Abstract
Aims
Pelvic floor muscle trauma and pudendal nerve injury have been implicated in stress urinary incontinence (SUI) development after childbirth. In this study, we investigated how combinations of these injuries affect recovery.
Methods
Sixty-seven female Sprague-Dawley rats underwent vaginal distension (VD), pudendal nerve crush (PNC), PNC and VD (PNC+VD), pudendal nerve transection (PNT), or served as unmanipulated controls. Four days, 3 weeks, or 6 weeks after injury, we simultaneously recorded pudendal nerve motor branch potentials (PNMBP), external urethral sphincter electromyography (EUS EMG), and transurethral bladder pressure under urethane anesthesia. The presence of a guarding reflex (increased frequency & amplitude of PNMBP or EUS EMG activity) during leak point pressure (LPP) testing was determined.
Results
Controls consistently demonstrated a guarding reflex. Four days after VD, EUS EMG activity was eliminated, but PNMBP activity reflected the guarding reflex; EUS EMG activity recovered after 3 weeks. Four days after PNC, both EUS EMG and PNMBP activity were eliminated, but demonstrated significant recovery at 3 weeks. Four days after PNC+VD both EUS EMG and nerve activity were eliminated, and little recovery was observed after 3 weeks with significant recovery of the guarding reflex 6 weeks after injury. Little recovery was observed at all time points after PNT. LPP results mirrored the reduction in EUS EMG activity.
Conclusion
Functional recovery occurs more slowly after PNC+VD than after either PNC or VD alone. Future work will be aimed at testing methods to facilitate neuroregeneration and recovery after this clinically relevant dual injury.
Keywords: Rat, stress urinary incontinence, electromyogram, bladder pressure, leak point pressure, cystometry, urodynamics, vaginal distension, nerve injury, guarding reflex
Introduction
During vaginal delivery, the soft tissues of the pelvic floor are compressed and injured (1), including the external urethral sphincter (EUS). This striated muscle of the urethra can become hypoxic during vaginal distension (2). In addition, the pudendal nerve can be compressed and stretched during vaginal childbirth in Alcock's canal proximal to where it innervates the EUS (3). Both of these injuries, along with other pelvic floor trauma are strongly correlated with the later development of stress urinary incontinence (SUI) (4;5). Therefore, among the many other pelvic injuries occurring during vaginal childbirth, the EUS and its innervation by the pudendal nerve can both be injured nearly simultaneously. We have hypothesized that simultaneous injury to the EUS slows or delays neuroregeneration and functional recovery of the injured pudendal nerve.
To simulate the injuries occurring during vaginal delivery in humans, a variety of animal models of SUI have been utilized, including vaginal distension (VD), pudendal nerve crush (PNC), and pudendal nerve transection (PNT) (6-9). These models have been used to demonstrate that recovery of urethral resistance to leakage is associated with EUS recovery and/or pudendal nerve regeneration (10;11). To investigate neuromuscular functional recovery after the various injuries simulating human childbirth trauma with a particular focus on the EUS and its innervation, we recorded the response to leak point pressure (LPP) testing using pudendal nerve motor branch potential (PNMBP) recordings to assess nerve injury and neuroregeneration, and EUS electromyography (EMG) to assess muscle injury and reinnervation, at several time points after VD, PNC, PNC+VD, and PNT.
Methods
Animal preparations
Sixty-seven female, virgin Sprague-Dawley rats (200-250g) were randomized into 5 groups: Controls, VD, PNC, PNC+VD, and PNT as approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Neuromuscular function recordings, including LPP, EUS EMG, and PNMBP, were performed in the VD and PNC groups either 4 days (n = 5) or 3 weeks (n = 5) after injury. In PNC+VD and PNT groups, recordings were made 4 days (n = 5), 3 weeks (n = 6), or 6 weeks (n = 6) after injury. Controls (n=13) consisted of unmanipulated age-matched animals. Immediately after the recordings, each rat was euthanized with intraperitoneal (i.p.) pentobarbital (100 mg/kg).
Childbirth simulation injury models
In order to perform the VD, PNC, PNC+VD and PNT procedures, rats were anesthetized with i.p. ketamine (100 mg/kg) and xylazine (10 mg/kg). For VD, the vagina was first accommodated with increasing sizes of bouge à boule urethral dilators. A modified 10F Foley balloon catheter was inserted into the vagina and the balloon was inflated with 3 ml water for 4 hours, as previously described (12). For PNC, the pudendal nerves were accessed from a postero-lateral gluteal approach in anesthetized animals. The ilium and sacrum were opened slightly, and the pudendal nerve was isolated and crushed bilaterally with a Castroviejo needle holder twice for 30 seconds as previously described (8). Animals in the PNC+VD group received both of the above procedures with PNC performed first. Rats in the PNT group underwent a procedure similar to that of PNC except that the pudendal nerve was transected bilaterally instead of being crushed. A segment of nerve (approximately 2 mm long) was removed from the transection site to prevent neuroregeneration. All rats received buprenophrine (0.1 mg/kg s.c.) for postoperative analgesia.
