Abstract
CPT-11 is a clinically used cancer drug, and it is a prodrug of the potent topoisomerase I inhibitor, SN-38 (7-ethyl-10-hydroxycamptothecin). To bypass the need for the in vivo conversion of CPT-11 and increase the therapeutic index, bifunctional derivatives of SN-38 were prepared for use in antibody-based targeted therapy of cancer. The general synthetic scheme incorporated an acetylene-azide click cycloaddition step in the design, a short polyethylene glycol spacer for aqueous solubility, and a maleimide group for conjugation. Conjugates of a humanized anti-CEACAM5 monoclonal antibody, hMN-14, prepared using these SN-38 derivatives were evaluated in vitro for stability in buffer and human serum, and for antigen-binding and cytotoxicity in a human colon adenocarcinoma cell line. Conjugates of hMN-14 and SN-38 derivatives 16 and 17 were found promising for further development.
Introduction
SN-38 is the active drug form of the clinically used anticancer agent, CPT-11 (Camptosar; irinotecan; 7-ethyl-10-{4-[1-piperidino]-1-piperidino}carbonyoxycamptothecin), and belongs to the class of 20(s)-camptothecin (CPT) group of compounds that act as potent topoisomerase I inhibitors. 1–3 SN-38 is 2 to 3 orders of magnitude more potent than CPT-11 in vitro.4 The latter has shown antitumor activity clinically in colorectal, lung, cervical, and ovarian cancers.5,6
The in vivo conversion of CPT-11 to SN-38 by human liver carboxylesterase is inefficient.7 In liver, SN-38 is further transformed to its β-glucuronide, SN-38G. Once excreted into bile, SN-38G is reconverted to SN-38 by intestinal β-glucuronidase,8 resulting in severe delayed diarrhea in patients undergoing the CPT-11 treatment.9 Oxidative degradations, mediated by cytochrome P450, give rise to metabolites, some of which are poorer substrates than CPT-11 for human carboxylesterase.10 Further, at physiological pH, CPTs exist in equilibrium with the corresponding lactone opened form, with the carboxylate form possessing ~10% of the potency of intact lactone form; this equilibrium is further shifted to the inactive carboxylate form due to the stabilization of the latter by complexation with human serum albumin.11,12 The pharmacological activity of CPTs depends on the presence of the intact hydroxylactone moiety.12 Early findings in the CPT area showed that derivatization of 20-hydroxyl group of CPT considerably minimized the undesirable lactone ring opening under physiological conditions.13
Erratic and patient-variable conversion of CPT-11 to the active drug and the complex in vivo metabolism14 of both CPT-11 and SN-38 result in reduced bioavailability of the active drug. These considerations have spawned considerable interest in utilizing SN-38 itself in water-soluble forms other than CPT-11. This then led to the attachment of parent CPT or 10-hydroxy-CPT, and later on SN-38, to hydrophilic polymers such as polyglutamic acid, poly HPMA, or polyethylene glycol.15–18 A liposomal formulation of SN-38 was also evaluated.19 In other approaches, the 10-hydroxyl group of the molecule was utilized in the form of an ether or an ester, with further attachment to dextran or to a peptide, respectively.20,21
We embarked on a project to target SN-38 selectively to tumor sites using tumor-selective mAbs, so as to increase both solubility and therapeutic index. SN-38 is a potent drug,22 with IC50 values in the nanomolar range in a number of tumor cell lines. There is considerable current interest in targeted therapies of cancer. The use of tumor-selective mAbs as carriers of therapeutic radionuclides or chemotherapy drugs or protein toxins has been investigated extensively in view of the opportunity for “patient-friendly” treatments. Several recent reviews describe the scope of these approaches.23–26 An advantage to using SN-38 in the antibody conjugate format is that the drug’s in vivo pharmacology is well established.
In this report, we describe the syntheses of bifunctional SN-38 derivatives and in vitro characteristics of their antibody conjugates. Selected derivatives were also evaluated as therapeutic agents in a number of preclinical models of solid tumors;27,28 the preclinical therapy data will be detailed elsewhere.
