Abstract
The first description of autoimmune pancreatitis and elevated serum immunoglobulin-G4 (IgG4) in 2001 heralded further reports of several related autoimmune diseases with raised IgG4 levels. It is now recognized that a spectrum of overlap syndromes associated with increased IgG4 and biopsy evidence of IgG4-producing plasma cells, which has now been convincingly linked with cholangitis, autoimmune hepatitis, Sjögren’s syndrome, nephritis and retroperitoneal fibrosis. Collectively, this disease cluster is referred to as IgG4-related systemic disease. The importance of making the correct diagnosis is underscored by the management of individuals with IgG4-related systemic disease. In the first instance, patients generally have a dramatic response to immunosuppressive therapy, whereas patients with other forms of cholangitis and pancreatitis do not. Also, surgical management of pancreatic malignancy can be avoided once the correct diagnosis of IgG4-related disease has been made. In the present review, an overview of the current information regarding the role of IgG4 and IgG4-positive cells affecting the biliary system, pancreas and liver is provided.
Keywords: Autoimmune pancreatitis, Immunoglobulin G4-associated cholangitis, Immunoglobulin G4 hepatopathy, Immunoglobulin G4-related systemic disease
Abstract
La première description de la pancréatite autoimmune et des taux élevés d’immunoglobuline G4 sérique (IgG4) en 2001 ont suscité la publication d’autres rapports sur plusieurs autres maladies autoimmunes provoquant une augmentation des taux d’IgG4. On sait maintenant qu’un spectre de syndromes de chevauchement associés à une augmentation de l’IgG4 et à des données biopsiques sur les cellules plasmatiques produisant de l’IgG4, et qui sont reliés de manière décisive à la cholangite, à l’hépatite autoimmune, au syndrome de Sjögren, à la néphrite et à la fibrose rétropéritonéale. Collectivement, ce groupe de maladies est désigné par le terme maladie systémique reliée à l’IgG4. L’importance de poser le bon diagnostic est soulignée par la prise en charge des personnes atteintes d’une maladie systémique reliée à l’IgG4. Dans le premier cas, les patients réagissent généralement de façon remarquable à la thérapie immunosuppressive, tandis que ceux atteints d’autres formes de cholangite et de pancréatite n’y réagissent pas. De plus, il est possible d’éviter une prise en charge chirurgicale de la malignité pancréatique lorsque le diagnostic de maladie reliée à l’IgG4 est bien posé. Dans la présente analyse, les auteurs donnent un aperçu de l’information courante sur le rôle de l’IgG4 et des cellules positives à l’IgG4 touchant le système biliaire, le pancréas et le foie.
Traditionally, the overlap syndromes of autoimmune hepatitis with either primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC) present us with a conundrum. By definition, biliary disease should be absent in patients with autoimmune hepatitis but the definition of the so-called ‘overlap syndromes’ made room for both hepatitic and biliary disease. Despite the ongoing debate of classification, the recognition of these overlap syndromes is important because patients with features of autoimmune hepatitis and biliary disease often benefit from corticosteroid therapy, whereas immunosuppression is usually avoided in patients with PBC or PSC because of the limited benefit and potential for toxicity. These overlap conditions can manifest in different forms in patients with either PBC or PSC who develop flares of steroid-responsive hepatitis. Conversely, patients diagnosed with autoimmune hepatitis may subsequently develop PBC or PSC (1).
Cholangiographic studies suggest that up to 10% of patients presenting with autoimmune hepatitis have evidence of sclerosing cholangitis and these patients have a higher alkaline phosphatase level and tend to be younger in age (2). In a series of patients with PBC, approximately 5% fulfill criteria for overlap with autoimmune hepatitis and these patients benefit from corticosteroid treatment to prevent progressive fibrosis (3,4). In addition, immunoglobulin G4 (IgG4)-related systemic disease (ISD) may also present with a spectrum of biliary, hepatic and pancreatic diseases (5–9).
The observation of increased serum levels of IgG4 in patients with autoimmune pancreatitis (AIP) in 2001 was complemented by additional reports of tissue infiltration with abundant IgG4-positive cells in other organs (5–9). The ISD spectrum is characterized by increased levels of serum IgG4 and IgG4-rich lymphoplasmacytic infiltrate. This is associated with intense sclerosis that can lead to progressive damage that is potentially ameliorated by corticosteroid therapy (10,11). While the pancreas is the most commonly affected organ, the salivary glands, biliary tree, liver, retroperitoneum, lymph nodes, lungs, prostate and kidneys may also be involved (8,9,12–19). The present review provides an overview of ISD related to the pancreas, biliary system and the liver, and their specific clinical disease, such as AIP, IgG4-associated cholangitis (IAC) and IgG4-hepatitis.
