Abstract
BACKGROUND:
Liver stiffness measurement (LSM) by transient elastography has recently been validated for the evaluation of liver fibrosis in chronic liver diseases. The present study focused on cases in which liver biopsy and LSM were discordant.
METHODS:
Three hundred eighty-six patients with chronic hepatitis C who underwent a liver biopsy between December 2004 and April 2007 were studied. First, the optimal cut-off value of LSM was selected for the determination of cirrhosis based on the receiver operating characteristic curve. Then, the cases in which liver histology and evaluation by LSM were discordant were selected. Laboratory test results such as serum total bilirubin concentration, prothrombin activity, albumin concentration, platelet count and the aspartate aminotransferase to platelet ratio index, together with the presence of esophageal varices, were analyzed.
RESULTS:
The optimal cut-off value was chosen to be 15.9 kPa for cirrhosis (fibrosis stage [F] 4) determination to maximize the sum of sensitivity (78.9%) and specificity (81.0%). There were 78 discordant cases: 51 patients showed an LSM of 15.9 kPa or higher and a fibrosis stage of F1 to F3 (high LSM group), and 27 patients had an LSM lower than 15.9 kPa and a fibrosis stage of F4 (low LSM group). Esophageal varices were seen in 11 patients in the high LSM group (n=51) and in no patients in the low LSM group (n=27) (P=0.0012). The aspartate aminotransferase to platelet ratio index was significantly higher in the high LSM group (1.49 versus 0.89, P=0.019). Other parameters did not differ significantly. However, platelet count, prothrombin activity and albumin concentration tended to be lower in the high LSM group.
CONCLUSIONS:
Patients with a high LSM need proper attention for cirrhosis, even if liver biopsy does not reveal cirrhosis.
Keywords: Hepatitis C, Liver biopsy, Liver fibrosis
Abstract
HISTORIQUE :
La mesure de l’élasticité hépatique (MÉH) par élastographie transitoire a récemment été validée pour évaluer la fibrose hépatique en présence d’une maladie hépatique chronique. La présente étude portait sur les cas où les résultats de la biopsie hépatique et de la MÉH divergeaient.
MÉTHODOLOGIE :
Les auteurs ont étudié 386 patients atteints d’hépatite C chronique ayant subi une biopsie hépatique entre décembre 2004 et avril 2007. D’abord, ils ont sélectionné la valeur seuil optimale de MÉH pour déterminer une cirrhose d’après la courbe de fonction d’efficacité du récepteur. Ensuite, ils ont retenu les cas à l’égard desquels l’histologie hépatique et l’évaluation par MÉH divergeaient. Ils ont analysé les résultats de tests de laboratoire comme la concentration bilirubinémique sérique totale, l’activité de la prothrombine, la concentration d’albumine, la numération plaquettaire et l’indice de ratio entre l’aspartate aminotransférase et les plaquettes, ainsi que la présence de varices œsophagiennes.
RÉSULTATS :
Les auteurs ont retenu la valeur seuil optimale de 15,9 kPa pour déterminer la cirrhose (stade de fibrose [F] 4) afin de maximiser la somme de sensibilité (78,9 %) et de spécificité (81,0 %). Ils ont repéré 78 cas divergents : 51 patients avaient une MÉH de 15,9 kPa ou plus et un stade de fibrose de F1 à F3 (groupe de MÉH élevé) et 27 patients, une MÉH inférieure à 15,9 kPa et un stade de fibrose de F4 (groupe de MÉH faible). Ils ont constaté des varices œsophagiennes chez 11 patients du groupe de MÉH élevé (n=51) et chez aucun patient du groupe de MÉH faible (n=27) (P=0,0012). L’indice de ratio entre l’aspartate aminotransférase et les plaquettes était considérablement plus élevé entre le groupe de MÉH élevé (1,49 par rapport à 0,89, P=0,019). Les autres paramètres ne différaient pas de manière significative. Cependant, la numération plaquettaire, l’activité de la prothrombine et la concentration d’albumine tendaient à être plus faibles dans le groupe de MÉH élevé.
CONCLUSIONS :
Les patients dont la MÉH est élevée doivent subir une évaluation de cirrhose, même si la biopsie hépatique n’en révèle pas la présence.