Results and Discussion
Synthetic chemistry: The initial approach
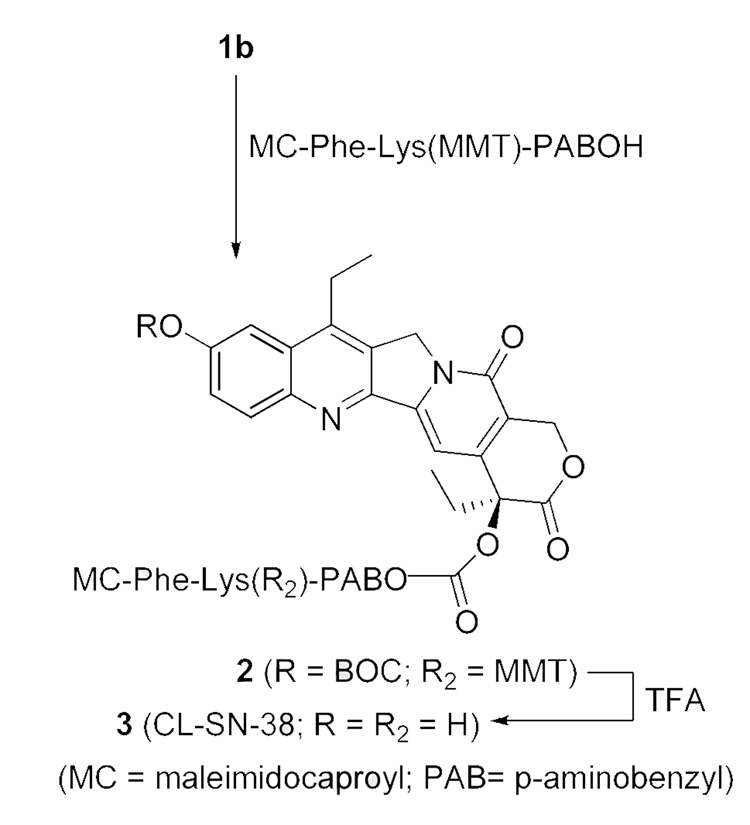
We originally set out to prepare a bifunctional SN-38, 3 (‘CL-SN-38’), using the sequence shown in Scheme-2. The synthesis was modeled on an approach described for a camptothecin (CPT) derivative wherein the lone 20-hydroxyl group of CPT was converted into a carbonate using a cross-linker that contained an intracellularly-cleavable dipeptide, Phe-Lys, and a maleimide.29 For application to SN-38, the more reactive phenolic hydroxyl group at the 10 position had to be protected, with deprotection performed after the carbonate formation at the 20-hydroxyl position. The maleimide-containing bifunctional linker precluded protection of the 10-hydroxyl as a silyl derivative, since fluoride-mediated deprotection was found to affect the maleimide group. The phenolic group was protected as the BOC derivative after determining that this group could be removed selectively in a short-duration TFA treatment.
Scheme 2.
Synthesis of the bifunctional SN-38, CL-SN-38 (3)
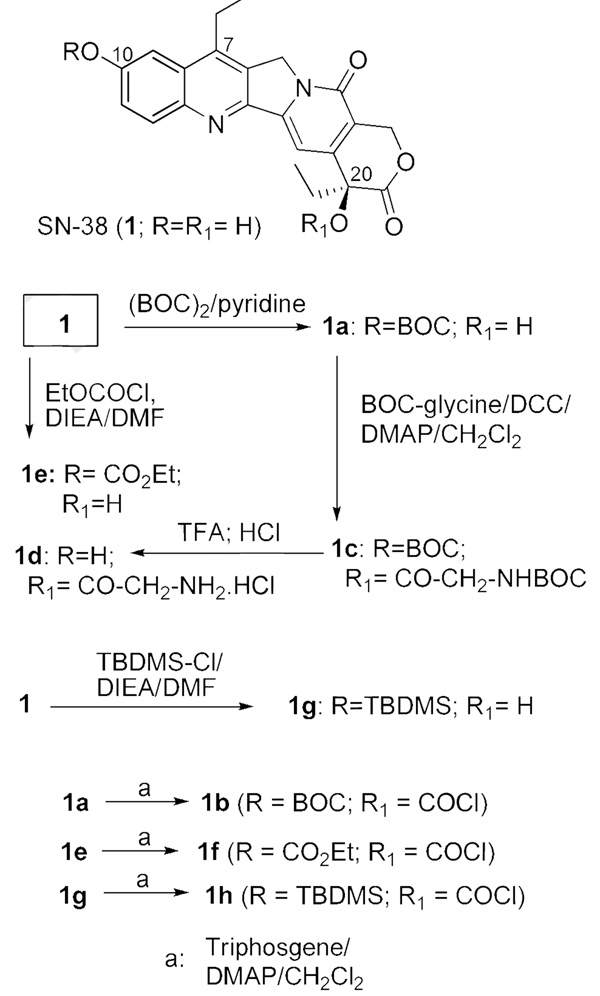
BOC-SN-38, 1a,18 was converted to its 20-O-chloroformate, 1b, using triphosgene and DMAP (Scheme 1), and the latter was reacted in situ for < 5 min with the known linker, MC-Phe-Lys(MMT)-PABOH 30 (Scheme 2). Initial experiments involving longer duration of reaction gave intractable products. We hypothesized that the maleimide functional group was incompatible to the conditions used in the SN-38-20-O-chloroformate formation, resulting in low yields. Thus, a short-duration reaction (< 5 min) at the carbonate-formation step was instituted, followed by immediate column chromatography to separate the product from DMAP and unreacted triphosgene. The best yield of the isolated product, 2, was ~ 30%, and in most runs, the product was obtained in low and variable yields, sometimes as low as 10%, despite attempted optimization. In the final step, the BOC group was removed by treating with TFA for 2–5 min, and isolating the product, 3 (‘CL-SN-38’), by solvent removal and precipitation with diethyl ether. The 20-carbonate was concomitantly deprotected to some extent, but the level of SN-38 so formed was limited to ≤ 20% by carrying out the TFA reaction for ≤ 5 min. The level of free SN-38 was further reduced to ≤ 10% by additional purification. The product, 3, was used as such for mAb conjugations, since underivatized SN-38 was removable during purification.
Scheme 1.