AIP
The term ‘autoimmune pancreatitis’ was originally introduced in 1995 (20) to describe a syndrome characterized by irregular narrowing of the main pancreatic duct, swelling of the pancreas and a favourable response to corticosteroid treatment (21). Now, AIP is associated with high levels of IgG4 and human leukocyte antigen (HLA) DRB1*0405-DQB1*0401 haplotype with biopsy evidence of abundant IgG4 plasma cell infiltrate, parenchymal fibrosis and obliterative phlebitis (10). Previously, AIP was initially linked with Sjögrens’ syndrome, PSC and retroperitoneal fibrosis (22), but recent studies (7,23) suggest that these are extrapancreatic manifestations of AIP associated with high serum IgG4 levels and tissue infiltration with abundant IgG4-positive cells. The diagnostic implications of making the correct diagnosis are underscored by reports that the pancreatic and extrapancreatic manifestations of AIP have a favourable response to corticosteroid therapy, whereas the diseases they mimic do not. For example, biliary strictures can completely resolve in patients with AIP with corticosteroid therapy (24,25); this seldom occurs in patients with PSC (26,27).
Clinical features of AIP
Over 85% of patients of with AIP are men and older than 50 years of age. AIP was originally mistaken for pancreatic cancer because patients often presented with painless jaundice with tight biliary stricturing. Patients usually have evidence of chronic pancreatic inflammation and imaging studies show a classical sausage-shaped pancreas, pancreatic edema and peripancreatic inflammation, and pancreatic stones in a few cases. However, some patients may present remotely months to years after the initial onset of disease with pseudotumours resembling pancreatic cancer. In other patients, the pancreas may become atrophic, sometimes with evidence of calcification. Accordingly, AIP should be suspected in patients with unexplained pancreatic disease, presenting with obstructive jaundice, pancreatic mass or enlargement, pancreatitis, pancreatic atrophy or exocrine insufficiency.
Diagnostic criteria for AIP
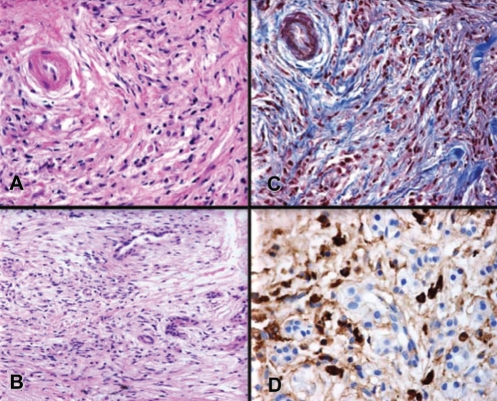
The original criteria developed by the Japan Pancreas Society (28) required the characteristic imaging features, such as a diffusely enlarged pancreatic gland with an irregular, attenuated pancreatic duct. The supportive criteria for AIP included elevated IgG4 levels and the histological features of lymphoplasmacytic sclerosing pancreatitis. This histological picture includes plasma cell infiltrate around small-sized ducts, swirling fibrosis centred around ducts and veins (storiform fibrosis), and obliterative phlebitis, where the infiltrate surrounds pancreatic venules while sparing arterioles (29–31). The immunostaining of pancreatic tissue revealed abundant IgG4-positive cells and also helped to distinguish AIP from alcoholic pancreatitis and the inflammatory infiltrate, which may be seen contiguous to pancreatic cancer (Figure 1) (7,32,33).
Figure 1).
Pancreatic histology of a 52-year-old woman with autoimmune pancreatitis. A Duct showing typical periductal lymphoplasmacytic inflammation and narrowing of the lumen. B Lymphoplasmacytic infiltration. C Periductal and interlobular fibrosis. D Immunohistochemical staining for immunoglobulin G4 showing marked immunoglobulin G4-positive plasma cell infiltrates. Courtesy of Dr Luis Uscanga, Teaching Department, INCMNSZ, Mexico City, Mexico
The histology, imaging, serology, organ involvement and response to steroid therapy diagnostic criteria reported by the Mayo Clinic (34) introduced additional criteria, namely extrapancreatic organ involvement, response to corticosteroids and autoantibodies reactive with nuclear antigens, carbonic anhydrase and lactoferrin (35,36). While the histological criteria are the gold standard for diagnosis of AIP, and are presumably present in all cases, the other features are invariably present but the diagnosis of AIP can be made with more confidence if patients have extrapancreatic manifestations and the disease is responsive to corticosteroid therapy (Table 1).
TABLE 1.
