Sacktor, and Y. Dudai, unpubl.), are expected to unveil further the mechanisms by which PKMζ might maintain long- and very long-term memory in cortex.
Materials and Methods
Animals
Male Wistar rats (60 d old, 250–350 g) were caged individually at 22°C ± 2°C in a 12 h light–dark cycle. Water and food were available ad libitum unless otherwise indicated. All experiments were approved by the Weizmann Institute of Science Institutional Animal Care and Use Committee (IACUC).
Chemicals
The PKMζ inhibitor ZIP (myr-SIYRRGARRWRKL-OH) (Laudanna et al. 1998; Serrano et al. 2005) was dissolved in phosphate-buffered saline (PBS) to a concentration of 10 nmol/μL (Pastalkova et al. 2006). A scrambled peptide, sZIP (myr-RLYRKRIWRSAGR-OH) (Pastalkova et al. 2006) at the same concentration or the vehicle was used in the control groups, as specified in the text. Both ZIP and sZIP were purchased from Quality Controlled Biochemicals. The serine/threonine protein kinase inhibitor H-7 [1-(5-isoquinolinesulphonyl)-2-methylpiperazine] (Sigma-Aldrich), was dissolved in PBS to a final concentration of 17 nmol/μL (Holahan and Routtenberg 2007).
Behavioral procedures
Conditioned taste aversion (CTA) was induced and tested as previously described (Rosenblum et al. 1993, 1997). Briefly, unless otherwise indicated, rats were deprived of water for 24 h, and then trained over 3 d to obtain their daily water ration within 10 min from two pipettes, each containing 10 mL of tap water. On day 5, water was replaced with the tastant solution (saccharin 0.1% or NaCl 0.45%, the CS). This was followed 40 min later by an i.p. injection of LiCl (0.15 M, 2% body weight, the US). Testing was performed at the times indicated in the text, by presenting the rats with an array of six pipettes, three each with 5 mL of the relevant taste and three each with 5 mL water. In this test situation, the rats explore, sample, and subsequently consume or avoid the contents of several pipettes, none of which contains by itself sufficient liquid to satiate their thirst. The aversion index (AI) was defined as ([water consumed] / [water + taste consumed] × 100) (Rosenblum et al. 1993). All the drinking and testing procedures were done in the home cages.
Surgery and targeted microinfusions
Rats were anesthetized with 0.4 mL/kg Pental, restrained in a stereotaxic apparatus, and implanted bilaterally with stainless steel guide cannulae (23 gauge) aimed 1.0 mm above the gustatory neocortex (AP + 1.3 mm, L ± 5.4 mm, V 5.4 mm relative to Bregma [Paxinos and Watson 1998]). The cannulae were positioned in place with acrylic dental cement and secured by two skull screws. A stylus was placed inside the guide cannulae to prevent clogging. Rats were allowed 1 wk to recuperate before being subjected to experimental manipulations. For microinfusions, the stylus was removed, and a 28-gauge injection cannula, extending 1.0 mm from the tip of the guide cannula, was inserted. The injection cannula was connected via PE20 tubing to a Hamilton microsyringe driven by a microinfusion pump (CMA/100; Carnegie Medicin). Microinfusions were performed bilaterally in a 1 μL volume per hemisphere delivered over 1 min. The injection cannula was left in position before withdrawal for an additional 1 min to minimize dragging of the injected liquid along the injection tract.
Statistics
t-Test (two-tail unpaired unless otherwise indicated) was used for comparison of two groups. One-way ANOVA and repeated-measures ANOVA were used for comparisons of more than two groups and in cases of repeated tests, respectively, with an α level of 0.05.
Acknowledgments
This research was supported by a grant from the U.S.–Israel Binational Science Foundation (BSF), Jerusalem (Y.D. and T.C.S.), by a grant from the Israeli Science Foundation, Jerusalem (Y.D.), and by National Institutes of Health Grants MH53576 and MH57068 (T.C.S.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1183309.