SN-38, variously derivatized at 10 and 20 positions
Conjugates of CL-SN-38 were prepared of humanized anti-CEACAM5 mAb, hMN-14 (labetuzumab), humanized anti-EGP-1 mAb, hRS7, and humanized anti-CD22 mAb, hLL2 (epratuzumab), used as control, and were found to exhibit significant and selective therapeutic effects in nude mice bearing human colonic and lung carcinoma xenografts.27 Briefly, in a lung metastatic model of human colon carcinoma in nude mouse, therapy with CL-SN-38 conjugate of the specific humanized anti-CEACAM5 mAb, hMN-14, increased median survival 2-fold to 74 days compared to treatments with equidoses of the conjugate of non-targeting control antibody, mixture of antibody and SN-38, or non-treatment control (P< 0.005). In the therapy of s.c. Calu-3 human lung adenocarcinoma xenografts in nude mice, the CL-SN38 conjugate of the specific, rapidly internalizing, humanized anti-EGP-1 mAb, hRS7, produced ‘cures’ in 80% of animals (no visible tumor) out to 110 days; in this experiment, treatment with equidose mixture of non-targeting control conjugate and non-treatment resulted in an 8.3-fold and a 14-fold increase in mean tumor volumes, respectively, on day 61.
While this approach helped establish the utility of mAb-SN-38 conjugates in preclinical models, it had certain shortcomings that precluded further development, namely: (1) The yield at the carbonate formation stage was low and irreproducible; and (2) solubility problem caused turbidity or precipitation during conjugations, resulting in significant protein loss. Even with 15% v/v of DMF or DMSO as co-solvent, low salt buffer, and gradual addition of the solution of the drug to reduced antibody, the protein recovery was only ~ 20% in many instances. By replacing maleimidocaproyl with a defined-PEG™ in the cross-linker, the solubility problem was overcome at the mAb conjugation stage (not shown). Still, the synthesis was impractical in the key aspects of yield, reproducibility, and scale-up. For our program, we needed a robust and higher-yield synthetic design.
Based on the hypothesis that the yield at the carbonate-forming step was hampered by the damage to the maleimide functional group, a substrate with Fmoc-protected version of the cross-linker without the maleimide group, namely Fmoc-Phe-Lys(MMT)-PABOH, was briefly examined for reacting with 1b. While the carbonate formation proceeded in ~ 60% yield, subsequent Fmoc deprotection liberated a free amino group under basic condition that was incompatible with the lactone group of SN-38. Both the yield and the quality of the product were unsatisfactory.
Azide-acetylene cycloaddition route
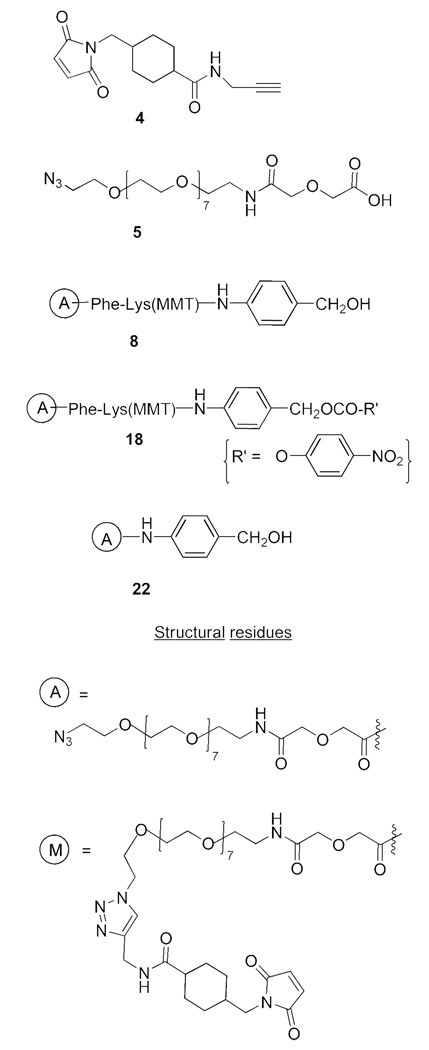
An azide-incorporated linker, containing the PABOH moiety, was conceptualized to overcome the synthetic hurdle in forming the 20-carbonate, with the subsequent Cu(I)-mediated ‘click’ cycloaddition31 enabling the introduction of the maleimide group. To this end, the commercially available O-(2-azidoethyl)-O’-(N-diglycolyl-2-aminoethyl)heptaethyleneglycol, 5, was used as a linker to introduce the azido group on to SN-38; the acetylenic counterpart for the click chemistry, 4, was prepared by reacting succinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (SMCC) with propargylamine. The provision of a short PEG moiety in the linker addressed the aqueous solubility issue. Structures of the relevant reagents used in the modified approach are shown in Chart 1. For simplicity, various azide- and maleimide-containing SN-38 derivatives are uniformly indicated with “A” and “M” descriptors, respectively, whose structures are also given in Chart 1.
Chart 1.