HISORt diagnostic criteria for autoimmune pancreatitis (AIP) and immunoglobulin G4 (IgG4)-associated cholangitis (IAC)
| AIP | IAC |
|---|---|
Histology
|
Histology
|
Imaging
|
Imaging
|
Serology
|
Serology
|
Other organ involvement
|
Other organ involvement
|
Response to steroid therapy
|
Response to steroid therapy
|
HISORt Histology, imaging, serology, organ involvement and response to steroid therapy; HPF High-power field. Adapted with permission from reference 43
Treatment of AIP
Persistent pancreatic enlargement or mass, intrahepatic biliary strictures, obstructive jaundice with distal biliary stricture, pancreatitis with pancreatic duct stricture, and uncontrolled diabetes and weight loss are all indications for therapy (37). Most patients respond with oral prednisone 40 mg daily for four weeks followed by a taper of 5 mg per week, during a period of eight weeks (Table 2). Generally, patients show complete resolution or marked improvement in the manifestations of disease (Table 2). Of note, a trial of corticosteroid therapy should not be used as a substitute for a rigorous search for etiology, and should be given only to patients with a negative evaluation for known etiologies of pancreatic and biliary disease, especially cancer.
TABLE 2.
Preferred Mayo Clinic initial steroid treatment protocol for autoimmune pancreatitis and immunoglobulin G4 (IgG4)-associated cholangitis
| Initial steroid regimen |
| Prednisone 40 mg/day oral for 4 weeks, then taper by 5 mg/week for a total of 11 weeks of treatment |
| Imaging |
Follow-up evaluation
|
| Laboratory evaluation |
Initial
|
Follow-up evaluation
|
ALT Alanine aminotransferase; AP Alkaline phosphatase; AST Aspartate aminotransferase; CA Carbohydrate antigen 19-9; ERCP Endoscopic retrograde cholangiopancreatography. Adapted with permission from reference 43
Resolution of symptoms often occurs quite rapidly in AIP, where obstructive jaundice usually resolves within two to three weeks. However, serological normalization of serum IgG4, and radiological resolution of pancreatic mass or enlargement, may take weeks to months (38). Symptomatic, radiological, serological or histological relapse may present during treatment, or after withdrawal of treatment. Symptomatic relapse is usually associated with radiological and serological relapses, whereas serological relapse may be observed in patients without symptoms or radiological evidence of disease activity (35).
Relapse with pancreatic and extrapancreatic disease occurs in approximately one-third of patients (39) and continued immunosuppressive therapy or at least close monitoring is recommended following complete remission, especially in patients lacking morphological and serological resolution (40).
Prognosis for AIP
The long-term prognosis for AIP is unknown, but it seems that patients generally do not experience exocrine and endocrine pancreatic insufficiency as seen in patients with alcohol-related chronic pancreatitis. Moreover, it is not clear if some patients develop pancreatic malignancy following presentation, and whether AIP would predispose to an increased incidence of pancreatic cancer (41).
IAC
More than four decades previously, two cases of PSC with pancreatic involvement were reported to be compatible with the diagnosis of IAC (42). It is likely that other reports of sclerosing cholangitis responsive to corticosteroid therapy may have also met the diagnostic criteria for AIP. Previously, IAC has been referred to using several different definitions that include PSC mimicking chronic pancreatitis, inflammatory pseudotumour from sclerosing cholangitis, pancreatic pseudotumour with multifocal idiopathic fibrosclerosis, lymphoplasmacytic sclerosing pancreatitis with cholangitis, sclerosing pancreatocholangitis, atypical PSC associated with unusual pancreatitis, lymphoplasmacytic sclerosing cholangitis without pancreatitis, AIP-associated sclerosing cholangitis, and IgG4-related lymphoplasmacytic sclerosing cholangitis. It is now recognized that IAC is part of the spectrum of steroid-responsive, multisystem fibroinflammatory diseases with the characteristic lymphoplasmacytic infiltrate rich in IgG4-positive cells (17). Unlike biliary strictures in patients with PSC, the lesions in patients with IAC respond well to steroids, and some stictures have been shown to resolve completely after immunosuppressive treatment.
