Abstract
BACKGROUND:
Type 2 diabetes mellitus (T2D) in children and adolescents is a growing public health concern. Although the prevalence of T2D in First Nations children has been documented to be as high as 1% in central Canada, no paediatric data are available for any Aboriginal community in British Columbia (BC).
OBJECTIVE:
To determine the prevalence of obesity, glucose intolerance and the metabolic syndrome in children living in a remote BC First Nations community.
METHODS:
Children who were six to 18 years of age and living in the community of Hartley Bay, BC, participated in the study. A medical history, a physical examination and a 2 h oral glucose tolerance test were completed. Overweight was defined as a body mass index between the 85th and 95th percentiles, and obese was defined as a body mass index greater than or equal to the 95th percentile, which were standardized for age and sex.
RESULTS:
Thirty of 34 children (88%) participated (mean ± SD age 11.8±3.4 years). Ten children (33%) were obese, and five (17%) were overweight. There were seven children (23%) with abnormal glucose tolerance as per the 2007 American Diabetes Association criteria: five with only impaired fasting glucose ([IFG] 5.6 mmol/L to 6.9 mmol/L), one with both IFG and impaired glucose tolerance and one with T2D. However, using the 2008 Canadian Diabetes Association criteria, two children (6.7%) had abnormal glucose tolerance (one with IFG plus impaired glucose tolerance and one with T2D) because no child met the definition for IFG alone (6.1 mmol/L to 6.9 mmol/L). Four children (13%) met the criteria for the metabolic syndrome.
CONCLUSIONS:
There is a high prevalence of the components of the metabolic syndrome, including overweight, obesity and abnormal glucose tolerance, in the children of this community.
Keywords: Aboriginal, Metabolic syndrome, Screening, Type 2 diabetes, Youth
Abstract
HISTORIQUE:
Chez les enfants et les adolescents, le diabète de type 2 (DT2) est une préoccupation de santé publique croissante. Bien que la prévalence de DT2 chez les enfants des Premières nations soit documentée comme pouvant atteindre 1 % dans les régions du centre du Canada, il n’existe pas de données pédiatriques sur les communautés autochtones de la Colombie-Britannique (C.-B.)
OBJECTIF:
Déterminer la prévalence d’obésité, d’intolérance au glucose et de syndrome métabolique chez les enfants d’une communauté éloignée des Premières nations, en C.-B.
MÉTHODOLOGIE:
Les enfants de six à 18 ans de la communauté de Hartley Bay, en C.-B., ont participé à l’étude. Ils ont fourni leurs antécédents médicaux et subi un examen physique ainsi qu’une épreuve d’hyperglycémie de deux heures provoquée par voie orale. L’embonpoint était défini comme un indice de masse corporelle situé entre le 85e et le 95e percentile, et l’obésité, comme un indice de masse corporelle supérieur ou égal au 95e percentile, normalisés selon l’âge et le sexe.
RÉSULTATS:
Trente des 34 enfants (88 %) ont participé (âge moyen±ÉT de 11,8±3,4 ans). Dix enfants (33 %) étaient obèses et cinq (17 %) faisaient de l’embonpoint. Sept enfants (23 %) présentaient une intolérance au glucose selon les critères de 2007 de l’American Diabetes Association : cinq enfants ne présentaient qu’une hyperglycémie modérée à jeun (HMJ, 5,6 mmol/L à 6,9 mmol/L), un, à la fois une HMJ et une intolérance au glucose et un autre, un DT2. Cependant, selon les critères de 2008 de l’Association canadienne du diabète, deux enfants (6,7 %) présentaient une intolérance au glucose (un, une HMJ et une intolérance au glucose et l’autre, un DT2) parce qu’aucun enfant ne respectait seulement la définition d’HMJ (6,1 mmol/L à 6,9 mmol/L). Quatre enfants (13 %) respectaient les critères de syndrome métabolique.
CONCLUSIONS:
On constate une forte prévalence des éléments du syndrome métabolique chez les enfants de cette communauté, y compris l’embonpoint, l’obésité et une intolérance au glucose.
