Abstract
BACKGROUND:
Although several clinical trials have evaluated the impact of n-3 polyunsaturated fatty acid (PUFA) on patients with attention-deficit hyperactivity disorder (ADHD), changes in plasma PUFA composition were not always assessed following n-3 supplementation. Furthermore, no reports are available on the efficacy of n-3 PUFA in Canadian youth with ADHD.
OBJECTIVES:
To determine fatty acid (FA) composition, and the efficacy and safety of n-3 PUFA supplementation on ADHD clinical symptoms in French Canadian primary school children.
PATIENTS AND METHODS:
The Strengths and Weaknesses in ADHD and Normal Behaviors (SWAN) and Conners’ questionnaires were used to assess changes in ADHD symptoms in 37 children (only 26 children completed the study from zero to 16 weeks). They were divided into two groups (A and B), and participated in a 16-week, double-blind, one-way, crossover randomized study. In the first phase, group A received the n-3 PUFA supplement and group B received n-6 PUFA (sunflower oil) as a placebo. During the second phase, group B received the active n-3 PUFA supplement that was continued in group A. FA composition and lipid profile were assessed during the phases of the study.
RESULTS:
FA differences between groups were observed in the 26 patients. Supplementation with n-3 PUFA resulted in significant increases in eicosapentaenoic and docosahexaenoic acids in group A, while group B was enriched with alpha-linolenic, gamma-linolenic and homo-gamma-linolenic acids. The n-3 PUFA supplement was tolerated without any adverse effects. A statistically significant improvement in symptoms was noted based on the parent version of the Conners’ questionnaire from baseline to the end of phase 1, and this amelioration continued from phases 1 to 2, although the latter changes from phases 1 and 2 were not statistically significant in any of the subscales except for the subscale measuring inattention in group B. The improvement was greater in patients from group A in phase 1 and in patients from group B in phase 2. A subgroup of eight patients (four in each group) displayed a statistically significant clinical improvement following the administration of the n-3 PUFA supplement, particularly for the inattention and global Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, total Conners’ subscales.
CONCLUSIONS:
A subgroup of children with ADHD who used n-3 PUFA supplements achieved and maintained symptom control. The data of the present study also supported n-3 PUFA safety and tolerability, but limited changes were noted in the FA profile in French Canadians with ADHD.
Keywords: ADHD, DHA, EPA, Fatty acids, Fish oil, Lipids
Abstract
HISTORIQUE :
Même si plusieurs essais cliniques ont porté sur les répercussions des acides gras polyinsaturés (AGPI) n-3 chez les patients ayant un trouble de déficit de l’attention avec hyperactivité (TDAH), on n’a pas toujours évalué les modifications à la composition plasmatique de l’AGPI après l’administration d’un supplément de n-3. De plus, il n’existe aucun rapport sur l’efficacité de l’AGPI n-3 chez les jeunes canadiens ayant un TDAH.
OBJECTIF:
Déterminer la composition des acides gras (AG) ainsi que l’efficacité et l’innocuité du supplément d’AGPI n-3 sur les symptômes cliniques de TDAH d’enfants canadiens français du primaire.
PATIENTS ET MÉTHODOLOGIE :
Les auteurs ont utilisé le questionnaire SWAN sur les forces et les faiblesses des symptômes de TDAH et des comportements normaux et le questionnaire de Conners pour évaluer les modifications aux symptômes de TDAH de 37 enfants (seulement 26 ont terminé l’étude de la semaine zéro à la semaine 16). Ils ont réparti les enfants en deux groupes (A et B), qui ont participé à une étude aléatoire transversale unilatérale à double insu de 16 semaines. Pendant la première phase, le groupe A a reçu le supplément d’AGPI n-3 et le groupe B, de l’AGPI n-6 (huile de tournesol) comme placebo. Pendant la deuxième phase, le groupe B a reçu le supplément d’AGPI n-3 actif, qui a été maintenu dans le groupe A. Les auteurs ont évalué la composition d’AG et le profil lipidique pendant les deux phases de l’étude.
RÉSULTATS :
On a observé des différences d’AG chez les 26 patients des deux groupes. Le supplément d’AGPI n-3 a entraîné une augmentation significative d’acide eicosapentanoïque et docosahexanoïque au sein du groupe A, tandis que le groupe B avait davantage d’acides alpha-linoléniques, gamma-linoléniques et homo-gamma-linoléniques. Le supplément d’AGPI n-3 était toléré sans effets indésirables. Les auteurs ont remarqué une diminution statistiquement significative des symptômes d’après la version du questionnaire de Conners des parents entre le début et la fin de la phase 1, et cette diminution s’est poursuivie entre les phases 1 et 2, même si elle n’était statistiquement significative dans aucune des sous-échelles sauf celle qui mesurait l’inattention au sein du groupe B. La diminution des symptômes était plus importante au sein du groupe A pendant la phase 1 et du groupe B pendant la phase 2. Un sous-groupe de huit patients (quatre de chaque groupe) ont présenté une amélioration clinique statistiquement significative après l’administration du supplément d’AGPI n-3, notamment pour ce qui est de l’indice d’inattention et de l’indice global des sous-échelles de Connors, conformément au Manuel diagnostique et statistique des troubles mentaux, quatrième édition.
CONCLUSIONS :
Un sous-groupe d’enfants ayant un TDAH qui ont pris le supplément d’AGPI n-3 ont réussi à contrôler leurs symptômes et à maintenir ce contrôle. Ces données soutiennent également l’innocuité et la tolérabilité de l’AGPI n-3, mais les changements observés étaient limités dans le profil d’AG des Canadiens français ayant un TDAH.
Attention-deficit hyperactivity disorder (ADHD) is the most common behaviour disorder diagnosed in children (1), and thus, it represents an important public health problem. The incidence reported in the Canadian population varies from 2% to 12% (2,3). In a study (3) conducted in 1999 in a Montreal (Quebec) suburb, the incidence rate of ADHD was 7.23% in boys and 1.94% in girls. This proportion is especially impressive if one considers that ADHD is often accompanied by psychiatric comorbidities (1,4). Moreover, longitudinal studies (4–6) have shown that behaviour disorders persist into adulthood in approximately 40% of children, and may be responsible for significant psychological and psychosocial morbidities.
Several studies (4,7–10) have measured the beneficial effects of psychostimulants in the treatment of ADHD. Efficacy rates of 70% to 90% have been observed in short-term randomized clinical trials, but not in long-term studies with methylphenidate or amphetamines. Although pharmacological intervention was effective after two to five years of treatment (11–13), it resulted in side effects that persisted even after five years. As a result, many parents prefer alternative treatments for the management of ADHD symptoms in their children. This is a consistent finding in Canada (14), the United States (4,15–18) and elsewhere in the world (19,20). Approximately 7% to 64% of ADHD children are treated with a variety of alternative approaches (15,21–25).