References
- Accolla R., Bathellier B., Petersen C.C.H., Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J. Neurosci. 2007;27:1396–1404. doi: 10.1523/JNEUROSCI.5188-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras S.G., Maren S., Fanselow M.S. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J. Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar A., Dudai Y. Potential role of protein kinase C in conditioned taste aversion in the rat insular cortex. Neurosci. Lett. 1997;48(Supp):S5–S6. [Google Scholar]
- Bahar A., Dudai Y., Ahissar E. Neural signature of taste familiarity in the gustatory cortex of the freely behaving rat. J. Neurophysiol. 2004;92:3298–3308. doi: 10.1152/jn.00198.2004. [DOI] [PubMed] [Google Scholar]
- Bontempi B., Laurent-Demir C., Destrade C., Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Buxbaum J.D., Dudai Y. A quantitative model for the kinetics of cAMP-dependent protein kinase (type II) activity: Long-term activation of the kinase and its possible relevance to learning and memory. J. Biol. Chem. 1989;264:9344–9351. [PubMed] [Google Scholar]
- Crick F. Memory and molecular turnover. Nature. 1984;312:101. doi: 10.1038/312101a0. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Memory from A to Z. Keywords, concepts, and beyond. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Morris R.G.M. To consolidate or not to consolidate: What are the questions? In: Bulhuis J. J., editor. Brain, perception, memory. Advances in cognitive sciences. Oxford University Press; Oxford, UK: 2000. pp. 149–162. [Google Scholar]
- du Villard X.D., Her C., Mac Leod P. Qualitative discrimination of sweet stimuli: Behavioral study on rats. Chem. Senses. 1981;6:143–148. [Google Scholar]
- Eisenberg M., Kobilo T., Berman D.E., Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Escobar M.L., Alcocer I., Bermudez-Rattoni F. In vivo effects of intracortical administration of NMDA and metabotropic glutamate receptors antagonists on neocortical long-term potentiation and conditioned taste aversion. Behav. Brain Res. 2002;129:101–106. doi: 10.1016/s0166-4328(01)00329-1. [DOI] [PubMed] [Google Scholar]
- Griffiths S., Scott H., Glover C., Bienemann A., Ghorbel M.T., Uney J., Brown M.W., Warburton E.C., Bashir Z.I. Expression of long-term depression underlies visual recognition memory. Neuron. 2008;58:186–194. doi: 10.1016/j.neuron.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Grossman S.E., Fontanini A., Wieskopf J.S., Katz D.B. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J. Neurosci. 2008;28:2864–2873. doi: 10.1523/JNEUROSCI.4063-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Holahan M.R., Routtenberg A. Post-translational synaptic protein modification as substrate for long-lasting, remote memory: An initial test. Hippocampus. 2007;17:93–97. doi: 10.1002/hipo.20245. [DOI] [PubMed] [Google Scholar]
- Jones M.W., French P.J., Bliss T.V.P., Rosenblum K. Molecular mechanisms of long-term potentiation in the insular cortex in vivo. J. Neurosci. 1999;19:1–8. doi: 10.1523/JNEUROSCI.19-21-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D.B., Simon S.A., Nicolelis M.A.L. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J. Neurosci. 2002;22:1850–1857. doi: 10.1523/JNEUROSCI.22-05-01850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Koh M.T., Bernstein I.L. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav. Neurosci. 2005;119:388–398. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Lamprecht R., Hazvi S., Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J. Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C., Mochly-Rosen D., Liron T., Constantin G., Butcher E.C. Evidence of ζ protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J. Biol. Chem. 1998;273:30306–30315. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- Ling D.S.F., Benardo L.S., Serrano P.A., Blace N., Kelly M.T., Crary J.F., Sacktor T.C. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Ling D.S.F., Benardo L.S., Sacktor T.C. Protein kinase Mζ enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- Lisman J.E. A mechanism for memory storage insensitive to molecular turnover: A bistable autophosphorylating kinase. Proc. Natl. Acad. Sci. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Rex C.S., Gall C.M. LTP consolidation: Substrates, explanatory power, and functional significance. Neuropharmacology. 2007;52:12–23. doi: 10.1016/j.neuropharm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Malenka R.C., Bear M.F. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Noda K., Ueda Y., Hanaki H., Saido T.C., Ikuta T., Kuroki T., Tamaoki T., Hirai S., Osada S., et al. UCN-01, an anti-tumor drug, is a selective inhibitor of the conventional PKC subfamily. FEBS Lett. 1995;359:259–261. doi: 10.1016/0014-5793(95)00042-8. [DOI] [PubMed] [Google Scholar]
- Nunez-Jaramillo L., Delint-Ramirez I., Bermudez-Rattoni F. PKC blockade differentially affects aversive but not appetitive gustatory memories. Brain Res. 2007;1148:177–182. doi: 10.1016/j.brainres.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Nunez-Jaramillo L., Jimenez B., Ramirez-Munguia N., Delint-Ramirez I., Luna-Illades C., Tapia R., Bermudez-Rattoni F. Taste novelty induces intracellular redistribution of NR2A and NR2B subunits of NMDA receptor in the insular cortex. Brain Res. 2008;1215:116–122. doi: 10.1016/j.brainres.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Pastalkova E., Serrano P., Pinkhasova D., Wallace E., Fenton A.A., Sacktor T.C. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic; San Diego, CA: 1998. [DOI] [PubMed] [Google Scholar]
- Reymann K.G., Frey J.U. The late maintenance of hippocampal LTP: Requirements, phases, “synaptic tagging,” “late associativity” and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Meiri N., Dudai Y. Taste memory: The role of protein synthesis in gustatory cortex. Behav. Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Rosenblum K., Berman D.E., Hazvi S., Lamprecht R., Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J. Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R.S., Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J. Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J.H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J. Cell Biol. 1985;100:835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S., Navakkode S., Sacktor T.C., Frey J.U. Synaptic tagging and cross-tagging: The role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. J. Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P., Yao Y., Sacktor T.C. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J. Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R., Sacktor T.C., Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMζ. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Shi S., Hayashi Y., Esteban J.A., Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Kandel E.R. Memory: From mind to molecules. Freeman; New York: 2000. [Google Scholar]
- Tapper D.N., Halpern B.P. Taste stimuli: A behavioral categorization. Science. 1968;161:708–710. doi: 10.1126/science.161.3842.708. [DOI] [PubMed] [Google Scholar]
- Yao Y., Kelly M.T., Sajikumar S., Serrano P., Tian D., Bergold P.J., Frey J.U., Sacktor T.C. PKMζ maintains late-long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J. Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]