With the increase in obesity rates among youth, there has been a commensurate rise in the incidence of type 2 diabetes (T2D) worldwide (1). Of particular concern is the appearance and increasing prevalence of T2D and impaired glucose tolerance (IGT) among Aboriginal youth (2,3). In addition, the prevalence of diabetes is greater among girls (4), who may go on to be mothers with T2D or gestational diabetes; predisposition to develop T2D is higher in the children of these mothers (5). Therefore, this trend is likely to continue unless long-term sustainable strategies are developed to change the current situation (6). Effective prevention and management of T2D is essential given that the onset of T2D during childhood is associated with severe and early onset of microvascular complications (7–9).
In adults, T2D is closely associated with dyslipidemia and cardiovascular disease. The coexistence of central obesity and at least two additional criteria (high blood pressure, high triglyceride level, low high-density lipoprotein [HDL] cholesterol level and high fasting glucose) is referred to as ‘the metabolic syndrome’ (10), and affects 24% of the United States’ adult population (11). Compared with its prevalence in adults, the overall prevalence of the metabolic syndrome in children and adolescents is relatively low (3% to 4%); however, this syndrome is reported to be increasing rapidly in obese adolescents (12,13). This raises the possibility that a high prevalence of childhood obesity and diabetes will translate into an epidemic of premature cardiovascular disease in young adults.
The first paediatric case of T2D in Canada was reported in 1984 (14); however, among Aboriginal communities, Canadian prevalence data remain limited to reports in Ojibwa-Cree in Manitoba and northwestern Ontario (14–17), with few studies of First Nations or Aboriginal children in other regions and none of Pacific coast groups. Recently, the authors of the present paper were invited by a remote coastal First Nations community in British Columbia (BC) to collaborate and determine the scope of the problem in their children, and to develop culturally sensitive and geographically relevant programs to address the critical issue of childhood obesity and diabetes.
Hartley Bay (Gitga’at) is a remote First Nations fishing community on the Pacific coast of BC. The population of the village varies over time, and ranges from approximately 180 to 300 individuals. The community is accessible only by boat or float plane. There are no roads, and walking in the community is limited to the small network of boardwalks around which the community is built.
The investigators have a long-standing working relationship with the Tsimshian Nation, which includes the communities of Hartley Bay (Gitga’at), Kitkatla (Gitkxaahla) and Port Simpson (Lax Kw’alaams). The relationship was established through Brighter Smiles – a community-driven cooperative program initially developed for the reduction of dental caries and well-child surveillance (18). Following identification, by a Brighter Smiles team, of one child with asymptomatic T2D, the Hartley Bay (Gitga’at) community requested diabetes screening for all their children. The objective was to determine the prevalence of obesity, glucose intolerance and the components of the metabolic syndrome among these children to provide the requisite information to work collaboratively to develop sustainable prevention and treatment programs. In the current article, the results of the community of Hartley Bay, BC, are presented.
METHODS
The project was reviewed and approved by both the Children’s and Women’s Institute Research Review Committee and the University of British Columbia Clinical Research Ethics Board located in Vancouver, BC. It was a community-based participatory action research project, and also had the approval of the Band Council (elected), the hereditary band chief, the band elders (hereditary), the community health director and the community as a whole. Care was taken to ensure that parents and children were educated and that consent was truly informed. Parents or caregivers gave written informed consent and youth gave written assent. This process required two trips to the community before the actual screening. The process for obtaining community approval (although the screening had been requested by them) and for obtaining consent or assent, and the logistics of travelling by float plane to the community with all of the equipment and personnel necessary to complete the screening, is described elsewhere (19,20).
All children six to 18 years of age were invited to participate in the study. There was a 100% consent rate. A family history was obtained, including history of T2D, gestational diabetes and cardiovascular risk factors. The medical history included symptoms for diabetes (eg, polyuria, polydipsia, nocturia and weight loss). The physical examination included height measured to the nearest 0.1 cm (Seca 214 Portable Stadiometer, Seca North America West, USA), weight recorded to the nearest 0.1 kg (Conair digital electronic scale, Conair Corporation, USA), waist circumference measured (two measurements averaged) at the midpoint between the lowest rib and iliac crest at the end of expiration to the nearest 0.1 cm (21), blood pressure measured three times and the average taken of the second two measurements, and examination for the presence of acanthosis nigricans.