Structures of some reagents and structural residues
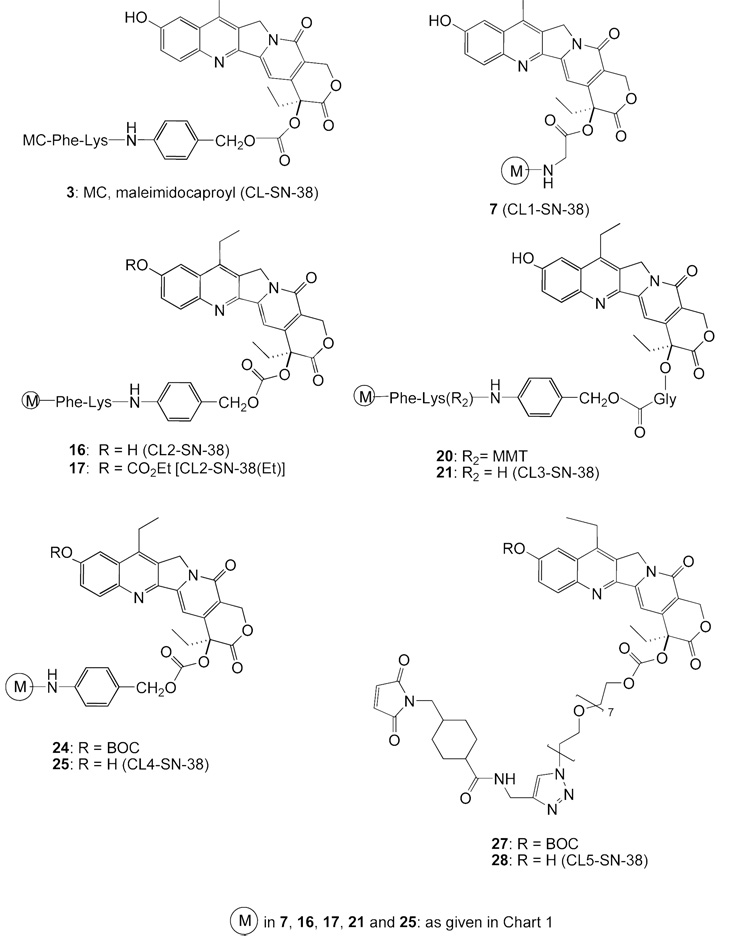
At the outset, our goal was to prepare a bifunctional SN-38 that contained an intracellularly-cleavable dipeptide, akin to that in ‘CL-SN-38’ (3) and other drug conjugates.29,30 Thus, the derivative ‘CL2-SN-38’ (16) was prepared as an alternative to 3. The choice of SN-38 as the drug for targeted therapy and the requirement for the liberation of the intact drug from the conjugate dictated that the hydroxyl group be derivatized as an ester or a carbonate. The utility of the stable, intracellularly-cleavable, dipeptide in both 3 and 16 must therefore be viewed in the context of the 20-carbonate (or ester) that is also a cleavable bond. To determine the putative need for the cleavable dipeptide in the linker in addition to the ester or carbonate, we prepared a series of bifunctional SN-38 derivatives CL1-SN-38 through CL5-SN-38 that differed in the nature of the linker at the 20-position and the presence of the cleavable peptide.Chart 2 gives the structures of the various bifunctional SN-38 products made.
Chart 2.
Structures of bifunctional SN-38 derivatives
Preparations using the modified approach
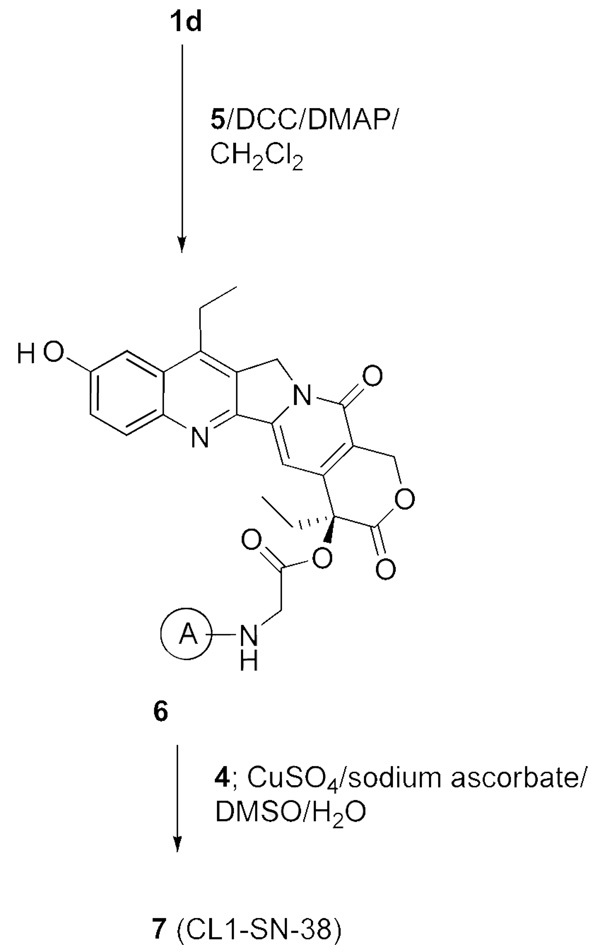
We first prepared ‘CL1-SN-38’, 7 (Scheme 3). In this, SN-38 was first derivatized as 20-O-glycinate, 1d, by a 3-step procedure of conversion to BOC-SN-38, ester formation using BOC-glycine, and removal of BOC groups, along the lines originally reported for 10-hydroxycamptothecin.16 The glycinate, 1d, was further transformed to the azido derivative, 6, which in turn was subjected to click cycloaddition with 4 to obtain CL1-SN-38, 7. The glycinate linkage, as in this product, has been used in the design of some water-soluble versions of CPT and SN-38.16,18
Scheme 3.
