Abstract
BACKGROUND:
There is little published information on baseline characteristics and therapeutic outcomes in hepatitis C virus (HCV)-infected Aboriginal Canadians. It is unclear what proportion of HCV-infected Aboriginal people receive therapy relative to other populations.
METHODS:
Adults with chronic HCV infection, quantifiable serum HCV-RNA levels and compensated liver disease were assigned, at the physician’s discretion, to either 24 or 48 weeks of treatment with peginterferon alpha-2a 180 μg/week plus ribavirin at a dose of 800 mg/day, or 1000 mg/day or 1200 mg/day in an open-label, expanded access program. The primary outcome was sustained virological response, defined as undetectable HCV-RNA by qualitative polymerase chain reaction (less than 50 IU/mL) at the end of 24 weeks of untreated follow-up. Baseline characteristics and outcomes in Aboriginal and non-Aboriginal patients were compared.
RESULTS:
A total of 2614 patients were eligible for the analysis; 44 individuals (1.7%) self-identified as being of Aboriginal heritage. The baseline characteristics of these two groups were similar. An overall sustained virological response was achieved in 47.7% and 46.5% of Aboriginal and non-Aboriginal patients, respectively. The overall frequencies of adverse events and laboratory abnormalities were similar between the two groups, although cytopenias occurred less frequently in Aboriginal patients.
INTERPRETATION:
Aboriginal patients were greatly under-represented in the present ‘community’-based treatment program, yet viral responses were similar to those of a non-Aboriginal cohort. To increase the uptake of HCV therapy in the Aboriginal population, clarification of the obstacles to treatment is warranted.
Keywords: Aboriginal, HCV, Interferon, Natives, Ribavirin, Treatment
Abstract
CONTEXTE :
Il existe peu de données publiées sur les caractéristiques de base des Autochtones, au Canada, et les résultats du traitement de l’hépatite C dans la population concernée. On ne sait pas vraiment dans quelle mesure les Autochtones infectés au virus de l’hépatite C (VHC) sont traités par rapport à d’autres populations.
MÉTHODE :
Des adultes atteints d’une infection chronique au VHC et présentant des concentrations sériques quantifiables d’ARN du VHC et une hépatopathie compensée ont été dirigés, à la discrétion du médecin, vers le traitement par le péginterféron alpha 2a, à raison de 180 μg/semaine, et la ribavirine, à raison de 800 mg/jour, de 1000 mg/jour ou de 1200 mg/jour, et ce, pour une durée de 24 ou de 48 semaines, dans le cadre d’un programme d’accès élargi, réalisé au su des parties. Le principal critère d’évaluation était la persistance de la réponse virologique, définie comme une concentration indécelable d’ARN du VHC, mesurée par une réaction en chaîne de la polymérase qualitative (moins de 50 UI/ml) à la fin d’une période de suivi de 24 semaines sans traitement. Il y a eu comparaison des caractéristiques de base des patients autochtones et des patients non autochtones, ainsi que des résultats obtenus.
RÉSULTATS :
Au total, 2614 patients étaient admissibles à l’analyse; 44 (1,7 %) personnes se sont déclarées d’ascendance autochtone. Les caractéristiques de base des deux groupes étaient comparables. Une réponse virologique persistante a été observée, dans l’ensemble, chez 47,7 % des Autochtones et 46,5 % des non-Autochtones. La fréquence générale des effets indésirables et des examens de laboratoire anormaux était à peu près la même dans les deux groupes, mais les cas de cytopénie étaient moins fréquents chez les patients autochtones que chez les patients non autochtones.
INTERPRÉTATION :
Les patients autochtones étaient nettement sous-représentés dans le programme communautaire de traitement, pourtant la réponse virologique s’est montrée comparable à celle notée chez les patients non autochtones. Afin d’accroître la fréquence du traitement de l’hépatite C dans la population autochtone, il serait justifié de cerner les obstacles à la pharmacothérapie.
The prevalence of hepatitis C virus (HCV) infection in Canada is greater in Aboriginal people than in the general population (1,2). This difference is presumed to result from more frequent exposure to HCV through high-risk behaviours such as injection drug use and tattooing, and through incarceration.