The use of n-3 polyunsaturated fatty acid (PUFA) supplements may represent an efficacious treatment for ADHD, with minimal side effects. ADHD may be secondary to essential fatty acid (EFA) deficiency (26) because children with ADHD more often present symptoms suggestive of a deficiency in n-3 PUFA, such as polydipsia, polyuria and eczema (25,27,28). Some studies (27–30) have suggested that children with ADHD have an abnormal PUFA profile and, in particular, a decrease in the plasma concentration of arachidonic acid, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Depletion of EFA may impact the brain, which contains the highest lipid content and requires n-3 PUFA for development, intercommunication and function; DHA plays a crucial role in brain development (31–33), whereas EPA is important mostly for function (34–36).
Several clinical studies have been performed to evaluate the efficacy and safety of n-3 PUFA in children with ADHD. Some of them did show an improvement in ADHD symptoms, although, according to Busch (37), who recently reviewed all of the studies performed in recent years, the results were not entirely conclusive. The present study was devised to investigate the efficacy of n-3 PUFA supplement when compared with a placebo on the core ADHD symptoms in French Canadian children.
METHODS
Study design
The present study was a 16-week, double-blind, one-way, crossover randomized study. The study sample was randomly assigned to two groups. The treatment consisting of n-3 PUFA supplement (NutriSanté Inc, Canada) was administered in two phases, each of eight weeks duration. In the first phase, group A received an active n-3 PUFA supplement and group B received equivalent quantities of n-6 PUFA (sunflower oil) as a placebo. During the second phase, group B received the active n-3 PUFA supplement that was continued in group A. The study protocol was approved by the Ethics Committee of CHU Sainte-Justine (Montreal, Quebec).
Dosing
To determine the dose of EPA to be administered to the subjects, the clinical trials conducted in children with ADHD were reviewed. In general, the doses of EPA that were used were probably too low compared with those used in adults. As reported by Peet and Stokes (38), dosages of 1 g/day to 2 g/day, particularly of EPA, are required to manage psychiatric and behavioural disorders, because adequate n-3 PUFA levels cannot be attained safely by diet alone. Therefore, in the present study, the nutritive supplement consisted of a calculated dose of 20 mg/kg/day to 25 mg/kg/day of EPA and 8.5 mg/kg/day to 10.5 mg/kg/day of DHA. This combination of EPA and DHA was used because of the positive results achieved in previous studies (25,39). Supplements used in the present study also contained phospholipids (PL) and tiny amounts of vitamin E. More precisely, each capsule of the active n-3 PUFA supplement consisted of 25 mg of PL, 250 mg of EPA, 100 mg of DHA and 3.75 U of alpha-tocopherol (vitamin E). Vitamin E was added to prevent oxidation of the fatty acids (FA) in the capsule. The total daily dose of the active n-3 PUFA supplement was administered once or twice per day according to the body weight of the children. Because the capsules could not be divided, participants weighing 16 kg to 25 kg received two capsules daily (500 mg EPA), those weighing 26 kg to 35 kg received three capsules daily (750 mg EPA), and those weighing 36 kg to 45 kg received four capsules daily (1000 mg EPA). The placebo capsule contained 500 mg of sunflower oil, with no n-3 PUFA, and was composed of 70% linoleic acid (a precursor of n-6 PUFA), 20% oleic acid, and palmitic and stearic acid (5% each). A similar amount of vitamin E (as per n-3 capsules) was included in the n-6 capsules.
Subjects
The study aimed to enroll 37 children from the ADHD clinic of CHU Sainte-Justine. The children were between six years 11 months of age and 11 years 11 months of age, with a Diagnostic and Statistical Manual of Mental Disorders, Fourth edition (DSM-IV) diagnosis of ADHD based on the results of the parent and teacher Conners’ questionnaires and a clinical evaluation. Preschool-aged children and adolescents were not included because ADHD is difficult to diagnose in preschool-aged children, and the varying behaviours of the adolescent period generally result in a heterogeneous presentation of symptoms. Therefore, the age groups selected for the current study present the least variability in the symptom expression of ADHD. Furthermore, subjects had an IQ score above 85, as evaluated by administering the Wechsler Intelligence Scale for Children, third edition. The parents of the subjects agreed to provide three blood samples from their children during the study.
Subjects diagnosed with mental health disorders (excluding those with characteristic comorbidity associated with ADHD), such as depression, anxiety, tic disorder, conduct disorder, specific learning disabilities and children receiving one or more of the medications, including a psychostimulant or nonstimulant (eg, atomoxetine), sedatives, anxiolytics and antipsychotics, were not included in the present study. Subjects with a medical condition requiring long-term treatment (leukemia or cancer), a chronic neurological condition (cerebral palsy or a metabolic disease) or a paroxysmal disorder (epilepsy); those with allergy to sunflower oil (contained in the placebo) or to fish (the active ingredient in the experimental treatment); and those with coagulation abnormalities, who were candidates for surgery during the duration of the study, or who received anticoagulants, were also excluded. Also, if more than one child in the same family had a diagnosis of ADHD, only one child was eligible to participate in the study and this child was selected at random. Subjects who consumed natural medicine products have an increased risk of hemorrhage, and those in whom a surgical procedure was planned were excluded. Patients who were found to consume fish, flaxseed oil and foods enriched with n-3 PUFA (eggs, or milk containing n-3 PUFA supplements) during the course of the study were also excluded. Every patient had a diagnosis of ADHD based on the medical interview, the medical examination and the results of the parent and teacher versions of the Conners’ questionnaires as conducted by two of the investigators (SB and MV) with the subjects and their families.
Efficacy assessments
The efficacy of the treatment was assessed using two measures: The Strengths and Weaknesses in ADHD and Normal Behaviors (SWAN) and Conners’ rating scales completed by the parents or caregivers and the teachers. To evaluate behaviour, the SWAN-F questionnaire, an adapted version of the SWAN, was used. The questionnaire includes the 18 symptoms of ADHD, as defined by the DSM-IV. Each criterion was evaluated according to a scale that ranges from −3 (very below average) to 3 (very above average). The day’s events that may have impacted the child’s behaviour were also documented at the end of the questionnaire. The SWAN questionnaire was completed once per week. A research assistant followed up with the parent and the teacher weekly, either by telephone or by e-mail. At the beginning of each study visit, the end of the first eight weeks and the end of the study, parents or caregivers completed a Conners’ parent rating scale.
Adverse event assessments
All side effects and health problems that the child presented during the week were documented by the research assistant, who called the families weekly. Finally, these children were all evaluated by one of the investigators (SB or MV) at the beginning of the study after eight weeks and at the end of the study to document clinical improvements or side effects.