Leak point pressure (LPP) with simultaneous neuromuscular physiological recordings
All rats were anaesthetized with urethane (1.2 g/kg) intraperitoneally. The pubic symphysis was exposed through a lower vertical midline incision where the rectus abdominis muscles were cut. The urethra was exposed by opening the pubic symphysis with forceps. Bipolar parallel platinum electrodes (30-gauge needles 2 mm apart) for recording EUS EMG were placed on the outside of the mid-urethra at the location of the EUS. The electrodes were connected to an amplifier (Model P511 AC Amplifier, Astro-Med, Inc., Providence, RI; band pass frequencies: 3 Hz - 3 KHz) and electrophysiological recording system (DASH 8X, Astro-Med; 10 KHz sampling rate).
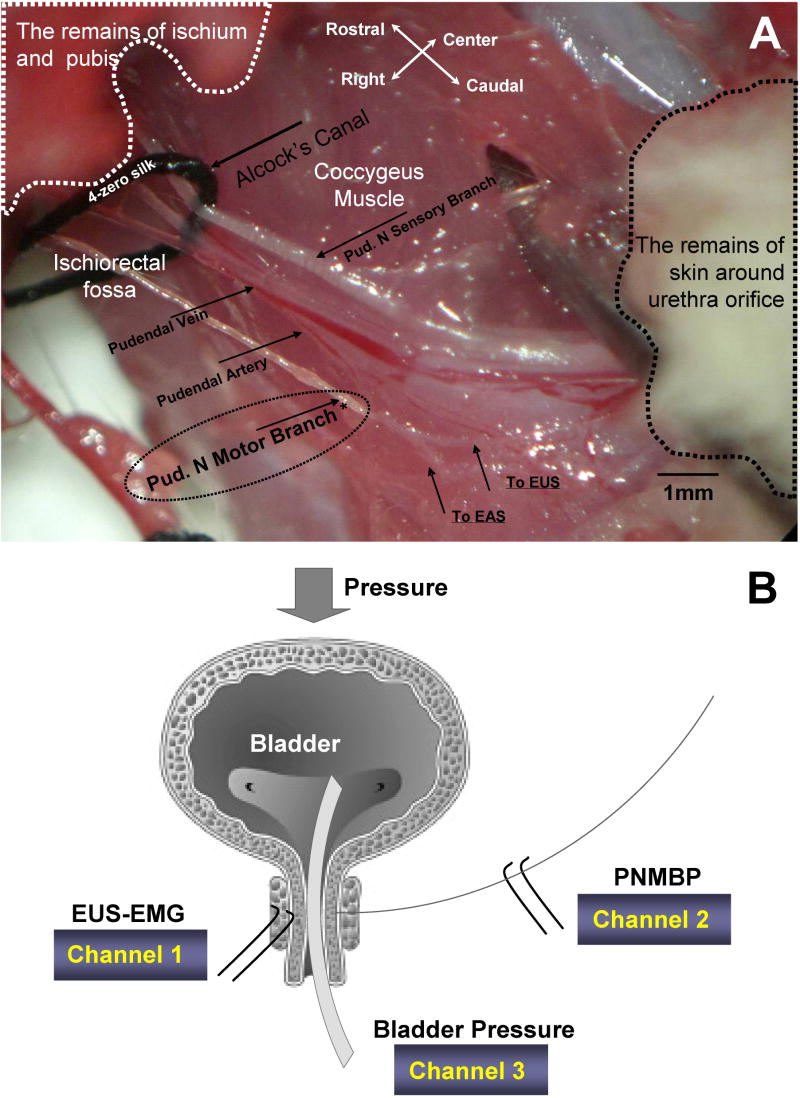
For PNMBP recordings (13), the pubis and ischium were partly removed using forceps to separate and enlarge the right side of the ischiorectal fossa where the pudendal nerve is located (Figure 1). The pudendal nerve motor branch to the EUS was identified on the dorsal side of the fossa and separated from the sensory and anal sphincter motor branches of the pudendal nerve using a glass dissecting needle under a surgical microscope. The pudendal motor branch to the EUS was guided over an identical set of recording electrodes that were placed in a warm (37°C) paraffin oil bath. As above, the electrodes were connected to an amplifier and the electrophysiological recording system. No PNMBP recordings could be made after pudendal nerve transection.