The diabetes screening protocol involved a standard 2 h oral glucose tolerance test (OGTT). Following a 12 h fast, an intravenous saline lock was inserted to avoid imposing two venipunctures on the children. Blood collected at baseline for glucose, and lipid profile (total cholesterol, triglycerides, HDL and low-density lipoprotein) was spun, aliquoted and stored in a −20°C freezer. Blood from the venipuncture was also immediately analyzed at the point-of-care for glucose (Ascensia Contour glucometer, Bayer Inc, Canada) and for glycated hemoglobin levels (Bayer DCA 2000 analyzer, Bayer Inc, Canada). The children then drank a 1.75 g/kg (maximum 75 g) glucose oral solution (ratio-GLUCOSE 75 g/300 mL, orange-flavoured [Ratiopharm, McKesson Canada]) and 2 h later, blood for glucose was collected. At the completion of the screening week, frozen samples were transported by float plane to BC Children’s Hospital in Vancouver for analysis.
Diagnostic criteria
Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), and then standardized for sex and age using data from the Centers for Disease Control and Prevention (USA) (22). Overweight was defined as a BMI between the 85th and 95th percentiles, and obesity as a BMI greater than or equal to the 95th percentile (23).
The metabolic syndrome was defined using the International Diabetes Federation (IDF) criteria (24) for children 10 to 16 years of age including:
- Obesity – 90th percentile or greater (or adult cut-off if lower) as assessed by waist circumference and the presence of two or more additional clinical features:
- ○ Triglycerides – 1.7 mmol/L or greater
- ○ HDL cholesterol – lower than 1.03 mmol/L
- ○ Blood pressure – 130 mmHg or greater (systolic) or 85 mmHg or greater (diastolic)
- ○ Fasting glucose – 5.6 mmol/L or greater or known T2D mellitus
The American Diabetes Association (ADA) criteria (25) were used for the diagnosis of diabetes to align with the IDF criteria for impaired fasting glucose (IFG):
Diabetes: fasting blood glucose of 7.0 mmol/L or greater, or 2 h OGTT glucose of 11.1 mmol/L or greater
IGT: 2 h OGTT glucose of 7.8 mmol/L to 11.0 mmol/L
IFG: fasting glucose of 5.6 mmol/L to 6.9 mmol/L
The number of children who met the 2008 Canadian Diabetes Association (CDA) (26) definition for IFG (6.1 mmol/L to 6.9 mmol/L) was also calculated to compare the results with previously published studies mentioned in the discussion section.
RESULTS
Although consent was obtained for all of the 34 eligible children, blood samples were obtained for 30 children (88%) because three were absent from the community, and one child failed to fast and had been scheduled for the date of departure and could not be rescheduled. There were 16 girls and 14 boys, ranging in age from 6.6 to 17.5 years (mean ± SD age 11.8±3.4 years). None of the children had symptoms suggestive of diabetes. Of the 30 children, 23 (77%) had a positive family history of diabetes and 22 (73%) had a family history of a comorbid medical condition such as myocardial infarction, stroke, dyslipidemia or microvascular complications. Ten children (33%) had evidence of acanthosis nigricans.
Five children (17%; two boys and three girls) were overweight and 10 children (33%; four boys and six girls) were obese. The prevalence of overweight and obesity was 50%. The prevalence of overweight or obesity in girls was 56% (nine of 16), and the prevalence of overweight or obesity in boys was 43% (six of 14).
Using the ADA criteria (25), five children had only IFG, one child had both IFG and IGT, and one child (the index case) had T2D. The prevalence of abnormal glucose tolerance in this population was 23%. The data on these seven children are presented in Table 1.
TABLE 1.