Neuropsychological tests
The neuropsychological tests included the continuous performance test (CPT) – a standardized measure of sustained attention. A ‘Go-No-Go’ test was used to measure impulsivity. These tests were administered by qualified neuropsychologists at the same time intervals as the blood tests.
Blood samples
Samples were collected at fasting, for the study of lipid profile and FA composition, as described previously (40–42). For transesterification, an internal standard consisting of nonadecanoic acid (C19:1) was precisely weighed, dissolved in 2 mL of methanol-hexane 4:1 (volume to volume) and added to plasma. Then acetyl chloride (200 μL) was slowly added. Each tube was tightly closed and subjected to methanolysis at 100°C for 1 h. After the tubes had been cooled, 5 mL of 6% potassium carbonate solution was slowly added to stop the reaction and neutralize the mixture. The tubes were then shaken and centrifuged, and an aliquot of the hexane upper phase was injected into the chromatograph using the Varian 8400 GC AutoSampler system (Varian Inc, Canada). The FAs were identified by comparison with the expected retention times of known standards and then analyzed with Galaxy Chromatography Data System software (Varian Inc, USA).
The lipid profile was determined by measuring triglycerides (TG), PL, total and free cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol (HDL-C), apolipoproteins (apo) A-I and B and antioxidant (vitamin A, beta-carotene and vitamin E) levels as described previously (42).
Diet assessment
A detailed dietary survey was conducted by telephone interview for the 24 families (11 from group A and 13 from group B) who participated in the study.
Statistical methods
To evaluate the effect of the treatment on Conners’ scores in each of the groups (A and B) and the individual changes in scores of the Conners’ subscales from three time points, baseline to phase 1, phase 1 to phase 2, and baseline to phase 2 were computed. To test whether the mean change in scores was different from zero, the Wilcoxon’s signed rank test for paired data was used. An alpha level of 0.05 was used to indicate that there was a statistically significant change in a score from two time points. A similar procedure was applied for SWAN scores.
The estimate of pair-wise Pearson coefficients among lipid measures (alpha-linolenic [ALA], DHA and EPA) and selected subscales of the SWAN (inattention, impulsivity, oppositional defiant disorder and total), and Conners’ (global, inattention, hyperactivity, impulsivity and total) questionnaires was used to assess whether components of the FA composition in children correlated with the severity of their ADHD symptoms. Correlations were estimated at baseline and after phases 1 and 2.
Statistical analyses were performed using R version 2.5.1 (citation R) (R Development Core Team, Austria <www.R-project.org>).
RESULTS
A total of 37 subjects were enrolled in the study. Twenty-six children succeeded in completing 16 weeks of the study (13 in each group). Eleven subjects withdrew their participation during the study: eight during phase 1 (five from group A and three from group B) and three during phase 2 (one from group A and two from group B) of the study. This included two subjects who withdrew after a few days because they were not able to swallow the capsule. In none of these children was withdrawal justified by side effects; withdrawal in all cases was due to the fact that parents and mostly teachers believed that symptoms of ADHD were better controlled by psychostimulant medication. The baseline characteristics of the study population are summarized in Table 1.
TABLE 1.
Characteristics of the study participants
| Parameters | ADHD (n=26)
|
|
|---|---|---|
| Group A: n-3 PUFA (nine boys, four girls) | Group B: Placebo (nine boys, four girls) | |
| Age, years | 9.27±0.40 | 9.09±0.50 |
| Weight, kg | 32.40±2.71 | 34.52±3.53 |
| Height, cm | 133.21±3.18 | 134±3.90 |
Data presented as mean ± SD. ADHD Attention-deficit hyperactivity disorder; PUFA Polyunsaturated fatty acid.
Efficacy
Despite efforts to facilitate parent and teacher participation in the study, an important number of questionnaires were not completed. During phase 1 of the study, 21 teachers and 24 parents completed the SWAN questionnaire. During phase 2, this questionnaire was completed by 17 teachers and 22 parents.
The Conners’ questionnaire was completed by 33 of 37 parents before the beginning of the study, 26 of 29 parents (13 in each group) after phase 1, and 20 of 26 parents (10 in each group) of the subjects who completed the study.
Analysis of the teacher-completed SWAN questionnaire revealed no significant differences in the symptoms of ADHD subjects in groups A or B during the 16-week study. In contrast, an improvement, particularly in inattention, was seen in the parent SWAN questionnaire. However, the improvement was not statistically significant (data not shown). For subjects in group B, only impulsivity showed a tendency to improvement, but this finding did not reach statistical significance (data not shown).
The results from the parent version of the Conners’ questionnaire showed that subjects, from baseline (time 0) to the end of phase 1 (time 1), showed a statistically significant improvement in several of the Conners’ subscales measuring symptoms of inattention and impulsivity in both groups (Table 2). In general, during this time frame, subjects in group A showed a greater, but not statistically significant, improvement in comparison with subjects in group B.
TABLE 2.
Mean changes in Conners’ subscales from baseline following n-3 polyunsaturated fatty acid (PUFA) supplementation
| Conners’ subscales | Baseline values
|
Differences in % relative to baseline values
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Time 0
|
Time 0 to time 1
|
Time 1 to time 2
|
Time 0 to time 2
|
||||||
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | ||
| A | Oppositional | 64.3 | 61.7 | −3.7 | −4.9 | −2.3 | −7.1 | −6.5 | −9.5* |
| B | Cognitive problems/inattention | 77.0 | 71.4 | −7.5* | −7.3* | −2.4 | −6.4 | −9.3* | −12.1* |
| C | Hyperactivity | 68.1 | 67.0 | −8.8* | −2.6 | −1.4 | −2.6 | −10.1* | −8.9* |
| D | Anxious-shy | 57.2 | 54.1 | −5.2* | −3.7* | −1.9 | −0.6 | −7.1 | −7.3 |
| E | Perfectionism | 54.9 | 53.8 | −5.8 | −3.0 | −2.1 | −6.0 | −8.1* | −6.6* |
| F | Social problems | 67.1 | 61.0 | −9.8* | −7.6* | 1.6 | −0.1 | −7.7 | −6.5 |
| G | Psychosomatic | 60.0 | 55.1 | −4.8 | −3.0 | −4.0 | −3.6 | −6.3 | −4.1 |
| H | ADHD index | 74.6 | 70.8 | −7.6* | −6.5* | −3.8 | −5.1 | −11.2* | −10.4* |
| I | Conners’ global index: Restless–impulsive | 69.8 | 68.2 | −7.9* | −6.5* | −1.0 | −4.3 | −10.7* | −10.0* |
| J | Conners’ global index: Emotional lability | 59.5 | 55.5 | −4.3 | −0.9 | 2.0 | −4.7 | −4.5 | −3.3 |
| K | Conners’ global index: Total | 67.9 | 65.3 | −7.3* | −4.8 | −0.2 | −4.7 | −9.5* | −8.6* |
| L | DSM-IV symptoms: Inattentive | 76.4 | 71.3 | −9.1* | −7.3* | −2.4 | −8.3* | −10.9* | −14.8* |
| M | DSM-IV symptoms: Hyperactive–impulsive | 67.7 | 66.9 | −7.2* | −3.1 | −0.8 | −2.4 | −8.9* | −7.1* |
| N | DSM-IV symptoms: Total | 74.2 | 71.1 | −8.8* | −5.4 | −2.3 | −6.7 | −11.2* | −12.1* |
Conners’ questionnaires were used to assess changes in attention-deficit hyperactivity disorder (ADHD) symptoms in 26 children, divided into two groups A and B, participating in a 16-week, double-blind, one-way, crossover randomized study. In the first phase, group A received n–3 PUFA supplement and group B received n-6 PUFA (sunflower oil) as placebo. During the second phase, group B received the active n–3 fatty acid supplement that was continued in group A.