Synthesis of the bifunctional SN-38, CL1-SN-38 (7)
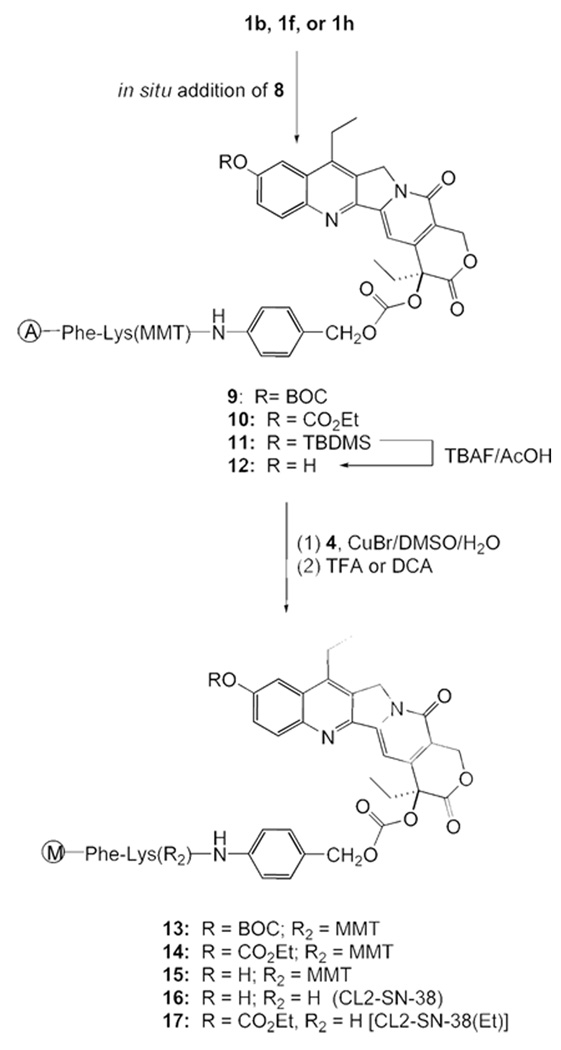
Scheme 4 shows the preparation of ‘CL2-SN-38’, 16. Although we continued with the ‘BOC’ protection for the 10-hydroxyl group, it was imperative to find an alternate protecting group so as to avoid the impractical 5-min deprotection as well as the yield reduction in the final step. The click chemistry approach enabled the silyl protecting group to be examined. Accordingly, we prepared the TBDMS derivative of SN-38, 1g; transformed it to the azide-appended SN-38, 11; and cleaved off the silyl group to afford 12. Click cycloaddition, followed by mild deprotection using dichloroacetic acid, furnished CL2-SN-38. Separating the carbonate-forming step from the maleimide introduction conferred certain robustness in yield reproducibility. Yields were generally in the range of 70%–80% at the carbonate formation and the click cycloaddition steps in a number of preparations. Furthermore, the final product, after removing the protecting groups, could be readily purified by flash chromatography.
Scheme 4.
Synthesis of the bifunctional SN-38, CL2-SN-38 (16) & CL2-SN-38(Et) (17)
The 10-carbonate variant, 17, designated as CL2-SN-38(Et), was designed as the CL2-SN-38 equivalent. The phenolic carbonate in this derivative was expected to cleave readily under physiological conditions, based on a report on the differential cleavage rates of 10-carbonate vs. 20-carbonate in 10,20-bis carbonate derivatives of 10-hydroxycamptothecin.32 The original rationale for preparing 17 was to avoid the use of the BOC protecting group. Unexpectedly, this derivative enhanced the stability of the conjugate, as discussed in a later section.
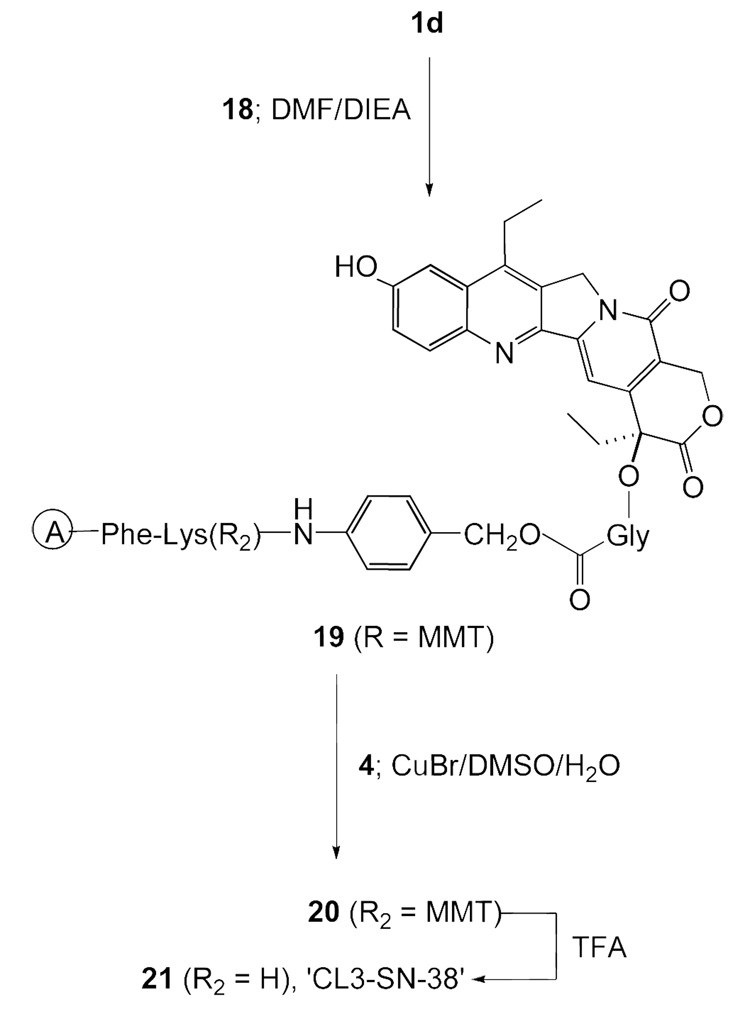
Scheme 5 depicts a bifunctional SN-38, 21 (‘CL3-SN-38’), possessing an ester linkage at the 20-position as well as the cleavable dipeptide, Phe-Lys. This product has structural similarities to both CL1-SN-38 and CL2-SN-38, and was designed as an improved version of both of these structures. Lysosomal cleavage of the peptide bond will liberate SN-38-20-O-glycinate from the conjugate, which can undergo ester hydrolysis chemically or enzymatically in lysosome or cytosol.