Characteristics of children with abnormal glucose tolerance
| Age, years | Sex | Glucose intolerance | Fasting BG* | 2 h BG† | BMI %ile‡ | # of MS criteria | Acanthosis nigricans |
|---|---|---|---|---|---|---|---|
| 17.4 | F | IFG | 5.8 | 4.7 | 96.0 | 2 | Yes |
| 13.0 | M | IFG | 6.0 | 6.9 | 97.7 | 2 | Yes |
| 17.2 | F | IFG | 6.0 | 5.6 | 95.2 | 3 | No |
| 13.3 | F | IFG | 5.6 | 6.1 | 95.1 | 5 | No |
| 14.4 | M | IFG | 5.7 | 7.7 | 92.5 | 1 | Yes |
| 11.7 | M | IFG + IGT | 6.1 | 7.8 | 98.4 | 3 | Yes |
| 10.4 | M | T2D | 6.6 | 12.1 | >99.0 | 3 | Yes |
Blood glucose (BG) measured after 12 h fast;
BG measured 2 h after a standard oral glucose tolerance test;
Body mass index (BMI) percentile (%ile) for age and sex. F Female; IFG Impaired fasting glucose; IGT Impaired glucose tolerance; M Male; MS The metabolic syndrome; T2D Type 2 diabetes
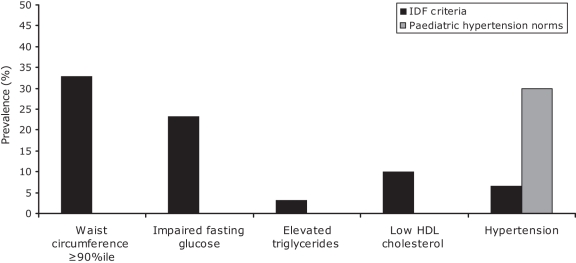
Using the IDF criteria, four children (13%) met the criteria for the metabolic syndrome; specifically, three children met three criteria and one child met all five criteria for the metabolic syndrome. In addition, five children had one metabolic syndrome component and three children had two components. The prevalence of each metabolic syndrome component is presented in Figure 1.
Figure 1).
Prevalence of the components of the metabolic syndrome. %ile Percentile; HDL High-density lipoprotein; IDF International Diabetes Federation
Using the paediatric definitions of the National High Blood Pressure Education Program Working Group (USA) for elevated blood pressure (27), which take into account age, sex and height percentile, nine children (Figure 1) had an abnormal blood pressure – either hypertension (95th percentile or greater) or ‘prehypertension’ (between the 90th and 95th percentiles).
DISCUSSION
Obesity is a major modifiable risk factor for T2D, and across Canada, the prevalence of childhood obesity is rising (28). Our study population had a prevalence of overweight and obesity similar to previously reported data among First Nations children. In Manitoba, a First Nations community had a high prevalence of overweight and/or obese children (64% of girls and 60% of boys) (29).
The children of Hartley Bay have a prevalence of glucose intolerance that is higher than previously reported in other First Nations communities. Screening of 256 children, 10 to 19 years of age, in two Eeyou Istchee (Eastern James Bay Cree) communities found no cases of prediabetes or T2D (30). Dean et al (15) screened 717 children in the remote northern Manitoba Ojibwa-Cree community of St Theresa Point First Nation and found a 1.1% overall prevalence of T2D (3.6% prevalence of T2D for girls 10 to 19 years of age) and 2.7% prevalence of IFG. Screening of 115 children from the Beausoleil First Nations Band of central Ontario revealed a 7% prevalence of IFG with no new cases of T2D (31). The high prevalence of IFG documented in the present study is likely related to the use of a lower cut-off for IFG (5.6 mmol/L or greater) recommended by both the ADA and the IDF. Using the 2008 CDA definition (26) for IFG (6.1 mmol/L or greater), the rate of glucose intolerance in Hartley Bay (6.7%) would have been more consistent with that observed by Dean et al (15). This screening study provides evidence to support the validity of the current CDA clinical practice guidelines for screening in children and adolescents in that the two Gitga’at youth determined to have abnormal glucose tolerance (one child with both IFG and IGT and one child with T2D) were 10 years of age or older and were both obese.
The prevalence of acanthosis nigricans observed in this population (33%) was slightly higher than that previously reported in First Nations children of central Ontario (communities of Beausoleil, Georgina Island and Mnjikaning) (21%), in which no glucose intolerance was confirmed (32). Of the seven children found to have glucose intolerance in our population, five (71%) had acanthosis nigricans.
Using the 2007 IDF criteria for the metabolic syndrome in children and adolescents (24), we documented a prevalence of 13% in this small population. One limitation of the IDF criteria is that the definition of elevated systolic and diastolic blood pressure is based on adult reference ranges and does not use previously published norms based on age, sex and height percentile (27). Using these paediatric-specific definitions for hypertension, seven additional children met the criteria for hypertension and this would have resulted in one additional case of the metabolic syndrome, increasing the percentage to 16.7%. This is much lower than the 40.5% reported by Kaler et al (33) in an Alberta community.
Limitations
While the absolute sample size in the present study is small, it represents 88% of school-aged children in the community, and is indicative of the commitment and considerable support from the community toward this project. Also, due to the complex interlinking of the families and the remoteness of the village, family medical history as well as social and cultural exposure are shared by many individuals. Nevertheless, for the community of Hartley Bay, these data are strong because of the magnitude of the issues seen.