The prognosis and clinical management of chronic liver diseases (CLDs) highly depend on the extent of liver fibrosis because life-threatening complications mainly occur in patients with cirrhosis (1,2). This is particularly true of chronic hepatitis C virus (HCV) infection. Patients with cirrhosis are at high risk for hepatocellular carcinoma, liver failure and resulting death (3–8). This emphasizes the need for early identification of cirrhosis to screen for or prevent complications.
Liver biopsy is currently considered to be the reference standard for the assessment of cirrhosis. However, it is an invasive procedure with rare, but severe, adverse events, including mortality (9). Its acceptance is limited, especially in asymptomatic patients. In addition, sampling error is common because only 1/50,000 of the organ is analyzed, and a false negativity of up to 30% was reported when compared with surgically resected liver as a reference standard (10–12). Therefore, there is a need to develop and validate noninvasive tests that can accurately reflect the full spectrum of hepatic fibrosis and cirrhosis, and their severity in liver diseases.
Both routine and specific biomarkers, together with combinations thereof, have been proposed as noninvasive indicators of the degree of liver fibrosis (13–16). However, these markers do not directly reflect the extent of fibrosis. Rather, they represent partial processes such as fibrogenesis and fibrolysis. Blood levels of some markers are affected by impaired metabolism in renal failure or cholestasis.
Transient elastography is a new, noninvasive and reproducible technique that measures tissue stiffness, which is mainly attributable to the extent of fibrosis. Liver stiffness measurement (LSM) by transient elastography has recently been validated for the evaluation of hepatic fibrosis in chronic liver diseases. However, various LSM cut-off values for cirrhosis have been reported to be between 12.5 kPa and 17.6 kPa (17–25), and no single cut-off value was accompanied by simultaneously high sensitivity and specificity. Ganne-Carrie et al (26) assessed 775 CLD patients of various etiologies and reported a sensitivity of 79% and a specificity of 95% using 14.6 kPa as a cut-off value for fibrosis stage (F) 4 (cirrhosis). In assessing cirrhosis, discordant results were found between liver biopsy and LSM in 80 of 1007 patients (7.9%). They re-analyzed the liver biopsy specimens and suggested that the main cause of the discordance was sampling variation of liver fibrosis assessed by liver biopsy.
Indeed, LSM is averaged over a volume that can be approximated by a cylinder 20 mm in height and 20 mm in diameter, which represents approximately 1% of the total liver volume and is 500-fold larger than the biopsy sample size. Therefore, LSM may be less likely to be affected by sampling error than liver biopsy and may be more relevant in the clinical assessment of CLD status. However, the discordance between liver biopsy and LSM has not been assessed in this respect.
In assessing the discordance between liver biopsy and LSM, it is difficult to choose an adequate reference standard. Because our primary aim was to compare liver biopsy with LSM in terms of clinical relevance, we compared the results of routine laboratory tests such as platelet count, albumin level and pro-thrombin activity, which are the earliest indicators of clinical cirrhosis (27–29). Our interest focused particularly on cases in which liver biopsy and LSM were discordant (ie, cases with a high LSM and noncirrhotic histology, and those with a low LSM and cirrhotic histology).
PATIENTS AND METHODS
Patients
Between December 2004 and April 2007, a total of 394 patients with chronic HCV underwent liver biopsy. Liver biopsies were indicated for the assessment of liver fibrosis before interferon therapy (n=186), overall prognosis in patients suspected of having cirrhosis (n=192) and liver function reservoir in patients with hepatocellular carcinoma before treatment (n=16). LSM was performed within two weeks after liver biopsy. All patients were positive for serum HCV-RNA and showed at least transiently elevated serum alanine aminotransferase levels. Patients with ascites or hepatitis B virus coinfection were excluded from the current study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the University of Tokyo’s (Tokyo, Japan) institutional review board (registration number 960). All patients fulfilling these criteria were enrolled after providing their written and informed consent. LSM by elastography was compared with the stage of liver fibrosis, laboratory test results and other patient characteristics.
Methods
LSM was performed using FibroScan (Echosens, France), a new medical device based on elastometry. The investigators previously underwent a training period in which each had performed at least 50 measurements. The procedure was totally noninvasive and was performed on the right lobe of the liver through the intercostal space. Only LSMs obtained in at least eight successful acquisitions with a success rate of at least 60% were considered reliable.