Scheme 5.
Synthesis of the bifunctional SN-38, CL3-SN-38 (21)
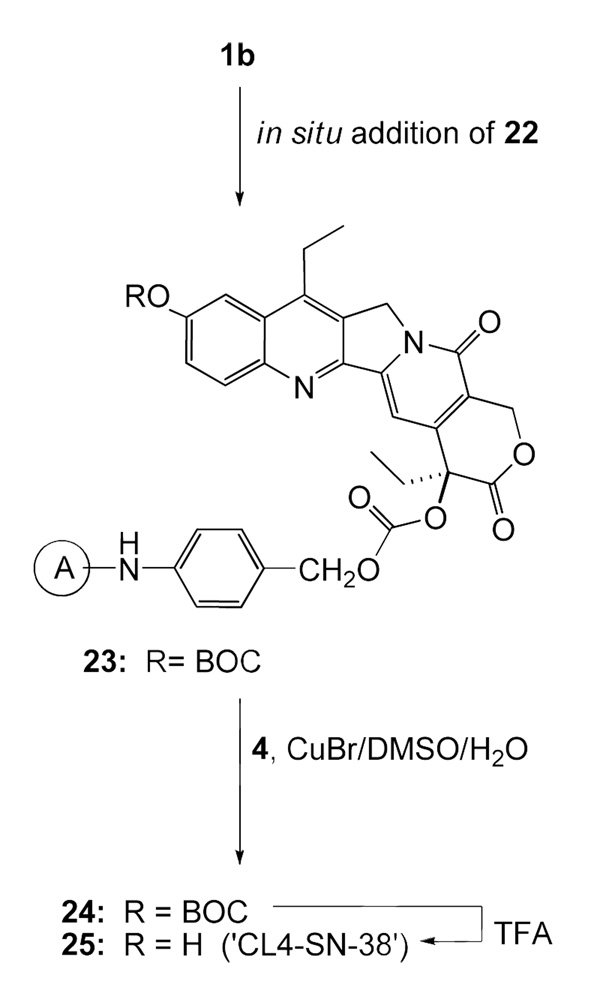
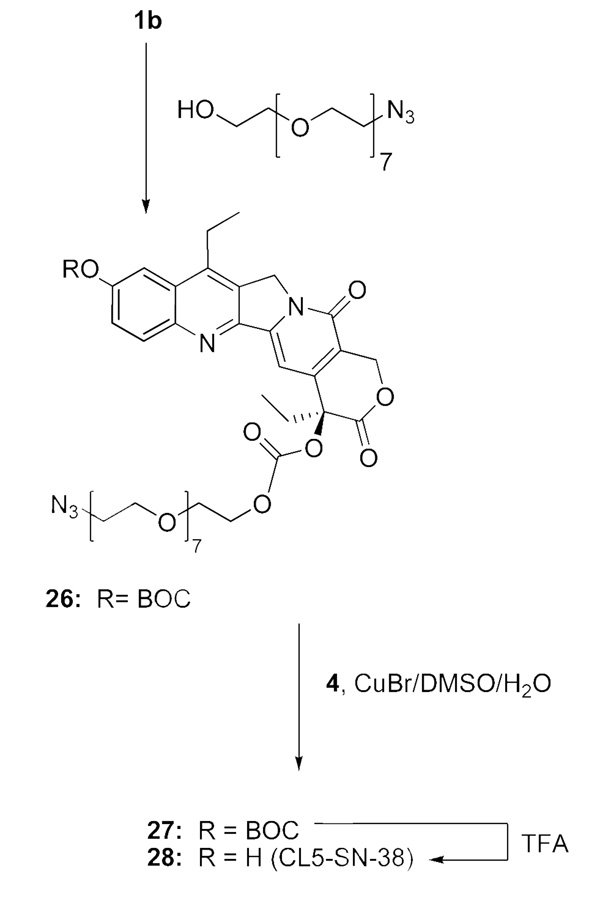
SN-38 derivative 25 (‘CL4-SN-38’; Scheme 6), which has a carbonate linking at the 20 position, does not have the ‘Phe-Lys’ cleavable peptide, but possesses the PABO residue, and the latter is attached to the PEG azide. Its synthesis is straightforward. A simpler carbonate, 28 (Scheme-7), was prepared by directly attaching O-(2-azidoethyl)heptaethyleneglycol to the chloroformate 1b; the resultant product 26 was coupled to 4, and the click chemistry product 27 was deprotected with TFA to obtain 28. In principle, BOC protecting group of Scheme 6 and Scheme 7 can be replaced with TBDMS to further improve yields.