The combination of pegylated interferon alpha-2a and ribavirin has been reported to provide optimal rates of sustained virological response (SVR) in diverse populations enrolled in multinational studies, as well as in specific ethnic groups such as those of Middle Eastern descent and those from Asia (3,4). In contrast, African Americans achieve much lower rates of SVR than Caucasians, for reasons that are biological and socioeconomic (5–8). There is little published information on the characteristics and therapeutic outcomes of Aboriginal people with HCV infection. It is unclear whether the proportion of Aboriginal people treated for chronic HCV infection reflects the high prevalence of infection in this population. Moreover, the treatment success rate in this population is unknown. One group reported lower SVR rates in Alaskan natives (9). However, it is unclear whether these results apply to the broader population of North American Aboriginals or are indicative of the effect of limited resources for HCV treatment delivery in the community setting. The Canadian Pegasys Expanded Access Program (EAP) enrolled more than 2500 patients in diverse settings. In the present retrospective subgroup analysis, we report the safety and efficacy outcomes of HCV treatment in Aboriginal Canadian participants in the EAP.
METHODS
Patients
Patients eligible for the Canadian Pegasys EAP were adults aged 18 years or older with chronic HCV infection confirmed by a quantitative test for HCV-RNA (Cobas Amplicor HCV Monitor Test v2.0 [Roche Diagnostics, USA], limit of quantitation 600 IU/mL). Patients with cirrhosis were eligible, provided they had compensated liver disease (Child-Pugh class A). Both interferon-naive and previously treated patients were eligible for enrolment. In the first phase of the EAP, all patients were required to have a liver biopsy. This procedure was optional in later phases of the program.
Patients were excluded if they had a history of decompensated liver disease, a hemoglobin concentration less than 100 g/L, a neutrophil count less than 1500 cells/mL, a platelet count less than 90,000/mL, or presented with evidence of infection with hepatitis B virus or HIV. Other exclusion criteria included a history of autoimmune disease, organ transplantation, uncontrolled major psychiatric conditions, active substance abuse or other serious chronic disease. Patients were enrolled after informed consent was obtained. Conduct of this EAP was initiated at each clinical site after first being reviewed and approved by a local research ethics board.
Study design
The EAP was an open-label, multicentre study in which eligible patients were assigned at the physician’s discretion to 24 or 48 weeks of treatment with subcutaneous peginterferon alpha-2a (Pegasys, Roche Pharmaceuticals Inc, USA) 180 μg/week plus oral ribavirin (Copegus, Roche Pharmaceuticals Inc). In the first phase of the program, all patients received ribavirin 800 mg/day. Physicians were allowed to prescribe ribavirin at a dose of 1000 mg/day (if a patient weighed less than 75 kg) or 1200 mg/day (if a patient weighed 75 kg or more) after evidence became available that these regimens were optimal in patients with HCV genotype 1 infection (10).
In the event of clinically significant laboratory abnormalities or adverse events, the dosage of peginterferon alpha-2a or ribavirin was adjusted according to established guidelines, which were previously published (11).
Assessments and outcomes
Serum HCV-RNA levels were determined at baseline and at week 12 by quantitative polymerase chain reaction (PCR) assay (Cobas Amplicor HCV Monitor Test v2.0, limit of quantitation 600 IU/mL). Samples from patients with unquantifiable HCV-RNA were retested using a more sensitive qualitative PCR assay (Cobas Amplicor HCV Monitor Test v2.0, limit of detection 50 IU/mL). All HCV-RNA assays were performed at the virology laboratory of the BC Centre for Disease Control.
An early virological response (EVR) at week 12 was defined as undetectable HCV-RNA by qualitative PCR, or a 2log10 reduction or more in HCV-RNA relative to the base-line value by quantitative PCR. SVR was defined as undetectable HCV-RNA (less than 50 IU/mL) by qualitative PCR 24 weeks after the last injection of peginterferon alpha-2a. Week 4 assessment for rapid virological response was not performed because this was not standard practice when the present study was conducted.
Monitoring for adverse events and safety assessments (physical examination and laboratory evaluations) was conducted at regular intervals throughout the study. Because it was an EAP, requirements for reporting adverse events were less stringent than those in phase III registration trials. During the early phases of the trial, Health Canada required that all suspected laboratory abnormalities be reported. After the approval of peginterferon alpha-2a in Canada, reporting of neutropenia and thrombocytopenia was not required. In contrast, reporting of hemoglobin abnormalities (an adverse event associated with ribavirin) was required throughout the study.
Statistical analysis
The present analysis consists of a descriptive comparison of data from patients self-identifying as Aboriginal with data from all other patients enrolled in the EAP. The term ‘Aboriginal’ included North American Indians (also known as First Nations Canadians), Metis and Inuit.