In addition, there are currently no published norms for waist circumference in paediatric Aboriginal children, and therefore, the referenced waist circumference percentiles for children of Caucasian European background were used in the present study (24). Population studies of Canadian First Nations children are required to develop appropriate reference ranges for all anthropometric parameters to better determine prevalence of the metabolic syndrome and subsequent cardiovascular and diabetes risk.
CONCLUSIONS
There is a high prevalence of the components of the metabolic syndrome, including overweight, obesity and abnormal glucose tolerance, in the children of this community. The 2007 IDF criteria for the metabolic syndrome (24) may underestimate prevalence because the criteria for hypertension are based on adult cut-offs rather than paediatric-specific norms. Although these data cannot be extrapolated to other Pacific coast communities at this time, these results may encourage other rural and remote First Nations communities to take notice of the need to address obesity, T2D and the metabolic syndrome in their children.
The community of Hartley Bay was informed of these results, through presentations, along with general information on the pathophysiology and treatment of type 2 diabetes and the metabolic syndrome. For those children identified to have components of the metabolic syndrome, appropriate medical follow-up was established, including ongoing home glucose monitoring. Investigators are working collaboratively with this remote and rural community to develop lifestyle-modification programs that are culturally and environmentally appropriate. Future steps include implementation and evaluation of a school-based program called Action Schools! BC <www.actionschoolsbc.ca> to promote healthy eating and regular physical activity in these communities. More extensive physical activity, physical fitness and nutritional information will be collected at the time of program implementation. The collaborative team believes this project to be a long-term (20 years) commitment with an ongoing evaluation component to gain a better understanding of the natural history of diabetes progression in these children. Our goal is to develop programs that will involve the whole community, and effect change that the community is willing to implement and sustain over the long term – for generations to come.
Acknowledgments
The authors thank the community of Hartley Bay for their collaborative partnership, and Dr Rozmus for his assistance with obtaining informed consent and assent from participants.
Footnotes
FUNDING: The present study was funded by The Lawson Foundation (London, Ontario) and the University of British Columbia (Vancouver, British Columbia) Special Populations Fund. Dr Wahi received support through the Canadian Paediatric Society Resident Advocacy Grant. Dr Panagiotopoulos is the recipient of the Child & Family Research Institute Clinician Scientist Award.
CONTRIBUTIONS: Dr Wahi received financial support to perform this research from the Canadian Paediatric Society Resident Advocacy Grant, and she participated in data collection, analysis and manuscript preparation. Dr Zorzi participated in data collection, analysis and manuscript preparation. Dr Macnab participated in study concept and design and manuscript preparation. Dr Panagiotopoulos participated in the concept and design; data collection, analysis and interpretation of data; drafting and revising of the manuscript and approved the manuscript as submitted. Drs Wahi and Zorzi contributed equally to the work. All authors and the community of Hartley Bay have approved the final draft of this manuscript for submission.