Figure 1.
A. The anatomy of the pudendal nerve motor branch as seen in the neurophysiological recordings, showing the pudendal nerve (Pud. N) motor and sensory branches as well as the pudendal artery & vein. * indicates location of PNMBP recordings. B. Schematic diagram of external urethral sphincter (EUS) electromyogram (EMG), pudendal nerve motor branch potential (PNMBP), and bladder pressure recordings during leak point pressure testing. EAS = external anal sphincter.
To perform LPP testing, a polyethylene catheter (PE-90) was inserted into the bladder via the urethra, and was connected to both a pressure transducer (model P122; Astro-Med, Inc.) and syringe pump (model 200; KD Scientific, New Hope, PA). Bladder pressure was referenced to air pressure at the level of the bladder. As the bladder was filled with saline at 5 ml/h, bladder pressure, EUS EMG, and PNMBP were recorded. For LPP testing, an increase in intravesical pressure was made when the bladder was approximately half full by gradually pressing a cotton swab on the bladder until urine leaked. At the moment of urine leakage, the cotton swab and all external pressure were removed. Following the LPP test, the bladder was emptied. If an active bladder pressure contraction was induced by LPP testing, the results were not analyzed and the test was repeated. The test was repeated 6 - 8 times in each animal. LPP was calculated as baseline pressure subtracted from peak pressure as previously described (14).
Data analysis
Quantitative assessment of PNMBP and EUS EMG signals was performed by determining the mean rectified amplitude of the potential and the mean frequency of motor unit firing. Power supply interference (60 Hz and 120 Hz) was filtered with a digital band pass filter (59 - 61 Hz and 119 - 121 Hz; Myosotic SignaPoint 2007, Myosotic LLC, Woodinville, WA). A threshold for differentiating noise from neuromuscular activity was set at 0.2 μV, according to the noise amplitude observed in multiple recordings of the tonic state.
During LPP testing, tonic activity before the LPP test and peak activity at the pressure peak were identified and archived in 3 or 4 one second samples for each rat (AstroVIEWX, Astro-Med, Inc.; Figure 2). Increases in amplitude (ΔμV) and frequency (ΔHz) during LPP were calculated by subtracting baseline (tonic) activity from peak LPP activity. Positive ΔμV and ΔHz were taken to indicate a response to bladder filling or LPP testing. Mean values were calculated for each animal and were used to calculate a mean and standard error for each group. The Wilcoxon Signed Rank test was used to compare control EMG and PNMBP data before and during LPP (Sigma Stat, Systat, Inc., Point Richmond, CA). A one way ANOVA followed by a Holm-Sidak posthoc test was used to compare LPP, ΔμV, ΔHz of each experimental group to the control group. A value of P<0.05 was used to indicate a statistically significant difference between groups in both cases.
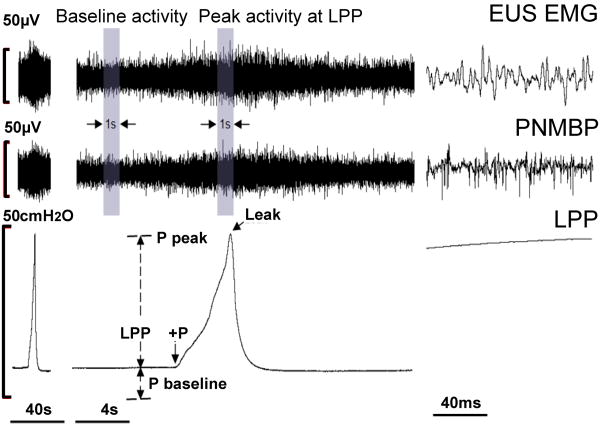
Figure 2.
Example of a leak point pressure (LPP) test with simultaneous external urethral sphincter electromyogram (EUS EMG) and pudendal nerve motor branch potential (PNMBP) recordings in a control rat at three different time scales. The two shaded bars (baseline activity and peak activity at LPP) were used to calculate increased frequency (ΔHz) and amplitude (ΔμV) in both EUS EMG and PNMBP during LPP. LPP is calculated as the baseline pressure (P baseline) subtracted from the peak pressure (P peak). +P indicates beginning of externally applied pressure to the bladder.
Results
Both EUS EMG and PNMBP demonstrate tonic activity prior to LPP testing (Figure 2). When a pressure increase was applied to the bladder during an LPP test, both EUS EMG and PNMBP activity increased significantly in controls (Table 1) indicating the presence of a guarding response in the urethra to an increase in bladder pressure.