Liver biopsy was performed under ultrasonography by experienced hepatologists with a 16 G Bard Monopty needle (Medicon Inc, Japan). The sample length was checked immediately after the procedure. When the sample length was less than 15 mm, the biopsy was judged to be inappropriate and was repeated. Liver biopsy specimens were fixed in formalin and were paraffin-embedded. All biopsy specimens were analyzed by two experienced hepatopathologists blinded to the clinical data. Liver fibrosis was staged using a scale of 0 to 4 (F0, no fibrosis; F1, mild fibrosis; F2, moderate fibrosis; F3, severe fibrosis; F4, cirrhosis). The length and number of portal tracts (whether it contained five or more tracts) and the presence of fragmentation were checked based on the criteria by Regev et al (10).
Esophageal varices were evaluated by reviewing the reports of upper gastrointestinal endoscopies or multidetector computed tomography (CT) scans performed within three years before the LSM. CT scan findings of esophageal wall thickening, intraluminal protrusions or irregularities, or nodular enhancement within the esophageal wall were considered to be indicative of the presence of esophageal varices (30,31).
In the analysis of discrepant cases, the optimal LSM cut-off value for the determination of cirrhosis was selected, as determined by liver biopsy histology, to maximize the sum of sensitivity and specificity based on the Youden index (32). Then, the cases in which there was a discordance between liver histology and evaluation by LSM were selected. There were two groups of discordant cases – those determined to be cirrhotic (F4) by LSM but noncirrhotic (F1 to F3) by histology, and those determined to be noncirrhotic by LSM but cirrhotic by histology. Laboratory test results of serum total bilirubin concentration, prothrombin activity, albumin concentration, platelet count and the aspartate aminotransferase to platelet ratio index (APRI) (14), together with the presence of esophageal varices, were compared between the two groups using unpaired Student’s t test for continuous variables and Fisher’s exact probability test for categorical variables. All tests were two-sided, with a significance level of 5%. Statistical analyses were performed with SPSS version 12 (SPSS Inc, USA).
RESULTS
Patient characteristics
A total of 394 patients underwent LSM within two weeks after liver biopsy. Eight patients were excluded because of unsuccessful measurements, which were mainly due to obesity (three patients had fewer than eight valid measurements and five had a success rate lower than 60%). Thus, 386 patients were included in the current analysis. Their characteristics at the time of the LSM are summarized in Table 1. There were 277 men and 109 women, with a mean (± SD) age of 68.2±9.5 years. The frequency distribution of fibrosis stages on the precedent liver biopsy was F1 in 29 patients (7.5%), F2 in 56 patients (14.5%), F3 in 82 patients (21.2%) and F4 in 219 patients (56.7%). The median biopsy specimen length was 16.2 mm.
TABLE 1.
Baseline patient characteristics (n=386)
| Variable | Value |
|---|---|
| Age, years, mean ± SD | 68.2±9.5 |
| Male sex, n (%) | 227 (58.8) |
| Body mass index, kg/m2, median (range) | 22.9 (14.3–34.0) |
| Alcohol consumption >80 g/day, n (%) | 40 (10.4) |
| Aspartate aminotransferase, U/L, median (range) | 54 (10–444) |
| Alanine aminotransferase, U/L, median (range) | 47 (6–506) |
| Albumin, g/L, median (range) | 37 (24–52) |
| Total bilirubin, μmol/L, median (range) | 13.7 (5.1–59.9) |
| Platelet count, ×109/L, median (range) | 116 (33–462) |
| Prothrombin activity, %, median (range) | 78.7 (41.7–100.0) |
| Liver biopsy finding, n (%) | |
| Fibrosis stage (F) 1 | 29 (7.5) |
| F2 | 56 (14.5) |
| F3 | 82 (21.2) |
| F4 | 219 (56.7) |
| Qualities of biopsy specimen | |
| Length, mm, median (range) | 16.2 (15.2–17.3) |
| ≥5 portal tracts, n (%) | 354 (96.2) |
| Not fragmented, n (%) | 334 (90.8) |
| Liver stiffness, kPa, median (range) | 20.1 (3.3–75) |
Receiver operating curves
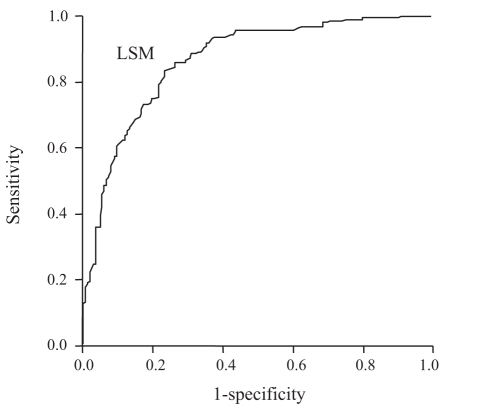
Figure 1 shows the diagnostic value (receiver operating characteristic curve) of LSM for the prediction of histologically diagnosed cirrhosis. The area under the receiver operating characteristic (AUROC) curve is the most commonly used method of summarizing overall accuracy; an area of 1 represents a perfect test and an area of 0.5 or lower represents a noninformative test. The AUROC curve of LSM was 0.87 (95% CI 0.83 to 0.90). The optimal cut-off value was chosen to be 15.9 kPa for F4 determination to maximize the sum of sensitivity (78.9%) and specificity (81.0%) (Table 2).