Scheme 6.
Synthesis of the bifunctional SN-38, CL4-SN-38 (25)
Scheme 7.
Synthesis of the bifunctional SN-38, CL5-SN-38 (28)
Azide-acetylene cycloaddition reaction was carried out using 5 mol % of cupric sulfate and 50 mol % of sodium ascorbate in the preparation of CL1-SN-38. In other derivatives, in view of sluggish conversion, 1–2 molar equivalents of cuprous bromide were used. Use of a 3-fold excess of the acetylenic reagent, 4, led to near-quantitative conversion in the cycloaddition reaction, within 30 min in most cases, as documented by HPLC analyses of reaction mixtures.
Characterizations of the intermediates and the final products were facilitated by comparisons with the well-separated 1H NMR signals in the spectra of simpler SN-38 derivatives 1a, 1e and 1g; the acetylenic reagent 4; and the cross-linkers 8 and 22.
Antibody conjugations
Interchain disulfides of mAbs were reduced with DTT or TCEP, generating 7–8 thiol groups on the antibody, as determined by Ellman’s assay.33 The reduced mAbs were conjugated to maleimide-containing biunctional SN-38 derivatives. The conjugations were done at a slightly acidic pH of 6.5 for a short duration of 20 min. Table 1 shows the SN-38 substitutions in representative conjugate preparations. The average SN-38/mAb molar substitution ratio (MSR) was calculated from determinations of SN-38 concentration by absorbance at 366 nm (correlated to standards) and mAb concentration by absorbance at 280 nm, the latter corrected for SN-38 absorbance at 280 nm. MALDI mass spectra of reduced mAb and the conjugates showed a series of fragmented peaks corresponding to half IgG and light and heavy chains, with molecular ion peak frequently being too weak to observe; these fragmentations complicate the determinations of mean drug/mAb molar substitutions by this method.
Table 1.
Representative SN-38/mAb MSRa
| mAbb | Conjugate | MSR |
|---|---|---|
| hMN-14c | hMN-14-7d | 7.7 |
| hMN-14-16d | 6.8 | |
| hMN-14-17d | 6.3 | |
| hMN-14-21d | 5.5 | |
| hMN-14-25d | 4.1 | |
| hMN-14-28d | 6.9 | |
| hRS7e | hRS7-7 | 5.3 |
| hRS7-16 | 6.3 | |
| hPAM4f | hPAM4-16 | 5.7 |
| hLL2g | hLL2-7 | 7.4 |
| hLL2-16 | 6.4 | |
| hLL2-17 | 5.5 | |
| hA20h | hA20-16 | 6.1 |
MSR, molar substitution ratio
monoclonal antibody
humanized anti-CEACAM5 mAb, hMN-14 (labetuzumab)
bifunctional SN-38 derivatives were: CL1-SN-38 (7), CL2-SN-38 (16), CL2-SN-38[Et](17), CL3-SN-38 (21), CL4-SN-38(25), and CL5-SN-38 (28)
humanized anti-EGP-1 mAb, hRS7
humanized anti-MUC-1 mAb, hPAM4
humanized anti-CD22 mAb, hLL2 (epratuzumab)
humanized anti-CD20 mAb, hA20 (veltuzumab).
In this report, in vitro data are described for the conjugates of the humanized anti-CEACAM5 mAb, hMN-14. Similar data were generated with SN-38 conjugates of other humanized mAbs (not shown).
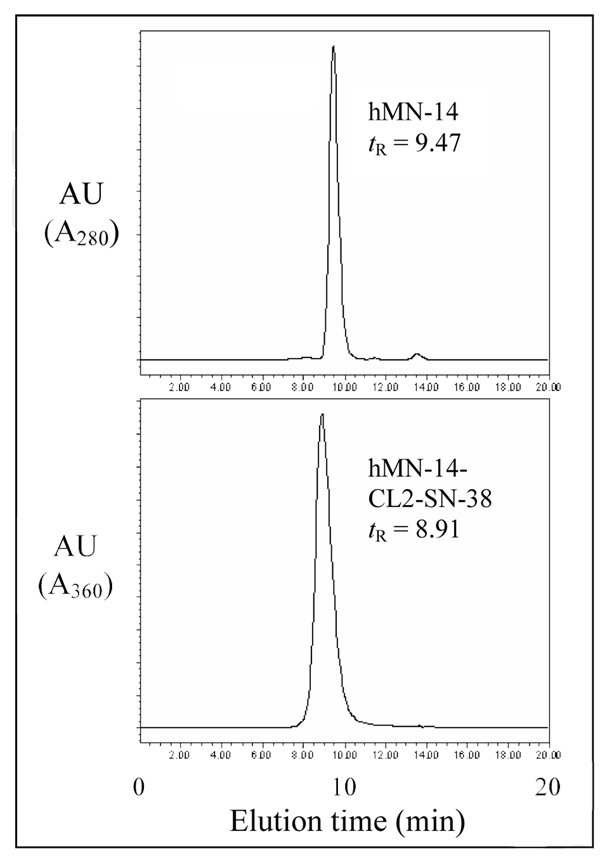
Figure 1 shows the comparative HPLCs of a humanized anti-CEACAM mAb, hMN-14, and its CL2-SN-38 conjugate with a mean drug substitution of 6.1.