P<0.05 from baseline values. DSM-IV Diagnostic and Statistical Manual of Mental Disorders, Fourth edition
From phases 1 to 2 (time 2), subjects in groups A and B showed further improvement in their symptoms. Although group B subjects tended to improve in several subscales than those in group A, the extent to which they did was not statistically significant except for subscale DSM-IV – inattention (Table 2).
Clinical evaluation
At the end of the study, families were asked whether they wished to continue with the n-3 PUFA supplement, restart or begin taking a psychostimulant. Twenty-three of the 26 families (88%) who completed the study voluntarily decided to continue taking the supplement, given the apparently beneficial effects of n-3 PUFA.
The behaviour and academic performance of the 26 subjects who completed the study were reviewed by the investigators on each visit, in the presence of the parents and the child. Based on these clinical observations, it was concluded that six subjects had no significant improvement, while 12 subjects reported minimal amelioration. However, at the end of the study, eight subjects (four each in groups A and B) were evaluated as having made a significant clinical improvement following the administration of the n-3 PUFA supplement. Behavioural changes were compared between baseline (time 0) and the end of phase 2 (time 2) for the Conners’ global index – total, DSM-IV symptoms – inattention, DSM-IV symptoms – hyperactive-impulsive and DSM-IV symptoms – total, in these eight children relative to 18 others who also completed the study. A statistical significance was measured for DSM-IV symptoms – inattention and DSM-IV symptoms – total, which indicated a greater improvement in the eight subjects. The P value resulting from the comparison of the improvements in the two scales between the two groups was 0.0009 for DSM-IV symptoms – inattention and 0.0059 for DSM-IV symptoms – total.
For the SWAN questionnaire, formal statistical comparisons between the two groups were not performed because the number of questionnaires completed made it difficult to draw a quantitative conclusion.
Adverse effects
The n-3 PUFA supplement was well tolerated. During the 16 weeks of the study, no serious treatment-related adverse events were reported in the subjects.
Dietary survey
Analysis of the diet questionnaire completed by telephone interview with 24 families revealed that families whose children participated in the study had diets typical of the Quebec population (43–45). Furthermore, these families did not consume less or exceed the average consumption of foods rich in n-3 PUFA when compared with the general population (data not shown).
FA analysis
Before administration of the n-3 PUFA supplement, the previous study (46) showed that the concentrations of plasma TG and PL were lower, whereas those related to total cholesterol, HDL-C and apo A-I were higher in ADHD patients compared with healthy age- and sex-matched controls. The proportion of plasma EPA and DHA was higher, but that of oleic and ALA acids was lower. As expected from these findings, the proportions of both total saturates and PUFA were higher and lower, respectively, in ADHD patients than in controls (46).
As noted in Tables 3 and 4, the supplementation of n-3 PUFA in group A relative to the placebo group B, for a period of eight weeks, enhanced the proportion of n-3 PUFA at the expense of n-6 PUFA, thereby resulting in a significant decline of the n-6 to n-3 ratio. The rise in n-3 PUFA was probably due to the percentage increase of the n-3 family reflected by the elevation of EPA, docosapentaenoic acid and DHA. In view of the increase in n-3 PUFA family (EPA, docosapentaenoic acid and DHA), a decline was noted in the ALA to EPA ratio. An analysis of lipids (TG, cholesterol, PL, low-density lipoprotein-cholesterol and HDL-C), apo (A-I and B), liposoluble vitamins (retinol, alpha-tocopherol and beta-carotene) and malondialdehyde (an index of lipid peroxidation) was also carried out, and no differences were observed in their plasma concentrations (data not shown).
TABLE 3.
Plasma fatty acid (FA) profile in paediatric attention-deficit hyperactivity disorder patients in the first phase (time 0 to time 1)
| FA | Group A | Group B |
|---|---|---|
| 14:0 | 0.85±0.07 | 0.88±0.09 |
| 16:0 | 20.50±0.45 | 19.95±0.48 |
| 17:0 | 0.30±0.01 | 0.28±0.01 |
| 18:0 | 7.36±0.19 | 7.33±0.13 |
| 22:0 | 0.95±0.05 | 0.96±0.04 |
| 24:0 | 0.80±0.08 | 0.68±0.06 |
| 16:1 | 1.30±0.08 | 1.40±0.14 |
| 18:1 (n-7) | 1.27±0.05 | 1.27±0.06 |
| 18:1 (n-9) | 16.92±0.71 | 16.84±0.38 |
| 22:1 (n-9) | 0.65±0.07 | 0.67±0.06 |
| 24:1 (n-9) | 1.02±0.05 | 0.97±0.04 |
| LA; 18:2 (n-6) | 30.14±0.72* | 33.64±0.94 |
| 18:3 (n-6) | 0.28±0.03* | 0.44±0.04 |
| 20:3 (n-6) | 1.32±0.05† | 1.76±0.12 |
| 20:4 (n-6) | 6.78±0.25 | 7.25±0.27 |
| ALA; 18:3 (n-3) | 0.57±0.06 | 0.58±0.03 |
| EPA; 20:5 (n-3) | 2.77±0.33‡ | 0.61±0.16 |
| DPA; 22:5 (n-3) | 0.85±0.06‡ | 0.49±0.04 |
| DHA; 22:6 (n-3) | 3.22±0.20‡ | 1.78±0.20 |
Following the supplementation of group A with n-3 polyunsaturated FA and group B with placebo for a eight-week period, blood was drawn and plasma FA were separated by gas chromatography. FA proportion is expressed as the percentage of the total amount of FA present. FA contributing less than 0.2% of the total have been omitted. Student’s t test (two-tailed) was used to compare differences between means (± SEM).