Clinical presentation of IAC
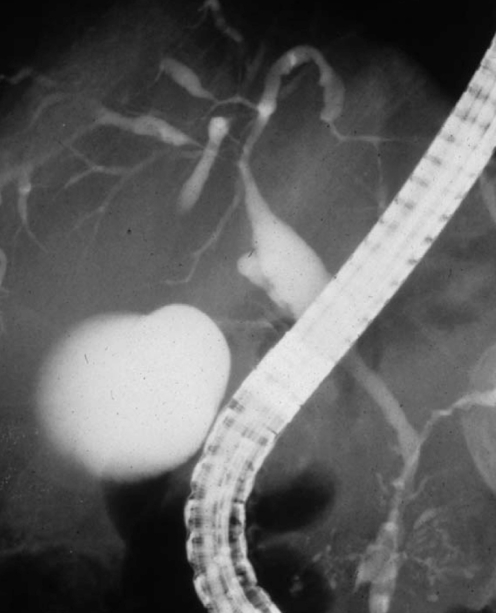
The largest study of IAC reported to date describes 53 patients from the Mayo Clinic database of AIP created in 1999 (43). In contrast to the young patients with the overlap syndrome of PSC and autoimmune hepatitis (2), most patients with IAC were older (mean age of 62 years) and 85% were men. These patients frequently presented with obstructive jaundice (77%), and had associated AIP (92%), increased serum IgG4 levels (74%) and abundant IgG4-positive cells in bile duct biopsy specimens (88%). Biliary strictures were confined to the intrapancreatic bile duct in 51% and involvement of the proximal extrahepatic and intrahepatic ducts was observed in 49% of patients (Figure 2) (43).
Figure 2).
Endoscopic retrograde cholangiopancreatography of a 76-year-old man with autoimmune pancreatitis and immunoglobulin G4-associated cholangitis. Multiple strictures are present in the hilar hepatic and intrahepatic bile ducts and irregular narrowing of the main pancreatic duct. Courtesy of Dr Terumi Kamisawa, Department of Internal Medicine, Tokyo Metropolitan Komagome Hospital (Tokyo, Japan). Reproduced with permission from reference 66
Diagnosis of IAC
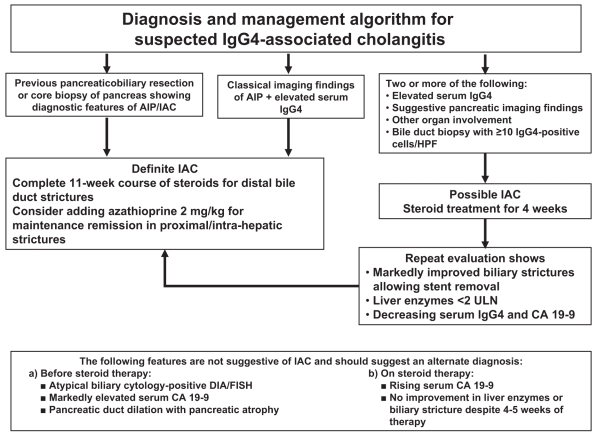
Early reports document the histological diagnoses of IAC after surgical resection, mainly following pancreatoduodenectomy (44–48). Indeed, obstructive jaundice is an uncommon initial feature in classic PSC, and this presentation should raise suspicion of IAC. Other clinical features that should prompt a diagnosis of IAC include the presence of biliary strictures with pancreatic duct irregularity, additional pancreatic manifestations or other organ involvement, and elevated serum IgG4 levels. Diagnosis is confirmed if biliary or other organ histology shows marked infiltration with IgG4-positive cells (greater than 10 cells/high-power field) or if the strictures resolve with corticosteroid treatment (Table 2 and Figure 3).
Figure 3).
Preferred algorithm for the diagnosis and management of immunoglobulin G4 (IgG4)-associated cholangitis. In all cases, a rigorous evaluation should be made to exclude biliary/pancreatic malignancy before initiating steroid therapy, which should not be used as a substitute for a thorough search for malignancy. AIP Autoimmune pancreatitis; CA Carbohydrate antigen 19-9; DIA Digital imaging analysis; FISH Fluorescent in-situ hybridization; HPF High-power field; IAC IgG4-associated cholangitis; ULN Upper limit of normal. Adapted from reference 43
Treatment of IAC
Currently, there is sufficient evidence to recommend a trial of corticosteroid therapy to treat bile duct lesions and pancreatitis in IAC. Resolution of jaundice, improvement in hepatic biochemistries and reversal of cholangiographic evidence of biliary stricturing have all been been reported with corticosteroid treatment (49–57). For example, Ghazale et al (43) reported the Mayo Clinic experience of treating IAC using corticosteroid therapy in 30 patients for initial therapy compared with surgical resection in 18 patients and conservative management in five patients. After a follow-up period in excess of two to three years, 53% of patients relapsed after corticosteroid withdrawal and 44% relapsed after surgery. Corticosteroid therapy normalized liver biochemistries in 61% of patients, and biliary stents were removed in 17 of 18 patients. Subsequently, 15 patients requiring further treatment for relapse after corticosteroid withdrawal responded to retreatment with corticosteroids, whereas seven patients treated with additional immunomodulatory drugs achieved steroid-free remission (43).