RESULTS
Of 2683 patients enrolled in the EAP, 2614 were eligible for the present analysis, including 44 individuals (1.7%) of self-identified Aboriginal heritage. The baseline characteristics of these two groups were generally similar (Table 1). Specifically, the key predictors of EVR and SVR were similar between groups.
TABLE 1.
Baseline characteristics of Aboriginal and non-Aboriginal patients
| Characteristic | Aboriginal, n=44 | Non-Aboriginal, n=2570 |
|---|---|---|
| Age, years, median (range) | 44 (25–58) | 47 (18–76) |
| Male, n (%) | 25 (57) | 1784 (69) |
| BMI, kg/m2, median (range) | 28 (22–37) | 27 (16–56) |
| Liver biopsy performed, n (%) | 43 (98) | 2182 (85) |
| Metavir fibrosis stage, n (%)* | ||
| F0 to F2 | 27 (63) | 1320 (60) |
| F3 | 9 (21) | 430 (20) |
| F4 | 5 (12) | 376 (17) |
| Other/unknown | 2 (5) | 56 (3) |
| HCV genotype, n (%) | ||
| 1 | 31 (70) | 1698 (66) |
| 2 | 4 (9) | 286 (11) |
| 3 | 9 (20) | 468 (18) |
| Other | 0 (0) | 118 (5) |
| Serum HCV-RNA level, IU/mL ×103, median (range) | 569 (14.2–11,600) | 733 (0.6–31,100) |
| Treatment naive, n (%) | 30 (68) | 1716 (67) |
| Previously treated, n (%) | 14 (32) | 854 (33) |
Only patients who had a liver biopsy were included in the calculation of percentages. BMI Body mass index; HCV Hepatitis C virus
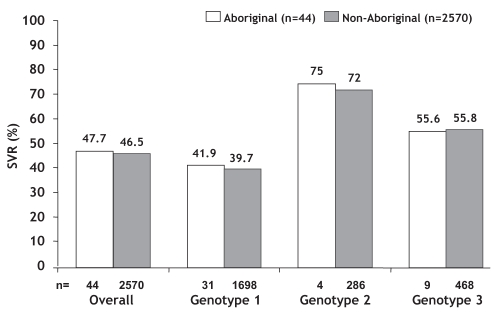
Among patients for whom a week 12 HCV-RNA assay result was reported (40 Aboriginal, 2458 non-Aboriginal), an EVR was achieved in 66% and 78% of Aboriginal and non-Aboriginal individuals, respectively. An end-of-treatment virological response was achieved in 23 of 44 Aboriginal patients (52%) and in 1606 of 2570 non-Aboriginal patients (62%). The overall SVR rate was similar in Aboriginal (47.7%) and non-Aboriginal patients (46.5%) (Figure 1). SVR rates were similar by individual genotype as well. Virological relapse between the end of treatment and follow-up occurred in three of 23 (13%) Aboriginal patients and 497 of 1606 (31%) non-Aboriginal patients.
Figure 1).
Sustained virological response (SVR) rates in Aboriginal and non-Aboriginal patients. A total of 118 non-Aboriginal patients had a hepatitis C virus genotype other than 1, 2 or 3 (not shown). Approximately one-third of Aboriginal (n=14) and non-Aboriginal patients (n=854) had either relapsed after or did not respond to a previous course of treatment
When only previously treated patients were considered, there was an apparent discrepancy in outcomes. None of the 14 previously treated Aboriginal patients achieved an SVR, compared with 30% (260 of 854) of the previously treated non-Aboriginal patients.
In general, the overall frequencies of adverse events and serious adverse events were similar between groups (Table 2). Three Aboriginal patients experienced a serious adverse event during the study: gastritis (n=1), dehydration (n=1) and suicide attempt (n=1).
TABLE 2.