REFERENCES
- 1.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: An epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–72. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 2.Young TK, Reading J, Elias B, O’Neil JD. Type 2 diabetes mellitus in Canada’s first nations: Status of an epidemic in progress. CMAJ. 2000;163:561–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Acton KJ, Burrows NR, Moore K, et al. Trends in diabetes prevalence among American Indian and Alaska native children, adolescents, and young adults. Am J Public Health. 2002;92:1485–90. doi: 10.2105/ajph.92.9.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollex RL, Hanley AJG, Zinman B, et al. Metabolic syndrome in aboriginal Canadians: Prevalence and genetic associations. Atherosclerosis. 2006;184:121–9. doi: 10.1016/j.atherosclerosis.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: Follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9:83–8. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Paediatric Society, First Nations and Inuit Health Committee [Principal author: K Saylor] Risk reduction for type 2 diabetes in aboriginal children in Canada. Pediatr Child Health. 2004;9:477–9. [Google Scholar]
- 7.Yokoyama H, Okudaira M, Otani T, et al. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care. 1997;20:844–7. doi: 10.2337/diacare.20.5.844. [DOI] [PubMed] [Google Scholar]
- 8.Krakoff J, Lindsay RS, Looker HC, et al. Incidence of retinopathy and nephropathy in youth-onset compared with adult onset type 2 diabetes. Diabetes Care. 2003;26:76–81. doi: 10.2337/diacare.26.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared to type 1 diabetes. Diabetes Care. 2006;29:1300–6. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet PZ, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab. 2005;19:405–19. doi: 10.1016/j.beem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Retnakaran R, Zinman B, Connelly PW, et al. Nontraditional cardiovascular risk factors in pediatric metabolic syndrome. J Pediatr. 2006;148:176–82. doi: 10.1016/j.jpeds.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Dean HJ, Mundy RL, Moffatt M. Non-insulin-dependent diabetes mellitus in Indian children in Manitoba. CMAJ. 1992;147:52–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Dean HJ, Young TK, Flett B, Wood-Steiman P. Screening for type-2 diabetes in aboriginal children in northern Canada. Lancet. 1998;352:1523–4. doi: 10.1016/S0140-6736(05)60329-7. [DOI] [PubMed] [Google Scholar]
- 16.Harris SB, Perkins BA, Whalen-Brough E. Non-insulin-dependent diabetes mellitus among First Nations children – New entity among First Nations people of northwestern Ontario. Can Fam Physician. 1996;42:869–76. [PMC free article] [PubMed] [Google Scholar]
- 17.Dean HJ, Sellers EAC, Young TK. Type 2 diabetes in youth in Manitoba, Canada, 1986–2002. Can J Diab. 2003;27:449–54. [Google Scholar]
- 18.Harrison RL, Macnab AJ, Duffy DJ, Benton DH. Brighter Smiles: Service learning, inter-professional collaboration and health promotion in a First Nations community. Can J Public Health. 2006;97:237–40. doi: 10.1007/BF03405594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panagiotopoulos C, Rozmus J, Gagnon R, Macnab A. Diabetes screening of children in a remote First Nations community on the west coast of Canada: Challenges and solutions. Rural Remote Med. 2007;7:771. [PubMed] [Google Scholar]
- 20.Gagnon R, Gagnon F, Panagiotopoulos C. Aircraft loading and freezer enhancements: Lessons for medical research in remote communities. Air Med J. 2008;27:188–92. doi: 10.1016/j.amj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Douketis JD, Paradis G, Keller H, Martineau C. Canadian guidelines for body weight classification in adults: Application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ. 2005;172:995–8. doi: 10.1503/cmaj.045170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention 2000 CDC growth charts: United States<http://www.cdc.gov/growthcharts/> (Version current at January 20, 2009).
- 23.Barlow SE, the Expert Committee Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 24.The International Diabetes Federation Consensus Definition of the Metabolic Syndrome in Children and Adolescents (2007) <http://www.idf.org/home/index.cfm?unode=CF99300B-ACEF-4970-B59D-F11A5306427B> (Version current at January 20, 2009).
- 25.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42–7. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 26.Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S10–11. 162–7. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 28.Tremblay MS, Willms JD. Secular trends in the body mass index of Canadian children. CMAJ. 2000;163:1429–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Young TK, Dean HJ, Flett B, Wood-Steiman P. Childhood obesity in a population at high risk for type 2 diabetes. J Pediatr. 2000;136:365–9. doi: 10.1067/mpd.2000.103504. [DOI] [PubMed] [Google Scholar]
- 30.Dannenbaum D, Torrie J, Noel F, et al. Undiagnosed diabetes in 2 Eeyou Istchee (Eastern James Bay Cree) communities: A population-based screening project. Can J Diabetes. 2005;29:397–402. [Google Scholar]
- 31.Smith WG, Gowanlock W, Babcock K. Type 2 diabetes in First Nation children: A collaborative effort to assess and prevent disease. Paediatr Child Health. 2001;6:755–9. doi: 10.1093/pch/6.10.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith WG, Gowanlock W, Babcock K, et al. Prevalence of acanthosis nigricans in First Nations children in Central Ontario, Canada. Can J Diabetes. 2005;29:410–4. [Google Scholar]
- 33.Kaler SN, Ralph-Campbell K, Pohar S, et al. High rates of the metabolic syndrome in a First Nations Community in western Canada: Prevalence and determinants in adults and children. Int J Circumpolar Health. 2006;65:389–402. doi: 10.3402/ijch.v65i5.18139. [DOI] [PubMed] [Google Scholar]