The initial treatment and follow-up protocol recommended by the Mayo Clinic for patients with IAC is outlined in Table 2. It is important to mention that this protocol assumes that a rigorous evaluation has been performed to rule out cholangiocarcinoma (CCA) and other cancers, and that the diagnosis of IAC has been established based on clinical, laboratory and imaging features. Of note, a trial of corticosteroid therapy should not be substituted for a thorough search of other causes of biliary strictures (Figure 3).
Some patients are relatively refractory to therapy, which was underscored by a recent report (58) of a patient with AIP and IAC unresponsive to both corticosteroids and 6-mercaptopurine. The systemic manifestations of IgG4-associated disease resolved following rituximab therapy, a monoclonal antibody directed against the CD20 antigen on B lymphocytes, permitting removal of the patient’s biliary stents. This study highlights the importance of making the diagnosis of AIP or IAC even if the disease is not responsive to standard corticosteroid therapy, considering that biological therapies such as rituximab may be a treatment option for refractory or recurrent disease.
A management dilemma is the timing of removal of the biliary stent, because the patient with obstructive jaundice and stricture is unlikely to be treated with steroids alone, and there is obviously a risk for ascending cholangitis if the inflammatory stricturing recurs after stent withdrawal.
Predictors of relapse in AIP and IAC
The Mayo Clinic experience suggests that relapse of IAC occurrs in 40% of patients after completing an 11-week course of corticosteroids (43). Post hoc analysis showed that proximal extrahepatic and intrahepatic biliary stricturing was associated with a high rate of relapse, but no other specific clinical predictors of relapse were identified. Park et al (59) studied 40 patients with AIP who achieved symptomatic, radiological, and serological remission on prednisolone 0.5 mg/kg per day for one to two months followed by a gradual taper to a maintenance dose of 2.5 mg/day to 7.5 mg/day for an average of six months before stopping treatment. In this study, 30% of patients relapsed. While no clinical predictors for relapse were identified, evaluation of HLA class II alleles showed that relapse of AIP was associated with the substitution of aspartic acid to a nonaspartic acid residue, at residue 57 of HLA DQβ1 (nonrelapse group 29.6% versus relapse group 100%; P <0.001; OR 3.4; 95% CI 1.9 to 6.0).
Differences between IAC and PSC
Demographic, radiographic, histological and immunological factors help to differentiate IAC from PSC. For example, Nishino et al (60) reported that patients with IAC were considerably older at the time of diagnosis and serum IgG4 levels were significantly higher compared with PSC patients. They also found that patients with PSC had cholangiographic evidence of band-like strictures, a beaded appearance, and a ‘pruned-tree’ appearance; however, patients with IAC were more likely to have segmental strictures and strictures of the distal one-third of the common bile duct. Histological studies of the liver demonstrate that fibrous obliterative cholangitis is only found in patients with PSC and advanced fibrotic changes corresponding to Ludwig’s stages 3 and 4 are seldom observed in IAC patients. The extent of IgG4-positive plasma cell infiltration is far more pronounced in the liver of patients with IAC compared with PSC patients, and cholangiographic studies show no improvement in PSC cases following corticosteroid therapy; all IAC patients however tend to have demonstrable radiographic improvement in the biliary strictures (61).
Furthermore, CCA develops in up to 10% to 30% of patients with PSC (63); development of CCA however, has not been reported with IAC. These differences between PSC and IAC suggests that both entities have major differences in their underlying etiology and pathophysiology.
IgG4-ASSOCIATED HEPATITIS
Recently, a case report (12) was published of one patient with hepatitis and chronic cholecystitis, raised IgG4 levels and IgG4-bearing plasma cells in the gallbladder wall, with no evident pancreatic disease. Liver biopsy showed interface and lobular hepatitis with rosette formation and marked plasma cell infiltration, and the immunostaining of liver tissue showed abundant IgG4-reactive plasma cells. The international autoimmune hepatitis group score was 18 (pretreatment definitive diagnosis greater than 15) and standard treatment resulted in remission of symptoms and normalization of liver biochemistries with prednisolone 40 mg daily for four weeks, tapered by 5 mg weekly to 5 mg daily. Because liver dysfunction is frequently seen in AIP, albeit usually cholestatic or obstructive, investigators previously interested in studying ISD started to evaluate the liver disease in these patients as well. In one study from Japan (13), liver histology and IgG4-bearing plasma cell infiltrates were evaluated in 17 patients with AIP before and after treatment and were compared with 63 patients with autoimmune hepatitis, PBC, PSC and chronic viral hepatitis. Liver histology was classified into various patterns that included portal inflammation with or without interface hepatitis, large bile duct obstructive features, portal sclerosis, lobular hepatitis, and canalicular cholestasis as well as combinations of some of these histological features in the same liver.