Figure 1).
Receiver operating characteristic curve of liver stiffness measurement (LSM) accuracy for the diagnosis of cirrhosis in 386 patients. The area under the receiver operating characteristic curve of LSM was 0.87 (95% CI 0.83 to 0.90)
TABLE 2.
Cut-off value of liver stiffness measurement for cirrhosis
| Variable | Cirrhosis |
|---|---|
| Optimal cut-off, kPa* | 15.9 |
| Sensitivity, % | 78.9 |
| Specificity, % | 81.0 |
| Positive predictive value, % | 87.2 |
| Negative predictive value, % | 69.4 |
| Positive likelihood ratio | 4.15 |
*The optimal cut-off value was chosen to maximize the sum of sensitivity and specificity
Discordance analysis
Using the LSM cut-off value of 15.9 kPa, there were 78 cases of discordance between LSM and stage of fibrosis: 51 patients showed an LSM of 15.9 kPa or higher and a fibrosis stage of F1 to F3 (high LSM group), and 27 patients had an LSM lower than 15.9 kPa and a fibrosis stage of F4 (low LSM group). Among the patients in the high LSM group, the distribution of histological fibrosis stage was F2 in 10 patients and F3 in 41 patients. Clinical factors were compared between the two groups (Table 3). Esophageal varices were seen in 11 of 51 patients in the high LSM group and in none of the 27 patients in the low LSM group (P=0.0012). Fibrosis stages seen in these patients were F2 in two patients and F3 in nine. The APRI was significantly higher in the high LSM group (P=0.019), indicating higher aspartate aminotransferase levels or lower platelet count. Although other parameters did not differ significantly between the two groups, platelet count, pro-thrombin activity and albumin concentration tended to be lower in the high LSM group.
TABLE 3.
Differences in frequency distribution between the two discrepant groups
| Variable | High LSM* (n=51) | Low LSM† (n=27) | P |
|---|---|---|---|
| Esophageal varices, n (%) | 11 (21.6) | 0 (0) | 0.0012 |
| Albumin, g/L | 37 (24–45) | 39 (28–45) | 0.269 |
| Prothrombin activity, % | 79.2 (60.7–100.0) | 82.9 (63.0–97.1) | 0.754 |
| Total bilirubin, μmol/L | 12.0 (5.1–32.5) | 12.0 (5.1–30.8) | 0.813 |
| APRI | 1.49 (0.25–6.43) | 0.89 (0.22–4.94) | 0.019 |
| Platelet count, ×109/L | 112 (39–269) | 123 (46–255) | 0.062 |
| Qualities of biopsy specimen | |||
| Length, mm | 16.2 (15.2–16.8) | 16.4 (15.3–17.2) | 0.6246 |
| ≥5 portal tracts, n (%) | 49 (96.1) | 25 (92.6) | 0.606 |
| Not fragmented, n (%) | 46 (90.2) | 22 (81.5) | 0.302 |
Data are expressed as median (range), unless otherwise specified.