Table 1.
Amplitude and frequency of EUS EMG and PNMBP in control rats (n = 13) during LPP testing.
| EUS EMG | PNMBP | |||
|---|---|---|---|---|
| Baseline activity | Peak activity | Baseline activity | Peak activity | |
| Amplitude (μV) | 6.4 ± 0.7 | 10.8 ± 1.0* | 4.2 ± 0.5 | 6.5 ± 0.6* |
| Frequency (Hz) | 168 ± 15 | 287 ± 16* | 303.692 ± 39 | 593 ± 55* |
P < 0.01 compared to baseline activity.
Four days after VD, EUS EMG was weak and little response to LPP testing was observed (Figure 3). In contrast, both baseline PNMBP activity and the guarding response in PNMBP remained normal 4 days after VD (Figure 4). Both EUS EMG (PNC, PNC+VD, and PNT) and PNMBP (PNC and PNC+VD) were weak and little response was detected 4 days after nerve injury. Of the 5 rats that received PNC, only 1 responded to LPP testing with an increase in EUS EMG activity. It is possible that in this animal the pudendal nerve was incompletely injured. In the absence of neuromorphometric confirmation, we did not exclude these results from the analysis but did not consider this animal to be representative. Compared with controls, ΔHz and ΔμV of EUS EMG were significantly reduced 4 days after injury in all experimental groups (Figure 3). Similarly, compared with controls, ΔHz and ΔμV of PNMBP were significantly reduced 4 days after injury in all experimental groups except VD (Figure 4).
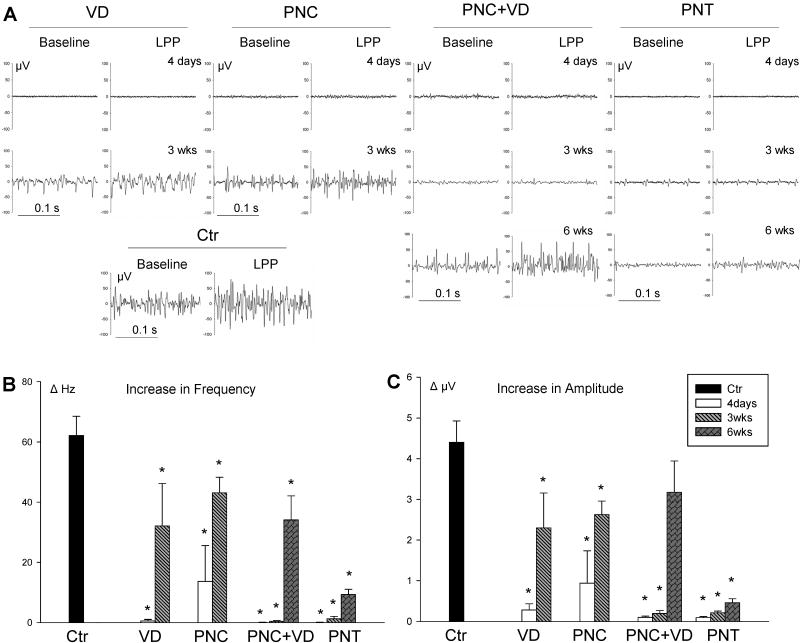
Figure 3.
External urethral sphincter electromyogram (EUS EMG). A. Examples of baseline and peak EUS EMG activity during LPP in control (Ctr) animals, 4 days and 3 weeks (3wks) after vaginal distension (VD) and pudendal nerve crush (PNC), and 4 days, 3 weeks, and 6 weeks (6 wks) after PNC+VD and pudendal nerve transection (PNT). B. Increase in frequency (ΔHz) of EUS EMG in response to LPP. C. Increase in amplitude (ΔμV) of EUS EMG in response to LPP. Only 1 of the 5 rats studied 4 days after PNC showed an EUS EMG response to LPP testing, accounting for the high variability observed in amplitude and frequency in that group. Each bar represents mean +/- standard error of the mean of results from 5-13 rats. * indicates a significant difference (P<0.05) compared to control.