Safety, tolerability and laboratory test abnormalities
| Variable | Aboriginal | Non-Aboriginal |
|---|---|---|
| Adverse events, n (%) | 17 (39) | 1063 (41) |
| Serious adverse events, n (%) | 3 (7) | 140 (5) |
| Neutropenia* (lowest neutrophil count at any time), n (%) | ||
| Grade 3 (500 cells/mL to 750 cells/mL) | 5 (14) | 288 (14) |
| Grade 4 (<500 cells/mL) | 0 (0) | 59 (3) |
| Peginterferon alpha-2a dosage reduction for neutropenia, n (%) | 5 (14) | 356 (18) |
| Thrombocytopenia* (lowest platelet count at any time), n (%) | ||
| Grade 3 (50,000/mL) | 0 (0) | 66 (3) |
| Peginterferon alpha-2a dosage reduction for thrombocytopenia, n (%) | 2 (5) | 95 (5) |
| Anemia† (hemoglobin <100 g/L at any time), n (%) | 2 (5) | 259 (10) |
| Ribavirin dosage reduction for anemia, n (%) | 2 (5) | 328 (13) |
Monitoring and reporting of neutrophil and platelet levels were required for a total of 37 Aboriginal and 2008 non-Aboriginal patients;
Monitoring and reporting of hemoglobin levels were required for all 44 Aboriginal and 2570 non-Aboriginal patients
In non-Aboriginal patients, the most commonly reported serious adverse events included pneumonia (n=13), anemia (n=7), therapeutic abortion (n=5), liver failure (n=5) and variceal hemorrhage (n=4). Depression (n=3), suicidal depression (n=1) and suicide attempt (n=2) were also reported as serious adverse events in non-Aboriginal patients.
The frequency of grade 3 neutropenia was similar between groups (Table 2). Of note, grade 4 neutropenia and grade 3 or 4 thrombocytopenia were not reported in Aboriginal patients. Anemia and the need for ribavirin dosage reduction were less frequently identified in the Aboriginal population.
DISCUSSION
There is very little published information on HCV treatment response in Aboriginal populations in North America. Our descriptive analysis revealed no clinically significant differences in treatment outcomes or adverse events between Aboriginal and non-Aboriginal individuals with the use of combination HCV antiviral therapy. No differences between these populations were identified that may have biased the results in favour of or against the Aboriginal population. As with all evaluations of HCV therapy, the population of individuals receiving therapy may be biased because they may be less burdened by medical, psychological, social, economic and substance use issues, which limit access to and success of HCV therapy.
Our SVR results are in contrast to those in a previous publication (9), which reported low SVR rates in a longitudinal study of Alaskan natives. In this previous study, 60 of 800 patients enrolled in the Alaska Native Tribal Health Consortium Hepatitis Research Program received interferon or interferon-ribavirin HCV antiviral therapy between 1992 and 2004 (eight patients received more than one course of treatment). Of 40 individuals who started a first course of combination therapy, 11 (27.5%) discontinued prematurely because of adverse events or noncompliance. Fourteen (35%) achieved an SVR, but only 10% of those with genotype 1 infection achieved an SVR despite completing a full course of therapy. These results prompted the authors to speculate that Alaskan natives with genotype 1 might respond less well to interferon plus ribavirin-based HCV therapy than Caucasians. In our judgement, a more likely explanation for the poor genotype 1 results in that study is related to the medications used and the dosing of the HCV drugs. The current standard of care for HCV consists of pegylated interferon alpha-2a plus ribavirin (12). This therapy is more effective in achieving an SVR than conventional interferon plus ribavirin treatment (13,14). The dosage of ribavirin and duration of treatment have a significant impact on outcomes, particularly in patients infected with HCV genotype 1 (10,15). The importance of optimal dosing was first formally recognized in treatment guidelines published in 2002 (16). Therefore, patients treated before that date may have received suboptimal dosing. Another possible explanation for the difference in outcomes between our analysis and those from the analysis in Alaskan natives may be related to genetic heterogeneity between various North American Aboriginal populations. There is mounting evidence that genetic polymorphisms influence therapeutic interferon responsiveness (17–20).
In our evaluation, the EVR and end-of-treatment virological response rates were lower in Aboriginal patients than in Caucasians. Further evaluation to confirm this phenomenon is justified. If confirmed, this would suggest greater resistance to an initial response to HCV therapy in Aboriginal patients versus Caucasians. The final SVR rates were similar, which may suggest that the post-treatment relapse rates may be lower in Aboriginal patients. Additional research on cytopenia complications of therapy within the Aboriginal population is justified, given our observations of low rates of severe anemia, neutropenia and thrombocytopenia.
According to Statistics Canada’s 2006 Census (21), 3.8% of the Canadian population is Aboriginal (2.2% North American Indians, 1.2% Metis, 0.2% Inuit). The prevalence of HCV infection in Aboriginal patients is five to eight times higher than that in the general population (1,2). Given this high prevalence and the fact that only 1.7% of our treated population were self-identified Aboriginal Canadians, this group was greatly under-represented in the present ‘community’-based treatment program. Access to and uptake of treatments for many medical conditions are lower in Aboriginal patients. Although there may be multiple obstacles to initiating HCV treatment in the Aboriginal population, including possible substance abuse and hepatitis B virus coinfection (which were exclusion criteria in the present EAP), as well as concurrent socioeconomic and mental health barriers, our work suggests that concerns about diminished therapeutic success should not be one. To increase the uptake of HCV therapy in the Aboriginal population, clarification of these obstacles is warranted.