The number of hepatic IgG4-bearing plasma cells was significantly higher in patients with AIP than in controls and the extent of IgG4 plasma cell infiltrate directly correlated with serum IgG4 concentration. Moreover, glucocorticoid therapy reduced IgG4-bearing plasma cell infiltration of the liver and ameliorated other histological findings. The authors, therefore, concluded that all liver biopsies of patients with AIP showed a spectrum of biliary and hepatic changes associated with infiltration of IgG4-bearing plasma cells. Moreover, the histological features were modulated by corticosteroid therapy, suggesting that the liver disease is amalgamated into the spectrum of pathology associated with ISD (13). However, the role of liver biopsy in patients with AIP or IAC is still controversial, especially in patients with biliary obstruction.
CONCLUSIONS
The recognition of AIP, IAC, IgG4-hepatitis and ISD as a spectrum of syndromes responsive to immunosuppressive therapy has provided clinicians with a clearly defined phenotype with reproducible serological and histological feaures. While serum levels of IgG4 may only be elevated in 75% of individuals with ISD, the vast majority of patients should have IgG4 reactive plasma cells detectable by histology to make a tissue diagnosis. Furthermore, patients should also display the characteristic clinical, histomorphological and imaging features discussed in the present article. Unlike the overlap syndromes associated with PBC and PSC with autoimmune hepatitis, the diagnostic features of ISD are more clear-cut. Therefore, clinicians do not have to resort to the International Autoimmune Hepatitis Group scoring criteria (64) (that are reportedly meant to be restricted to ‘research use’ only) or the recently published simplified criteria (65) to make informed decisions regarding a trial of immunosuppressive therapy (1–4). As stated, a corticosteroid trial should only be given to patients with a negative work-up for known etiologies for other pancreatic or biliary diseases, such as pancreatic and biliary carcinoma.
The studies described in the present review tell us that measurement of IgG4 levels should be performed in all newly diagnosed patients with atypical pancreatic disease, autoimmune hepatitis and biliary stricturing with an atypical clinical presentation. A trial of corticosteroids should be instituted in patients with elevated IgG4 levels or histological evidence of pronounced IgG4 plasma cell infiltrate on biopsy, once clinical, biochemical and cholangiographic assessments have ruled out other neoplastic diseases. Further clinical studies are also required to understand the pathogenesis as well as the long-term prognosis of these very interesting, newly recognized clinical spectrums of disease.
REFERENCES
- 1.Gish RG, Mason A. Autoimmune liver disease. Current standards, future directions. Clin Liver Dis. 2001;5:287–314. doi: 10.1016/s1089-3261(05)70167-7. [DOI] [PubMed] [Google Scholar]
- 2.Abdalian R, Dhar P, Jhaveri K, Haider M, Guindi M, Heathcote EJ. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: Evaluating the role of routine magnetic resonance imaging. Hepatology. 2008;47:949–57. doi: 10.1002/hep.22073. [DOI] [PubMed] [Google Scholar]
- 3.Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology. 1998;28:296–301. doi: 10.1002/hep.510280203. [DOI] [PubMed] [Google Scholar]
- 4.Poupon R, Chazouilleres O, Corpechot C, Chretien Y. Development of autoimmune hepatitis in patients with typical primary biliary cirrhosis. Hepatology. 2006;44:85–90. doi: 10.1002/hep.21229. [DOI] [PubMed] [Google Scholar]
- 5.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T. IgG4-positive plasma cells specifically infiltrate various organs in autoimmune pancreatitis. Pancreas. 2004;29:167–8. doi: 10.1097/00006676-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–4. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande V, Chicano S, Finkelberg D, et al. Autoimmune pancreatitis: A systemic immune complex mediated disease. Am J Surg Pathol. 2006;30:1537–45. doi: 10.1097/01.pas.0000213331.09864.2c. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Notohara K, Levy MJ, et al. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23–8. doi: 10.1038/modpathol.3800689. [DOI] [PubMed] [Google Scholar]
- 10.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–6. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14:3948–55. doi: 10.3748/wjg.14.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umemura T, Zen Y, Hamano H, et al. IgG4 associated autoimmune hepatitis: A differential diagnosis for classical autoimmune hepatitis. Gut. 2007;56:1471–2. doi: 10.1136/gut.2007.122283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: Association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–71. doi: 10.1002/hep.21700. [DOI] [PubMed] [Google Scholar]