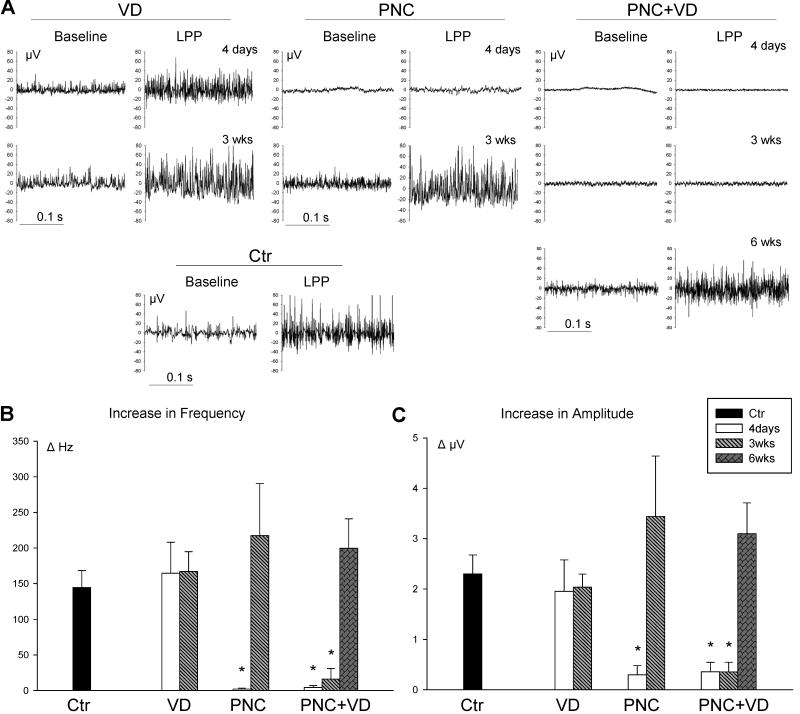
Figure 4.
Pudendal nerve motor branch potential (PNMBP). A. Examples of baseline and peak PNMBP activity during LPP. in control (Ctr) animals, 4 days and 3 weeks (3wks) after vaginal distension (VD) and pudendal nerve crush (PNC), and 4 days, 3 weeks, and 6 weeks (6 wks) after PNC+VD and pudendal nerve transection (PNT). B. Increase in frequency (ΔHz) of PNMBP in response to LPP. C. Increase in amplitude (ΔμV) of PNMBP in response to LPP. Each bar represents mean +/- standard error of the mean of results from 5-13 rats. * indicates a significant difference (P<0.05) compared to control.
Three weeks after VD or PNC, EUS EMG and PNMBP baseline activity demonstrated recovery. The response to LPP testing also showed recovery; however, ΔHz and ΔμV of EMG remained significantly reduced compared to controls (Figure 3). Less recovery of the neuromuscular response was demonstrated 3 weeks after PNC+VD or PNT: ΔHz and ΔμV of EUS EMG (PNC+VD and PNT) and PNMBP (PNC+VD) remained near zero and were significantly decreased compared to controls (Figures 3 and 4).
PNC+VD and PNT groups were observed 6 weeks after injury to determine if recovery occurs but is delayed compared to the VD and PNC groups. Six weeks after PNC+VD, the response to LPP demonstrated recovery, although ΔHz remained significantly decreased compared to controls. In contrast, 6 weeks after PNT, both ΔμV and ΔHz of EUS EMG remained near zero and were significantly decreased compared to controls, indicating a lack of a guarding response.
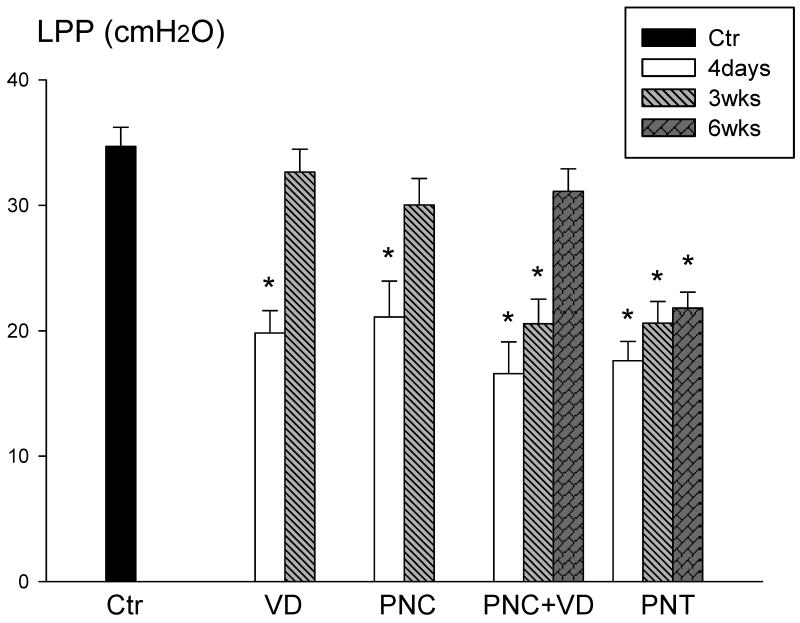
LPP results 4 days after injury in all experimental groups were significantly decreased compared to controls (Figure 5). Three weeks after VD or PNC, LPP results were similar to control values, mirroring the recovery of the PNMBP response, although not the EUS EMG response. Rats receiving PNC+VD had normal LPP values 6 weeks after injury, also paralleling the recovery of the PNMBP response. Six weeks after PNT, LPP values remained significantly decreased compared to controls, indicating lack of restoration of normal function.