Acknowledgments
The authors thank Hong Wang, Jennifer Lee and Rob Balshaw of Syreon Corporation (Vancouver, British Columbia) for their assistance with the statistical analysis, as well as Blair J Jarvis for providing editorial assistance. The present research was funded by Roche Canada (Mississauga, Ontario).
REFERENCES
- 1.Forrester L, Zaniewski G, Shi Y, et al. Incidence of acute hepatitis B and hepatitis C in the Canadian Aboriginal population, 1999–2000. Poster prepared by the Bloodborne Pathogens Section of the Blood Safety Surveillance and Health Care Acquired Infections Division of Health Canada. <http://www.phac-aspc.gc.ca/hcai-iamss/bbp-pts/pdf/hepbc_ab_e.pdf> (Version current at May 12, 2008).
- 2.Wu H-X, Wu J, Wong T, et al. Enhanced Hepatitis Strain Surveillance System Incidence and risk factors for newly acquired hepatitis C virus infection among Aboriginal versus non-Aboriginal Canadians in six regions, 1999–2004. Eur J Clin Microbiol Infect Dis. 2007;26:167–74. doi: 10.1007/s10096-007-0267-7. [DOI] [PubMed] [Google Scholar]
- 3.Missiha S, Heathcote J, Arenovich T, Khan K, Canadian Pegasys Expanded Access Group Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol. 2007;102:2181–8. doi: 10.1111/j.1572-0241.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 4.Derbala M, Aner A, Bener A, Lopez AC, Omar M, El Ghannam M. Pegylated interferon-alpha 2b-ribavirin combination in Egyptian patients with genotype 4 chronic hepatitis. J Viral Hepat. 2005;12:380–5. doi: 10.1111/j.1365-2893.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 5.Muir AJ, Bornstein JD, Killenberg PG, Atlantic Coast Hepatitis Treatment Group Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites N Engl J Med 20043502265–71.(Erratum in 2004;351:1268). [DOI] [PubMed] [Google Scholar]
- 6.Layden-Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343–50. doi: 10.1053/jhep.2003.50217. [DOI] [PubMed] [Google Scholar]
- 7.Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–8. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 8.Conjeevaram HS, Fried MW, Jeffers LJ, et al. Virahep-C Study Group Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–7. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Christensen C, Bruden D, Livingston S, et al. Hepatitis C treatment results in an Alaska native/American indian population Hepatology 2007424 Suppl 1654A–5A.(Abst) [Google Scholar]
- 10.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. PEGASYS International Study Group Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 11.Lee SS, Bain VG, Peltekian K, et al. Canadian PEGASYS Study Group Treating chronic hepatitis C with pegylated interferon alfa-2a (40 KD) and ribavirin in clinical practice Aliment Pharmacol Ther 200623397–408.(Erratum in 2006;23:1029). [DOI] [PubMed] [Google Scholar]
- 12.Dienstag JL, McHutchison JG.American Gastroenterological Association medical position statement on the management of hepatitis C Gastroenterology 2006130225–30.(Erratum in 2006;130:1018 and 2006;131:979). [DOI] [PubMed] [Google Scholar]
- 13.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 14.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson IM, Brown RS, Jr, Freilich B, et al. WIN-R Study Group Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: A randomized trial. Hepatology. 2007;46:971–81. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002 – June 10–12, 2002. Hepatology. 2002;36(5 Suppl 1):S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Borozan I, Feld J, et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437–44. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Borozan I, Sun J, Anand N, et al. Validation of a gene signature predicting response to treatment in chronic hepatitis C virus infections Hepatology 2007464 Suppl 1256A–7A.(Abst) [Google Scholar]
- 19.Layden-Almer JE, Kuiken C, Ribeiro RM, et al. Hepatitis C virus genotype 1a NS5A pretreatment sequence variation and viral kinetics in African American and white patients. J Infect Dis. 2005;192:1078–87. doi: 10.1086/432760. [DOI] [PubMed] [Google Scholar]
- 20.Feld JJ, Nanda S, Huang Y, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–63. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics Canada. Aboriginal Peoples Highlight Tables, 2006 Census. <http://www12.statcan.ca/english/census06/data/highlights/Aboriginal/index.cfm?Lang=E> (Version current at May 13, 2008).