*Liver stiffness measurement (LSM) of 15.9 kPa or higher and fibrosis stage (F) 1 to F3;
†LSM lower than 15.9 kPa and F4. APRI Aspartate aminotransferase to platelet ratio index
DISCUSSION
In the present study, we prospectively assessed transient elastography and showed that the AUROC for the prediction of F4 was as large as 0.89. The optimal cut-off value for F4 was found to be 15.9 kPa, with a sensitivity of 78.9% and a specificity of 81.0%. These figures indicate that LSM is a reliable diagnostic test for cirrhosis, as previously demonstrated (Table 4). However, the question remains as to whether the discordance between liver biopsy and LSM represents a random error or a systematic bias (ie, whether there is a tendency to over- or underestimate liver fibrosis in either procedure).
TABLE 4.
Cut-off values of liver stiffness measurement for cirrhosis
Liver biopsy has been used as the gold standard for the evaluation of other tests for liver fibrosis. However, the reliability of liver biopsy has been questioned. Using the METAVIR scoring system, Bedossa et al (33) showed that only 65% of samples from a 15 mm-long needle liver biopsy (which is the currently recommended length) were correctly classified in terms of fibrosis stage compared with the surgical sample. The concordance rate increased to 75% using 25 mm-long specimens. Therefore, the discordance found in the present study was likely caused by random sampling errors due to the small specimen size and the heterogeneity of fibrosis in the liver. However, this leads to a biased error in favour of overall overestimation when applied to the uppermost category (F4), and the proportion of overestimation depends on the size of random errors. Similarly, a portion of F4 cases are underestimated and misdiagnosed as F3. However, this is counterbalanced by an overestimation of F2 into F3; the overall effects on intermediate categories such as F3 are less biased.
When two tests differ in their diagnosis of cirrhosis, the test with the smaller number of sampling errors is less likely to overestimate the stage of fibrosis and more likely to be accurate (34). Assuming that sampling errors in liver biopsy and those in LSM are mutually independent, and that LSM is associated with fewer sampling errors, fewer noncirrhotic patients are falsely diagnosed to be cirrhotic by LSM than by liver biopsy. This becomes especially important, clinically, when the results of the two assessments differ. In the current analysis, esophageal varices, which are an important stigma of cirrhosis, were found in 11 of 51 patients (21.6%) in the high LSM group but in none of the 27 patients in the low LSM group. Esophageal varices not only cause death in cirrhotic patients directly by rupture but are also reported to indicate a risk of transition from the compensated to the decompensated stages of cirrhosis (35). Consequently, screening for esophageal varices is strongly recommended for patients with a high LSM, regardless of liver biopsy results.
APRI is reported to be an accurate predictor of cirrhosis, showing an AUROC curve of 0.89 in the training set and 0.88 in the validation set (14). In the present study, the APRI was significantly higher in the high LSM group than in the low LSM group, supporting the hypothesis that LSM is more accurate than liver biopsy histology. Serum albumin concentration was lower and bilirubin concentration was higher in the high LSM group than in the low LSM group, although the difference was not significant. These indexes are associated with the prognosis of cirrhotic patients and are built into the Child-Pugh classification (36). It is possible that LSM is also a good predictor of prognosis in CLD patients. Poynard et al (37) assessed the discordant results between biochemical markers and biopsy in patients with chronic HCV. Their results suggested that 97 of 154 (62.9%) discordant cases were due to biopsy failure. Our results also suggested that approximately two-thirds of discordant cases were due to liver biopsy failure rather than LSM failure.
Limitations
The present study has some limitations. First, a large proportion of patients had cirrhosis or precirrhosis. Most underwent liver biopsy for the evaluation of suspected cirrhosis or in preparation for interferon therapy, which required ruling out a diagnosis of cirrhosis. Second, we adopted the cut-off value of 15.9 kPa to maximize the sum of sensitivity and specificity in the current study population. This does not mean that this value is universally optimal. Finally, although the diagnosis of varices using a CT scan is fairly specific, it is not sensitive, which may have led to an underestimation of varices or portal hypertension.