Figure 5.
Leak point pressure (LPP) in control (Ctr) animals, 4 days and 3 weeks (3wks) after vaginal distension (VD) and pudendal nerve crush (PNC), and 4 days, 3 weeks, and 6 weeks (6 wks) after PNC+VD and pudendal nerve transection (PNT). Each bar represents mean +/- standard error of the mean of results from 5-13 rats. * indicates a significant difference (P<0.05) compared to control.
Discussion
Childbirth is the greatest risk factor for SUI in women, although the mechanistic pathway has not been identified. The maternal injuries of childbirth include anatomic changes as well as injury to nerves, muscles, and connective tissue structures (15). In this study, we applied several different simulated childbirth injuries to female rats, including muscle and/or nerve injury. We chose these models because each of these components of the pelvic floor are vulnerable to injury during delivery and are important in maintaining continence.
It has previously been demonstrated that bilateral transection of the pudendal nerves significantly reduces EUS EMG activity (16). Both VD and PNC alone have been shown to produce mild SUI lasting approximately 2 weeks (7-9;17). LPP results demonstrate an upward trend with increasing time after injury, suggesting that muscle or nerve function recovers, or compensatory changes in the urethra occur (12;18). To better simulate the compound injuries of childbirth, we developed the dual PNC+VD injury model.
Our findings suggest that EUS-EMG and PNMBP activity parallel urethral resistance to leakage as evident by our finding that LPP values as well as EMG and/or PNMBP activity decrease after VD and/or PNC and that LPP values improve at similar time points to EMG and PNMBP recovery. PNMBP activity in response to LPP testing was restored by 3 weeks after either VD or PNC alone. However, little recovery in PNBP activity was demonstrated 3 weeks after PNC+VD. This dual injury may produce a more useful model for the study of childbirth injuries in the development of SUI since it better simulates the compound maternal injuries of childbirth and gives a more durable dysfunction than either injury alone.
A significant decrease in LPP 6 weeks after PNT has been shown previously (7). However, PNT is not a model for the maternal injuries of childbirth since the pudendal nerve is more likely to undergo a potentially reversible crush injury rather than the irreversible transection injury of PNT. In our study, the PNT group served as a positive control for our experiment with injury and no neuroregeneration and presented a durable model of SUI since little spontaneous recovery occurs. This group also demonstrated that the response to LPP observed in the other groups in not due to artifact. The limited recovery of the EUS EMG that did occur after PNT may be mediated by other motor nerves, such as the levator ani nerve from the L6-S1 truck that innervates pelvic floor muscles close to the EUS (19;20).
Along with the passive anatomic coaptation of the urethra provided by pelvic support structures (21), the EUS and the pudendal nerve are important for prevention of urinary leakage during sudden increases in intra-abdominal pressure (22). For the simultaneous neuromuscular function recordings, we removed parts of pubis and ischium in order to perform open recordings of EUS EMG and PNMBP, eliminating the effects of passive pelvic support in preventing urine leakage. We also opened the peritoneum and directly added additional passive pressure to the bladder for the LPP test. This eliminated abdominal pressure transmission to the proximal urethra, which would decrease the chances of urine leakage (23). Therefore, in this model, continence is primarily provided by basic tonic or a reflex contraction of the EUS during LPP testing.
No sham controls were included in this study, although the control group served as a sham control for the pubic and peritoneal dissections. The main aim of this study was to determine if recovery time was different when PNC and VD injuries were applied in combination with each other, rather than to assess the effects of each individual injury. In a previous study, no significant differences were found in LPP between unmanipulated controls and shams (24). Therefore, the conclusions of this study are valid, despite the absence of sham groups.
The development of striated muscle force is accomplished by two mechanisms: recruitment of motor units, associated with an increase in amplitude of firing, and increased firing rate of motor units, associated with an increase in frequency of firing (25). Since both frequency and amplitude increased from baseline with LPP testing, we conclude that both mechanisms are involved in the urethral response to an increase in abdominal pressure.