- 14.Zamboni G, Luttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: A study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, Kawashima A, Fletcher JG, et al. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology. 2007;242:791–801. doi: 10.1148/radiol.2423060003. [DOI] [PubMed] [Google Scholar]
- 16.Cheuk W, Yuen HK, Chu SY, Chiu EK, Lam LK, Chan JK. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:671–81. doi: 10.1097/PAS.0b013e318157c068. [DOI] [PubMed] [Google Scholar]
- 17.Björnsson E, Chari ST, Smyrk TC, et al. IgG4 associated cholangitis: Description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547–54. doi: 10.1002/hep.21685. [DOI] [PubMed] [Google Scholar]
- 18.Takato H, Yasui M, Ichikawa Y, et al. Nonspecific interstitial pneumonia with abundant IgG4-positive cells infiltration, which was thought as pulmonary involvement of IgG4-related autoimmune disease. Intern Med. 2008;47:291–4. doi: 10.2169/internalmedicine.47.0411. [DOI] [PubMed] [Google Scholar]
- 19.Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: Do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–8. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 21.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–6. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 22.Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep. 2005;7:101–6. doi: 10.1007/s11894-005-0047-4. [DOI] [PubMed] [Google Scholar]
- 23.Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811–2. doi: 10.1111/j.1572-0241.2003.08758.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishino T, Toki F, Oyama H, et al. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005;30:76–82. [PubMed] [Google Scholar]
- 25.Kojima E, Kimura K, Noda Y, et al. Autoimmune pancreatitis and multiple bile duct strictures treated effectively with steroid. J Gastroenterol. 2003;38:603–7. [PubMed] [Google Scholar]
- 26.Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2333–7. doi: 10.1111/j.1572-0241.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Gluud C. Glucocorticosteroids for primary sclerosing cholangitis. Cochrane Database Syst Rev. 2004;3:CD004036. doi: 10.1002/14651858.CD004036.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Pearson RK, Longnecker DS, Chari ST, et al. Controversies in clinical pancreatology: Autoimmune pancreatitis: Does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Weber SM, Cubukcu-Dimopulo O, Palesty JA, et al. Lymphoplasmacytic sclerosing pancreatitis: Inflammatory mimic of pancreatic carcinoma. J Gastrointest Surg. 2003;7:129–37. doi: 10.1016/s1091-255x(02)00148-8. [DOI] [PubMed] [Google Scholar]
- 30.Kloppel G, Luttges J, Lohr M, et al. Autoimmune pancreatitis: Pathological, clinical, and immunological features. Pancreas. 2003;27:14–9. doi: 10.1097/00006676-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Abraham SC, Wilentz RE, Yeo CJ, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: Are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110–20. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Kamisawa T, Funata N, Hayashi Y, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–7. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004;28:1114. doi: 10.1097/01.pas.0000126634.43301.45. [DOI] [PubMed] [Google Scholar]
- 34.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: Introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42:39–41. doi: 10.1007/s00535-007-2046-8. [DOI] [PubMed] [Google Scholar]
- 35.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kino-Ohsaki J, Nishimori I, Morita M, et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren's syndrome. Gastroenterology. 1996;10:1579–86. doi: 10.1053/gast.1996.v110.pm8613065. [DOI] [PubMed] [Google Scholar]
- 37.Pickartz T, Mayerle J, Lerch MM. Autoimmune pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:314–23. doi: 10.1038/ncpgasthep0837. [DOI] [PubMed] [Google Scholar]
- 38.Chari ST, Murray JA. Autoimmune pancreatitis, Part II: The relapse. Gastroenterology. 2008;134:625–8. doi: 10.1053/j.gastro.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Kamisawa T, Yoshiike M, Egawa N, et al. Treating patients with autoimmune pancreatitis: Results from a long-term follow-up study. Pancreatology. 2005;5:234–8. doi: 10.1159/000085277. [DOI] [PubMed] [Google Scholar]
- 40.Kamisawa T, Okamoto A, Wakabayashi T, Watanabe H, Sawabu N. Appropriate steroid therapy for autoimmune pancreatitis based on long-term outcome. Scand J Gastroenterol. 2008;43:609–13. doi: 10.1080/00365520701731263. [DOI] [PubMed] [Google Scholar]
- 41.Kamisawa T, Okamoto A. Prognosis of autoimmune pancreatitis. J Gastroenterol. 2007;42:59–62. doi: 10.1007/s00535-007-2052-x. [DOI] [PubMed] [Google Scholar]
- 42.Bartholomew LG, Cain JC, Woolner LB, Utz DC, Ferris DO. Sclerosing cholangitis: Its possible association with Riedel’s struma and fibrous retroperitonitis. Report of two cases. N Engl J Med. 1963;269:8–12. doi: 10.1056/NEJM196307042690102. [DOI] [PubMed] [Google Scholar]