P<0.006;
P<0.002;
P<0.0001. ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; DPA Docosapentaenoic; EPA Eicosapentaenoic acid; LA Linoleic acid
TABLE 4.
Fatty acid (FA) classes and ratios of plasma FA in paediatric attention-deficit hyperactivity disorder patients in the first phase (time 0 to time 1)
| FA | Group A | Group B |
|---|---|---|
| Total n-3 | 7.44±0.58* | 3.49±0.36 |
| Total n-6 | 38.78±0.70* | 43.46±0.91 |
| Total n-7 | 2.58±0.07 | 2.67±0.16 |
| Total n-9 | 18.84±0.70 | 18.76±0.35 |
| Total unsaturated | 67.64±0.55 | 68.38±0.54 |
| Total saturated | 31.63±0.56 | 30.87±0.53 |
| Total monounsaturated | 21.64±0.75 | 21.66±0.46 |
| Total PUFA | 46.31±1.09 | 47.07±0.81 |
| Total trans | 0.42±0.04 | 0.40±0.03 |
| 16:1 (n-7)/18:2 (n-6) | 0.04±0.00 | 0.04±0.01 |
| 18:2 (n-6)/20:4 (n-6) | 4.53±0.21 | 4.74±0.25 |
| 20:3 (n-9)/20:4 (n-6) | 0.01±0.00 | 0.02±0.00 |
| 24:0/22:0 | 0.84±0.07 | 0.71±0.06 |
| 24:0/20:0 | 3.03±0.28 | 2.74±0.29 |
| PUFA/saturated | 1.48±0.05 | 1.54±0.05 |
| EFA (LA+ALA)/non-EFA | 0.45±0.01† | 0.52±0.02 |
| EPA/DHA | 0.81±0.07* | 0.33±0.05 |
| DHA/AA | 0.48±0.03* | 0.25±0.03 |
| ALA/EPA | 0.35±0.12* | 1.35±0.17 |
| ALA/LA | 0.02±0.00 | 0.02±0.00 |
| n-6/n-3 | 5.78±0.59* | 13.47±0.85 |
Following the supplementation of group A with n-3 polyunsaturated FA (PUFA) and group B with placebo for a eight-week period, blood was drawn and plasma FA were separated by gas chromatography. FA proportion is expressed as the percentage of the total amount of FA present. FA contributing less than 0.2% of the total have been omitted. Student’s t test (two-tailed) was used to compare differences between means (± SEM).
P<0.0001;
P<0.005. AA Arachidonic acid; ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; EFA Essential FA; EPA Eicosapentaenoic acid; LA Linoleic acid
All of these tests were performed again during the second phase of the study after 16 weeks following n-3 PUFA supplementation, and the data were compared with those of the placebo group that received n-3 PUFA during eight weeks. Limited changes were recorded between the groups as noted in Tables 5 and 6. Similarly, no significant alterations were evidenced in the profile of lipids, apo, vitamins and malondialdehyde (data not shown).
TABLE 5.
Plasma fatty acid (FA) profile in paediatric attention-deficit hyperactivity disorder patients in the second phase (time 1 to time 2)
| FA | Group A | Group B |
|---|---|---|
| 14:0 | 0.92±0.13 | 1.00±0.09 |
| 16:0 | 21.74±0.58 | 21.80±0.52 |
| 17:0 | 0.31±0.01 | 0.32±0.01 |
| 18:0 | 7.24±0.23 | 7.45±0.18 |
| 20:0 | 0.26±0.01 | 0.27±0.02 |
| 22:0 | 0.79±0.05 | 0.84±0.06 |
| 24:0 | 0.50±0.05 | 0.51±0.04 |
| 16:1 | 1.25±0.12 | 1.24±0.09 |
| 18:1 (n-7) | 1.36±0.05* | 1.22±0.02 |
| 18:1 (n-9) | 16.41±0.70 | 16.47±0.53 |
| 22:1 (n-9) | 0.43±0.04 | 0.47±0.04 |
| 24:1 (n-9) | 0.97±0.05 | 1.01±0.06 |
| LA; 18:2 (n-6) | 31.80±0.69 | 31.37±0.76 |
| 18:3 (n-6) | 0.25±0.02† | 0.36±0.03 |
| 20:3 (n-6) | 1.16±0.09 | 1.18±0.07 |
| 20:4 (n-6) | 6.08±0.34 | 6.26±0.45 |
| ALA; 18:3 (n-3) | 0.58±0.04 | 0.61±0.03 |
| EPA; 20:5 (n-3) | 2.50±0.30 | 2.33±0.24 |
| DPA; 22:5 (n-3) | 0.74±0.07 | 0.69±0.07 |
| DHA; 22:6 (n-3) | 2.93±0.25 | 2.66±0.22 |
Following the supplementation of group A with n-3 polyunsaturated FA (PUFA) for an additional period of eight weeks (16 weeks in total), while group B received n-3 PUFA instead of placebo for eight weeks, blood was drawn and plasma FA were separated by gas chromatography. FA proportion is expressed as the percentage of the total amount of FA present. FA contributing less than 0.2% of the total have been omitted. Student’s t test (two-tailed) was used to compare differences between means (± SEM).
P<0.02;
P<0.004. ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; DPA Docosapentaenoic; EPA Eicosapentaenoic acid; LA Linoleic acid
TABLE 6.