Tonic activity in the EUS EMG in rats exhibits a compound interference potential pattern during the continence stage (16). Spontaneous PNMBP in the rat has not been previously characterized, however we found the discharge pattern of the nerve in tonic and guarding states to be similar to EUS EMG activity with events occurring a fraction of a second earlier in the PNMBP than in the EUS EMG. This indicates that EUS EMG activity is primarily activated by the pudendal nerve. The lack of tonic EUS EMG activity at any time point after PNT indicates that no other nerve innervates the EUS. This observation is in contrast to previous studies in which extrapudendal innervation was suggested (26).
PNMBP baseline and peak activity during LPP were not significantly different from controls 4 days after VD, indicating that the pudendal nerve is not injured significantly in VD even though the EUS EMG signal was decreased at the same time point after VD. Previous work with VD in rats has demonstrated EUS (12) and urethral smooth muscle injury (6) as well as injury to distal nerve fascicles near the EUS (8). Both LPP and EUS EMG demonstrated recovery 3 weeks after VD, suggesting recovery of the nerve terminals, neuromuscular junctions (NMJs), or the EUS muscle itself. However, the recovered amplitude of EUS EMG remained significantly lower than that of controls, suggesting that maturation of the NMJs at the EUS is not yet complete. Alternatively, a decreased amplitude could reflect a pattern of neuromuscular activity indicative of neuroregeneration, as shown previously in the EUS (11). Future work will be done to clarify this result.
Regenerating axons can traverse the segment of injury in a few days and then regenerate along the distal nerve segment at a rate of 1-2 mm/day toward the EUS target (27). Once the pudendal nerve reinnervates the EUS, the EMG guarding response should be restored, provided there are no other injuries affecting the reflex. Three weeks after PNC, the PNMBP was restored to normal, suggesting the pudendal motoneurons had traversed the injury site. However, similar to the VD group, EUS EMG, while demonstrating recovery, was still significantly decreased compared to controls, suggesting that reinnervation of the NMJs was not yet complete.
In contrast to the PNC group, 3 weeks after PNC+VD, no recovery was present. However, 6 weeks after injury, LPP was restored and ΔμV and ΔHz in PNMBP and ΔμV in EUS EMG were not significantly different from controls. One might expect the PNC+VD recovery to parallel that of PNC alone, however, PNBMP recovery was significantly delayed after PNC+VD compared to PNC, indicating the delay is related to delays in neuroregeneration.
Delayed neuroregeneration after PNC+VD may be due to brain derived neurotrophic factor (BDNF) and neurotrophin 4 (NT-4), which have been shown to play to a major role in regeneration of injured motoneurons (28;29). BDNF and NT-4 are upregulated in target muscles after nerve injury and promote neuroregeneration. However, after muscle injury, the same neurotrophins are significantly decreased and can become undetectable (30). Therefore, the same factors both inhibit NMJ reformation (31), and promote neuroregeneration. We postulate that the concurrent muscle injury delays or slows neuroregeneration due to insufficient upregulation of neurotrophins. Delayed nerve recovery in turn will postpone reinnervation and muscle function recovery. Future work will investigate this possible mechanism for delayed recovery after PNC+VD.
Conclusions
In this study, we established simultaneous neuromuscular recordings of EUS EMG and PNMBP during LPP and observed differing recovery patterns for different simulated childbirth injury models. Both pudendal nerve and EUS function recover more slowly after PNC+VD than after either PNC or VD alone. Delayed recovery of pudendal nerve activity after PNC+VD indicates delayed or slowed neuroregeneration and explains the slowed recovery of nerve and muscle function. Future work will be aimed at investigating the mechanism of this effect as well as testing methods to facilitate neuroregeneration and recovery after this clinically relevant dual injury.
Acknowledgments
This work was supported in part by NIH RO1 HD38679-08, the Cleveland Clinic, and the Rehabilitation Research and Development service of the Department of Veterans Affairs.
Contributor Information
Hai-Hong Jiang, Email: jiangh@ccf.org.
Hui Q Pan, Email: panh@ccf.org.
A. Marcus Gustilo-Ashby, Email: gashbym@yahoo.com.
Bradley Gill, Email: gillb@ccf.org.
Jonathan Glaab, Email: jglaab08@jcu.edu.
Paul Zaszczurynski, Email: zaszczp@ccf.org.