Due to the lack of an appropriate gold standard for assessing the degree of liver fibrosis, the cross-sectional comparison between liver biopsy and LSM that was attempted in the current study has its limitations. Nevertheless, the results of the present study have suggested that LSM is less likely to underestimate cirrhosis than liver biopsy. Patients with a high LSM require proper attention, even if liver biopsy assessment determines no cirrhosis. After all, the primary purpose of assessing fibrosis in CLD patients is to predict decompensation, carcinogenesis and overall survival. The clinical relevance of LSM in this respect is to be confirmed in future prospective studies. If LSM is confirmed as an effective predictor of cirrhosis, liver biopsy may be reserved for the assessment of etiology or the grading of necroinflammatory activity.
CONCLUSION
Patients with a high LSM need proper attention for cirrhosis, even if liver biopsy does not reveal cirrhosis.
REFERENCES
- 1.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 2.Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: A retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: National surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–81. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 5.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: A prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–9. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: Time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–6. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311–6. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- 8.Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin Gastroenterol Hepatol. 2005;3(Suppl 2):S141–3. doi: 10.1016/s1542-3565(05)00713-5. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL. The role of liver biopsy in chronic hepatitis C. Hepatology. 2002;36(Suppl 1):S152–60. doi: 10.1053/jhep.2002.36381. [DOI] [PubMed] [Google Scholar]
- 10.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 11.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 12.Maharaj B, Maharaj RJ, Leary WP, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–5. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 13.Le Calvez S, Thabut D, Messous D, et al. The predictive value of Fibrotest vs. APRI for the diagnosis of fibrosis in chronic hepatitis C. Hepatology. 2004;39:862–3. doi: 10.1002/hep.20099. [DOI] [PubMed] [Google Scholar]
- 14.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y, Hoshida Y, Kato N, et al. A simple combination of serum type IV collagen and prothrombin time to diagnose cirrhosis in patients with chronic active hepatitis C. Hepatol Res. 2004;30:214–20. doi: 10.1016/j.hepres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology. 2002;36(Suppl 1):S57–64. doi: 10.1053/jhep.2002.36800. [DOI] [PubMed] [Google Scholar]
- 17.Castera L, Foucher J, Bertet J, Couzigou P, de Ledinghen V. FibroScan and FibroTest to assess liver fibrosis in HCV with normal aminotransferases. Hepatology. 2006;43:373–4. doi: 10.1002/hep.21019. [DOI] [PubMed] [Google Scholar]
- 18.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 19.de Ledinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–9. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 20.Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut. 2006;55:403–8. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazemi F, Kettaneh A, N’kontchou G, et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–5. doi: 10.1016/j.jhep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–73. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahon P, Thabut G, Ziol M, et al. Liver stiffness measurement versus clinicians’ prediction or both for the assessment of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2006;101:2744–51. doi: 10.1111/j.1572-0241.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 25.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 26.Ganne-Carrie N, Ziol M, de Ledinghen V, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–7. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C – a study of 527 patients at one establishment. J Viral Hepat. 2000;7:268–75. doi: 10.1046/j.1365-2893.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 28.Oberti F, Valsesia E, Pilette C, et al. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609–16. doi: 10.1053/gast.1997.v113.pm9352863. [DOI] [PubMed] [Google Scholar]
- 29.Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology. 2005;42:282–92. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 30.Balthazar EJ, Naidich DP, Megibow AJ, Lefleur RS. CT evaluation of esophageal varices. AJR Am J Roentgenol. 1987;148:131–5. doi: 10.2214/ajr.148.1.131. [DOI] [PubMed] [Google Scholar]
- 31.Kim YJ, Raman SS, Yu NC, To’o KJ, Jutabha R, Lu DS. Esophageal varices in cirrhotic patients: Evaluation with liver CT. AJR Am J Roentgenol. 2007;188:139–44. doi: 10.2214/AJR.05.1737. [DOI] [PubMed] [Google Scholar]
- 32.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Ratziu V, Charlotte F, Heurtier A, et al. LIDO Study Group Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 35.D’Amico G, Morabito A, Pagliaro L, Marubini E. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31:468–75. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 36.Lawson A, Hagan S, Rye K, et al. Trent HCV Study Group The natural history of hepatitis C with severe hepatic fibrosis. J Hepatol. 2007;47:37–45. doi: 10.1016/j.jhep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Poynard T, Munteanu M, Imbert-Bismut F, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–55. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]