- 43.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: Clinical profile and response to therapy. Gastroenterology. 2008;134:706–15. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: A variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–95. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 45.Stathopoulos G, Nourmand AD, Blackstone M, Andersen D, Baker AL. Rapidly progressive sclerosing cholangitis following surgical treatment of pancreatic pseudotumor. J Clin Gastroenterol. 1995;21:143–148. doi: 10.1097/00004836-199509000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Ectors N, Maillet B, Aerts R, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263–8. doi: 10.1136/gut.41.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieminen U, Koivisto T, Kahri A, Farkkila M. Sjogren’s syndrome with chronic pancreatitis, sclerosing cholangitis, and pulmonary infiltrations. Am J Gastroenterol. 1997;92:139–42. [PubMed] [Google Scholar]
- 48.Erdogan D, Kloek JJ, Ten Kate FJ, et al. Immunoglobulin G(4)-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008;95:727–34. doi: 10.1002/bjs.6057. [DOI] [PubMed] [Google Scholar]
- 49.Laitt RD, Hubscher SG, Buckels JA, Darby S, Elias E. Sclerosing cholangitis associated with multifocal fibrosis: A case report. Gut. 1992;33:1430–32. doi: 10.1136/gut.33.10.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chutaputti A, Burrell MI, Boyer JL. Pseudotumor of the pancreas associated with retroperitoneal fibrosis: A dramatic response to corticosteroid therapy. Am J Gastroenterol. 1995;90:1155–8. [PubMed] [Google Scholar]
- 51.Kazumori H, Ashizawa N, Moriyama N, et al. Primary sclerosing pancreatitis and cholangitis. Int J Pancreatol. 1998;24:123–7. doi: 10.1007/BF02788570. [DOI] [PubMed] [Google Scholar]
- 52.Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreatocholangitis responsive to steroid therapy. Lancet. 1999;354:43–4. doi: 10.1016/s0140-6736(99)00603-0. [DOI] [PubMed] [Google Scholar]
- 53.Horiuchi A, Kawa S, Hamano H, Ochi Y, Kiyosawa K. Sclerosing pancreatocholangitis responsive to corticosteroid therapy: Report of 2 case reports and review. Gastrointest Endosc. 2001;53:518–22. doi: 10.1067/mge.2001.110452. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-del Castillo CF, Sahani DV, Lauwers GY. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 27-2003. A 36-year-old man with recurrent epigastric pain and elevated amylase levels. N Engl J Med. 2003;349:893–901. doi: 10.1056/NEJMcpc030022. [DOI] [PubMed] [Google Scholar]
- 55.Kojima E, Kimura K, Noda Y, Kobayashi G, Itoh K, Fujita N. Autoimmune pancreatitis and multiple bile duct strictures treated effectively with steroid. J Gastroenterol. 2003;38:603–7. [PubMed] [Google Scholar]
- 56.Uehara T, Hamano H, Kawa S, Sano K, Honda T, Ota H. Distinct clinicopathological entity ‘autoimmune pancreatitis-associated sclerosing cholangitis’. Pathol Int. 2005;55:405–11. doi: 10.1111/j.1440-1827.2005.01845.x. [DOI] [PubMed] [Google Scholar]
- 57.Fukui T, Okazaki K, Yoshizawa H, et al. A case of autoimmune pancreatitis associated with sclerosing cholangitis, retroperitoneal fibrosis and Sjogren’s syndrome. Pancreatology. 2005;5:86–91. doi: 10.1159/000084494. [DOI] [PubMed] [Google Scholar]
- 58.Topazian M, Witzig TE, Smyrk TC, et al. Rituximab therapy for refractory biliary strictures in immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2008;6:364–6. doi: 10.1016/j.cgh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Park DH, Kim M-H, Oh HB, et al. Substitution of aspartic acid at position 57 of the DQß1 affects relapse of autoimmune pancreatitis. Gastroenterology. 2008;134:440–6. doi: 10.1053/j.gastro.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Nishino T, Oyama H, Hashimoto E, et al. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis. J Gastroenterol. 2007;42:550–9. doi: 10.1007/s00535-007-2038-8. [DOI] [PubMed] [Google Scholar]
- 61.Nakazawa T, Ohara H, Sano H, et al. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005;30:20–5. [PubMed] [Google Scholar]
- 62.Angulo P, Lindor KD. Primary sclerosing cholangitis. Hepatology. 1999;30:325–32. doi: 10.1002/hep.510300101. [DOI] [PubMed] [Google Scholar]
- 63.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–26. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 64.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report. Review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–38. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 65.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 66.Kamisawa T, Egawa N, Tsuruta K, et al. Primary sclerosing cholangitis may be overestimated in Japan. J Gastroenterol. 2005;40:318–9. doi: 10.1007/s00535-004-1543-2. [DOI] [PubMed] [Google Scholar]