References
- 1.Retzky SS, Rogers RMJ. Urinary Incontinence in Women. 47. Summit, NJ: Ciba-Geigy Corp.; 1995. [PubMed] [Google Scholar]
- 2.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005 May;98(5):1884–90. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 3.Lien KC, Morgan DM, DeLancey JO, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. American Journal of Obstetrics & Gynecology. 2005;192(5):1669–76. doi: 10.1016/j.ajog.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Dietz HP, Simpson JM. Does delayed child-bearing increase the risk of levator injury in labour? Aust N Z J Obstet Gynaecol. 2007 Dec;47(6):491–5. doi: 10.1111/j.1479-828X.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. British Journal of Surgery. 1990;77:1358–60. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 6.Lin AS, Carrier S, Morgan DM, Lue TF. The effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52(1):143–51. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 7.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25(4):388–96. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 8.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. Journal of Urology. 2003 Sep;170(3):1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson CL, Lin DL, Rao S, Damaser MS. Short-term functional and neuroregenerative response of the urethra to ovariectomy and vaginal distension in female rats. International Urogynecology Journal. 2005 Mar;16(2):119–25. doi: 10.1007/s00192-004-1237-6. [DOI] [PubMed] [Google Scholar]
- 10.Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. Am J Physiol Renal Physiol. 2007 Nov;293(5):F1614–F1621. doi: 10.1152/ajprenal.00176.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, et al. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19(1):53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;292:R1738–R1744. doi: 10.1152/ajpregu.00784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang HH, Tang W, Damaser MS, Song B. Society of Neuroscience. 2007. A simultaneous myoneurophysiologic study of the guarding reflex in female rat. 2007-A-100741. [Google Scholar]
- 14.Damaser MS, Kim FJ, Minetti GM. Methods of testing urethral resistance in the female rat. Adv Exp Med Biol. 2003;539(Pt B):831–9. doi: 10.1007/978-1-4419-8889-8_51. [DOI] [PubMed] [Google Scholar]
- 15.Rogers RG, Leeman LL. Postpartum genitourinary changes. Urol Clin North Am. 2007 Feb;34(1):13–21. doi: 10.1016/j.ucl.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourology & Urodynamics. 2006;25(4):388–96. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 17.Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, et al. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol. 2006 Dec;176(6 Pt 1):2711–5. doi: 10.1016/j.juro.2006.07.139. [DOI] [PubMed] [Google Scholar]
- 18.Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. American Journal of Physiology - Renal Physiology. 2007 Nov;293(5):F1614–F1621. doi: 10.1152/ajprenal.00176.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res. 1989 Jun 19;490(1):85–94. doi: 10.1016/0006-8993(89)90433-2. [DOI] [PubMed] [Google Scholar]
- 20.Dubrovsky B, Martinez-Gomez M, Pacheco P. Spinal control of pelvic floor muscles. Exp Neurol. 1985 May;88(2):277–87. doi: 10.1016/0014-4886(85)90191-8. [DOI] [PubMed] [Google Scholar]
- 21.Ashton-Miller JA, Delancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007 Apr;1101:266–96. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 22.Park JM, Bloom DA, McGuire EJ. The guarding reflex revisited. Br J Urol. 1997 Dec;80(6):940–5. doi: 10.1046/j.1464-410x.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanagho EA. The anatomy and physiology of micturition. Clin Obstet Gynaecol. 1978 Apr;5(1):3–26. [PubMed] [Google Scholar]
- 24.Cannon TW, Ferguson C, Wojcik EM, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU International. 2002;90(4):403–7. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 25.Sanders DB, Stalberg EV, Nandedkar SD. Analysis of the electromyographic interference pattern. J Clin Neurophysiol. 1996 Sep;13(5):385–400. doi: 10.1097/00004691-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, et al. Effects of pudendal nerve injury in the female rat. Neurourology and Urodynamics. 2000;19(1):53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000 Jun;23(6):863–73. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Yan Q, Matheson C, Lopez OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. Journal of Neuroscience. 1994;14(9):5281–91. doi: 10.1523/JNEUROSCI.14-09-05281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman B, Kleinfeld D, Ip NY, Verge VMK, Moulton R, Boland P, et al. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. Journal of Neuroscience. 1995 Feb;15(2):1044–56. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakuma K, Watanabe K, Sano M, Uramoto I, Nakano H, Li YJ, et al. A possible role for BDNF, NT-4, and TrkB in the spinal cord and muscle of rat subjected to mechanical overload, bupivacaine injection, and axotomy. Brain Research. 2001;907:1–19. doi: 10.1016/s0006-8993(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 31.Wells DJ, McKechnie BA, Kelkar S, Fallon JR. Neurotrophins regulate agrin-induced postsynaptic differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1999 Feb;96:1112–7. doi: 10.1073/pnas.96.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]