Fatty acid (FA) classes and ratios of plasma FA in paediatric attention-deficit hyperactivity disorder patients in the second phase (time 1 to time 2)
| FA | Group A | Group B |
|---|---|---|
| Total n-3 | 6.78±0.59 | 6.32±0.49 |
| Total n-6 | 39.53±0.85 | 39.40±0.68 |
| Total n-7 | 2.60±0.14 | 2.46±0.10 |
| Total n-9 | 18.03±0.69 | 18.18±0.51 |
| Total unsaturated | 66.94±0.64 | 66.36±0.64 |
| Total saturated | 32.31±0.61 | 32.76±0.56 |
| Total monounsaturated | 20.87±0.79 | 20.95±0.56 |
| Total PUFA | 46.38±1.19 | 45.80±0.84 |
| Total trans | 0.44±0.05 | 0.49±0.07 |
| 16:1 (n-7)/18:2 (n-6) | 0.04±0.00 | 0.04±0.00 |
| 18:2 (n-6)/20:4 (n-6) | 5.35±0.32 | 5.45±0.54 |
| 20:3 (n-9)/20:4 (n-6) | 0.01±0.00 | 0.01±0.00 |
| 24:0/22:0 | 0.62±0.05 | 0.61±0.03 |
| 24:0/20:0 | 1.90±0.20 | 1.95±0.15 |
| PUFA/saturated | 0.23±0.01 | 0.23±0.02 |
| EFA (LA+ALA)/non-EFA | 0.48±0.01 | 0.47±0.02 |
| EPA/DHA | 0.85±0.06 | 0.86±0.05 |
| DHA/AA | 0.49±0.04 | 0.43±0.02 |
| ALA/EPA | 0.26±0.04 | 0.32±0.05 |
| ALA/LA | 0.02±0.00 | 0.02±0.00 |
| n-6/n-3 | 6.06±0.40 | 6.75±0.60 |
Following the supplementation of group A with n-3 polyunsaturated FA (PUFA) for an additional period of eight weeks (16 weeks in total), while group B received n-3 PUFA instead of placebo for eight weeks, blood was drawn and plasma FA were separated by gas chromatography. FA proportion is expressed as the percentage of the total amount of FA present. FA contributing less than 0.2% of the total have been omitted. Student’s t test (two-tailed) was used to compare differences between means (± SEM). AA Arachidonic acid; ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; EFA Essential FA; EPA Eicosapentaenoic acid; LA Linoleic acid
Correlation between the biochemistry findings and the results of the Conners’ and SWAN questionnaires
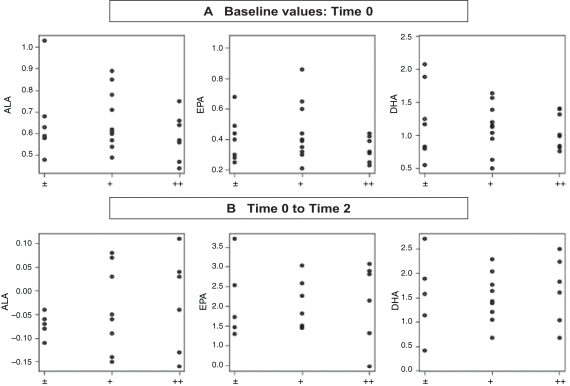
Using a Spearman correlation, no statistically significant correlation was found between the concentrations of ALA, DHA and EPA, and the results obtained from the SWAN and Conners’ questionnaires. However, ADHD symptoms were found to be more intense when the concentration of ALA was low and when the concentrations of EPA and DHA were elevated. No statistically significant correlation was found among the concentrations of ALA, DHA and EPA between time 0 (baseline) and time 2 (phase 2) and the response following administration of the supplement (Figure 1). An elevation in the concentration of ALA was found in six children, three of whom showed mild improvements, while the other three had a very good response to the n-3 PUFA supplement. In contrast, there was no correlation between the response to the administration of n-3 PUFA supplement and the increment in DHA and EPA, which was consistently present in all children (Figure 1).
Figure 1).
Score amelioration of the attention-deficit hyperactivity disorder (ADHD) children according to their n-3 polyunsaturated fatty acid (PUFA) levels. ADHD patients (n=26, who completed the study) have been distributed into three categories: light (±), good (+) and very good (++) clinical improvement, taking into account the percentage level of the three major n-3 fatty acids (alpha-linolenic acid [ALA], eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]). Comparisons were performed before the first phase or baseline (0) and after the second phase. The statistical values relative to the relationship between n-3 PUFA and improvement scores are ALA 0.489, EPA 0.055 and DHA 0.060
DISCUSSION
Clinical practice reveals the need to develop alternative treatments for children with ADHD who do not respond to standard psychostimulant treatment or whose families deprive them of treatment, fearful of the short- and long-term side effects of medication. Consequently, there is a significant percentage of parents who prefer alternative treatments for the management of ADHD symptoms in their children. The present study was, therefore, designed to investigate the effects of n-3 PUFA supplements, compared with the n-6 PUFA placebo, on the core ADHD symptoms in children following a 16-week, double-blind, one-way, crossover randomized study, using the SWAN and Conners’ rating scales completed by the parents or caregivers and the teachers.
An important issue of the present investigation was to determine whether n-3 PUFA can improve behavioural parameters in ADHD subjects. For behaviour assessment, we used the Conners’ parent rating scale that has been extensively used in various investigations and represents a suitable tool for behaviour evaluation in youths. The results from the parent version of the Conners’ questionnaire revealed that from baseline (time 0) to the end of phase 1 (time 1), the subjects’ ADHD symptoms showed a statistically significant improvement in several of the Conners’ subscales in both groups. Within this time frame, subjects in group A showed a greater, but not statistically significant, improvement in comparison with subjects in group B. From time 1 to time 2, further improvement was seen in both groups, which was more significant in patients from group B, although only statistically significant for subscale L in this group.
Moreover, our analysis of the teacher-completed SWAN questionnaire indicated no significant differences in ADHD symptoms during the duration of the study among subjects in groups A and B. In contrast, an improvement in the different scales and, in particular, scale A (inattention) was seen in the parent SWAN questionnaire, although the improvement was not statistically significant. Altogether, these data suggest that n-3 PUFA supplementation may result in improvements in ADHD symptomatology, but a positive effect of n-6 PUFA cannot be excluded according to our findings. It is possible that n-3 PUFA supplementation at higher levels may be necessary to achieve clear-cut improvements in ADHD symptomatology.
Based on the data relative to behaviour and academic performance in 26 subjects who completed the study, no significant improvement was recorded in six subjects, whereas 12 subjects reported minimal improvement. However, eight subjects (four each in groups A and B) exhibited a significant clinical improvement following the administration of the n-3 PUFA supplement, particularly for the inattention and global DSM-IV total Conners’ subscales. This improvement, particularly important in the attention dimensions, is limited in scope compared with two other studies (47,48) that reported an amelioration for all dimensions, including attention, hyperactivity and impulsivity. However, our cohort consisted of more children with ADHD, in which the inattentive type (20 of 37 [54%]) was more elevated than what is typically observed in ADHD, which may in part explain the observations.
The literature to date indicates variable effects on different mental health disorders and particularly on ADHD following treatment with n-3 PUFA. For example, two studies (49,50) comparing the use of a DHA supplement with a placebo showed no significant improvement in the symptoms of inattention in a group of children receiving DHA.
Improvements were seen in ADHD symptoms in several studies in which children were given a placebo or an n-3 PUFA supplement, which included EPA (51). However, these results are not always conclusive. Some of the studies that were performed were only based on parents’ (39,52) or teachers’ evaluation (47). This is important because a statistically significant improvement in ADHD symptoms was seen in our work and also in the study by Sinn and Bryan (48), only in the parent rating scales but not in the teacher rating scales. Similarly, Stevens et al (25) concluded that marked improvements were noted in multiple outcomes (rated by parents) in both groups, but a clear benefit was not observed from PUFA supplementation in all ADHD behaviour characteristics.
Thus, our results indicate a statistically significant trend toward improvement in the core symptoms of ADHD as assessed by Conners’ parent rating scales in children given an n-3 PUFA supplement. A clear clinical improvement was seen in eight of the 37 children (21.6%) in our study. However, if we take into consideration only the 26 children who completed our study, as per Sinn and Bryan’s study (48), we can note an improvement of approximately 30.7%, which is quite similar to their results. The amelioration of ADHD symptoms in both placebo and n-3 PUFA groups in the first eight weeks of the study (phase 0 to phase 1) also suggests the presence of a placebo effect. Nevertheless, the improvement measured in group A patients receiving the supplement is greater than in group B patients who were given the placebo. Moreover, further improvement during phase 2 was, in general, more evident and statistically significant for inattention in children from group B who received the n-3 PUFA supplement during eight weeks. Finally, based on clinical and statistical evaluations, we observed that eight of the 26 subjects (approximately 30%) who completed the study had a significant clinical improvement following the administration of the n-3 PUFA supplement.
Importantly, our young patients were not deficient in EFA. They did not display lower levels in arachidonic acid, EPA or DHA. Only the ALA proportion was somewhat decreased compared with controls. Therefore, future trials should attempt to include ALA in their n-3 PUFA supplements, in addition to EPA and DHA. This is particularly important because the symptoms of ADHD, including attention and behaviour, were improved after the administration of a combination of vitamin C and flax oil containing ALA – a precursor of DHA and EPA – in an open-label study (52).
There are a number of limitations regarding the present study that should be taken into account when interpreting the behavioural data. The high attrition rate resulted in a smaller sample size, which did not allow us to reach statistical significance for various variables. Moreover, the response of subjects to an n-3 PUFA supplement was monitored using parent and teacher behaviour rating scales. Although DSM-IV ADHD symptom rating scales constitute the most common method to estimate the response to medication as a primary outcome measure given its reliability and validity, clinical studies have started using measures of overall impairment and quality of life to assess the impact of treatment on patients with ADHD.
Our clinical evaluation with the families of the subjects revealed that at the end of the study, most families preferred to continue the use of the n-3 PUFA supplement rather than medication. One of the reasons may be attributed to the safety profile of the supplements because none of the subjects reported significant adverse effects. Another reason may be related to improved functioning as a result of a decrease in the deviant behaviours in day-to-day living. Although the present investigation did not assess quality of life, functional impairment and its correlation to DSM-IV ADHD symptoms, other studies have shown that, in patients with ADHD, the correlation between measures of functional impairments and symptoms of ADHD is modest (53).
Therapy with n-3 PUFA supplements was well tolerated in the current study. Furthermore, no differences were detected in the CPT scores of subjects at baseline or during the study. This is consistent with reports in the literature that CPT scores are not specific to the diagnosis of ADHD, and there is no significant correlation between the results on the behaviour rating scales and scores on CPT (54).
This is one of the few studies that included a detailed biochemical analysis of the lipid profile of children with ADHD. Unlike reports in the literature, abnormalities in plasma EFA, including the deficiency of DHA and EPA, were not observed in our cohort. On the contrary, the concentrations of the latter were higher, but the relative proportion of ALA was lower compared with normal controls, which suggests that French Canadian patients have satisfactory EFA consumption and intestinal fat absorption.
CONCLUSION
The results of the present study support the safety and tolerability of n-3 PUFA administration because most parents wanted their children to continue supplementation. Our findings also suggest that a subgroup of children with ADHD maintain and achieve symptom control using dietary supplementation with n-3 PUFA. Additional larger studies are needed to delineate the specific, clinical and biochemical characteristics of responders. In future trials, we will need to identify the patients who may benefit from n-3 PUFA supplementation and the type and doses of n-3 PUFA supplements. Duration of supplementation should also be taken into consideration because, in some recent studies (47,48), better results have been observed with longer supplement administration. We should also determine whether the latter should be given alone or in combination with conventional ADHD treatment (psychostimulants and atomoxetine) or the best tools used to evaluate the clinical results of PUFA supplementation. Finally, one should take environmental (including prenatal) and genetic factors into consideration, including the genes that regulate the metabolism of PUFA.
Acknowledgments
The present work was supported by JA DeSève Research Chair in Nutrition (EL) and NutriSanté Inc (Canada) (financial support and gift of n-3 and n-6 capsules). The authors thank the patients who participated in the trial and are grateful to G Beauséjour and C Rousseau for their technical help; M Beauchemin, L Chaib, ME Doucet, I Pelletier and E Tremblay for neurological statements; H Paquette and L Lortie for data collection; I Fortier for statistical supervising; and D Lebel, M Martel and D Fortier for their assistance.
REFERENCES
- 1.Jensen PS, Hinshaw SP, Swanson JM, et al. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): Implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22:60–73. doi: 10.1097/00004703-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Boyle MH, Offord DR, Racine Y, Fleming JE, Szatmari P, Sanford M. Evaluation of the revised Ontario Child Health Study scales. J Child Psychol Psychiatry. 1993;34:189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen D, Clapperton I, Gref P, Tremblay Y. Déficit d’attention/hyperactivité: Perception des acteurs et utilisation de psychostimulants. Montreal: Régie régionale de la santé et des services sociaux de Laval; 1999. [Google Scholar]
- 4.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 5.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry. 1993;50:565–76. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 6.Mannuzza S, Klein RG. Long-term prognosis in attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2000;9:711–26. [PubMed] [Google Scholar]
- 7.Biederman J, Spencer T, Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2004;7:77–97. doi: 10.1017/S1461145703003973. [DOI] [PubMed] [Google Scholar]
- 8.Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:780–8. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore A, Milne R. Methylphenidate in children with hyperactivity: Review and cost-utility analysis. Pharmacoepidemiol Drug Saf. 2001;10:85–94. doi: 10.1002/pds.564. [DOI] [PubMed] [Google Scholar]
- 10.Spencer T, Biederman J, Wilens T. Pharmacotherapy of attention deficit hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2000;9:77–97. [PubMed] [Google Scholar]
- 11.MTA Cooperative Group National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113:754–61. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- 12.MTA Cooperative Group National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: Changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762–9. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- 13.Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: Adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43:559–67. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Spigelblatt L, Laine-Ammara G, Pless IB, Guyver A. The use of alternative medicine by children. Pediatrics. 1994;94(6 Pt 1):811–4. [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics, Committee on Children With Disabilities Counseling families who choose complementary and alternative medicine for their child with chronic illness or disability. Pediatrics. 2001;107:598–601. [PubMed] [Google Scholar]
- 16.Baumgaertel A. Alternative and controversial treatments for attention-deficit/hyperactivity disorder. Pediatr Clin North Am. 1999;46:977–92. doi: 10.1016/s0031-3955(05)70167-x. [DOI] [PubMed] [Google Scholar]
- 17.Chan E, Rappaport LA, Kemper KJ. Complementary and alternative therapies in childhood attention and hyperactivity problems. J Dev Behav Pediatr. 2003;24:4–8. doi: 10.1097/00004703-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kemper KJ. Dietary supplements for attention-deficit/hyperactivity disorder – a fishy business? J Pediatr. 2001;139:173–4. doi: 10.1067/mpd.2001.116932. [DOI] [PubMed] [Google Scholar]
- 19.Gross-Tsur V, Lahad A, Shalev RS. Use of complementary medicine in children with attention deficit hyperactivity disorder and epilepsy. Pediatr Neurol. 2003;29:53–5. doi: 10.1016/s0887-8994(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 20.Stubberfield T, Parry T. Utilization of alternative therapies in attention-deficit hyperactivity disorder. J Paediatr Child Health. 1999;35:450–3. doi: 10.1046/j.1440-1754.1999.355401.x. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs T, Birbaumer N, Lutzenberger W, Gruzelier JH, Kaiser J. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Appl Psychophysiol Biofeedback. 2003;28:1–12. doi: 10.1023/a:1022353731579. [DOI] [PubMed] [Google Scholar]
- 22.Heywood C, Beale I. EEG biofeedback vs placebo treatment for attention-deficit/hyperactivity disorder: A pilot study. J Atten Disord. 2003;7:43–55. doi: 10.1177/108705470300700105. [DOI] [PubMed] [Google Scholar]
- 23.Lubar JF, Swartwood MO, Swartwood JN, O’Donnell PH. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995;20:83–99. doi: 10.1007/BF01712768. [DOI] [PubMed] [Google Scholar]
- 24.Nash JK. Treatment of attention deficit hyperactivity disorder with neurotherapy. Clin Electroencephalogr. 2000;31:30–7. doi: 10.1177/155005940003100109. [DOI] [PubMed] [Google Scholar]
- 25.Stevens L, Zhang W, Peck L, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. 2003;38:1007–21. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- 26.Colquhoun I, Bunday S. A lack of essential fatty acids as a possible cause of hyperactivity in children. Med Hypotheses. 1981;7:673–9. doi: 10.1016/0306-9877(81)90014-1. [DOI] [PubMed] [Google Scholar]
- 27.Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr. 2000;71(1 Suppl):327S–30S. doi: 10.1093/ajcn/71.1.327S. [DOI] [PubMed] [Google Scholar]
- 28.Stevens LJ, Zentall SS, Abate ML, Kuczek T, Burgess JR. Omega-3 fatty acids in boys with behavior, learning, and health problems. Physiol Behav. 1996;59:915–20. doi: 10.1016/0031-9384(95)02207-4. [DOI] [PubMed] [Google Scholar]
- 29.Richardson AJ, Puri BK. The potential role of fatty acids in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2000;63:79–87. doi: 10.1054/plef.2000.0196. [DOI] [PubMed] [Google Scholar]
- 30.Stevens LJ, Zentall SS, Deck JL, et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–8. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 31.Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids. 2007;77:247–50. doi: 10.1016/j.plefa.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–49. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Champeil-Potokar G, Chaumontet C, Guesnet P, Lavialle M, Denis I. Docosahexaenoic acid (22:6n-3) enrichment of membrane phospholipids increases gap junction coupling capacity in cultured astrocytes. Eur J Neurosci. 2006;24:3084–90. doi: 10.1111/j.1460-9568.2006.05185.x. [DOI] [PubMed] [Google Scholar]
- 34.Song C, Li X, Kang Z, Kadotomi Y.Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: Involved with PLA2 activity and corticosterone secretion Neuropsychopharmacology 200732736–44.(Erratum in 2007;32:1207). [DOI] [PubMed] [Google Scholar]
- 35.Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J Lipid Res. 2004;45:1112–21. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–6. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Busch B. Polyunsaturated fatty acid supplementation for ADHD? Fishy, fascinating, and far from clear. J Dev Behav Pediatr. 2007;28:139–44. doi: 10.1097/01.DBP.0000267560.34199.e0. [DOI] [PubMed] [Google Scholar]
- 38.Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65:1051–59. doi: 10.2165/00003495-200565080-00002. [DOI] [PubMed] [Google Scholar]
- 39.Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:233–9. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 40.Levy E, Thibault LA, Roy CC, Bendayan M, Lepage G, Letarte J. Circulating lipids and lipoproteins in glycogen storage disease type I with nocturnal intragastric feeding. J Lipid Res. 1988;29:215–26. [PubMed] [Google Scholar]
- 41.Levy E, Thibault L, Garofalo C, et al. Combined (n-3 and n-6) essential fatty deficiency is a potent modulator of plasma lipids, lipoprotein composition, and lipolytic enzymes. J Lipid Res. 1990;31:2009–17. [PubMed] [Google Scholar]
- 42.Levy E, Rizwan Y, Thibault L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–15. doi: 10.1093/ajcn/71.3.807. [DOI] [PubMed] [Google Scholar]
- 43.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–40. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 44.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–16. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 45.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 46.Spahis S, Vanasse M, Bélanger SA, Ghadirian P, Grenier E, Levy E. Lipid profile, fatty acid composition and pro- and anti-oxidant staus in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2008;79:47–53. doi: 10.1016/j.plefa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Richardson AJ, Montgomery P. The Oxford-Durham study: A randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360–6. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- 48.Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28:82–91. doi: 10.1097/01.DBP.0000267558.88457.a5. [DOI] [PubMed] [Google Scholar]
- 49.Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder – a placebo-controlled double-blind study. Eur J Clin Nutr. 2004;58:467–73. doi: 10.1038/sj.ejcn.1601830. [DOI] [PubMed] [Google Scholar]
- 50.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189–96. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- 51.Richardson A. Omega-3 fatty acids in ADHD and related neurodevelopmental disorders. Int Rev Psychiatry. 2006;18:155–72. doi: 10.1080/09540260600583031. [DOI] [PubMed] [Google Scholar]
- 52.Joshi K, Lad S, Kale M, et al. Supplementation with flax oil and vitamin C improves the outcome of Attention Deficit Hyperactivity Disorder (ADHD) Prostaglandins Leukot Essent Fatty Acids. 2006;74:17–21. doi: 10.1016/j.plefa.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Perwien AR, Kratochvil CJ, Faries DE, Vaughan BS, Spencer T, Brown RT. Atomoxetine treatment in children and adolescents with attention-deficit hyperactivity disorder: What are the long-term health-related quality-of-life outcomes? J Child Adolesc Psychopharmacol. 2006;16:713–24. doi: 10.1089/cap.2006.16.713. [DOI] [PubMed] [Google Scholar]
- 54.Halperin JM. The clinical assessment of attention. Int J Neurosci. 1991;58:171–82. doi: 10.3109/00207459108985433. [DOI] [PubMed] [Google Scholar]