Figure 1.
Size-exclusion HPLC of unmodified hMN-14 (top) and of hMN-14-CL2-SN-38 conjugate with a drug molar substitution of 6.1 (bottom). Unmodified hMN-14 was detected by absorbance at 280 nm. The HPLC profile of the conjugate was detected at the absorbance wavelength of SN-38, namely 360 nm; thus, the peak eluting near the antibody position corresponds to antibody substituted with SN-38. Abbreviation: tR, retention time.
Drug release from conjugates
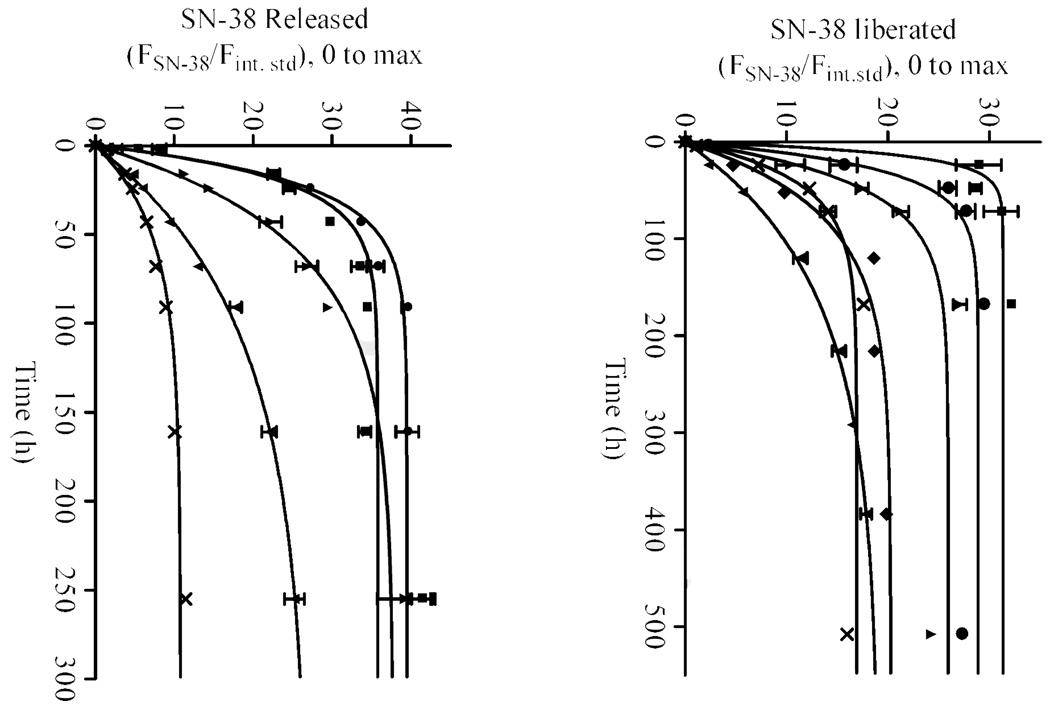
Figure 2 and Table 2 describe the stabilities of hMN-14 conjugates of the SN-38 derivatives in buffer and human serum. The procedure was adapted from the reported method for quantifying SN-38 in sera of patients undergoing the CPT-11 treatment.34 The hMN-14 conjugate of CL1-SN-38 (7), with the glycinate linkage, was the least stable under physiological conditions in vitro; this was counter-intuitive, as the ester was expected to be more stable than a carbonate. The hMN-14 conjugates of CL5-SN-38 (28) CL2-SN-38 (16), CL2-SN-38 (Et) (17) and CL4-SN-38 (25), all containing carbonate at the 20-position, with variations in the linkers, were more stable than the conjugate of 7. This suggested that perhaps hydrophobicity and/or steric hindrance due to the linker conferred certain stability. This effect might be operative in view of the attachment of the drug in the interchain regions of the antibody, and perhaps such an effect might not be evident in conjugates involving drug attachments to the lysine groups of mAb and the exposure to the more hydrophilic milieu. The most stable conjugate was the hMN-14 conjugate of 17; its in vitro half-life of 66 h in human serum resembles that of conjugates with cross-linkers containing the acid-sensitive hydrazone bond.35,36 Conjugates of both CL3-SN-38 (21) and CL1-SN-38 (7) contain glycinate at the 20 position, but their different linker compositions dictate their differential stabilities. A 5-fold greater stability, in buffer, for the conjugate of 21 vs. the conjugate of 7 lends credence to the speculation of the role of hydrophobicity/steric hindrance in drug-release kinetics.
Figure 2.
Kinetics of SN-38 release from various hMN-14-SN-38 conjugates incubated in PBS, pH 7.4 (top) or human serum (bottom) at 37 °C. Y-axis displays the ratios of the reverse-phase HPLC peak areas due to SN-38 and the fixed amount of internal standard (10-hydroxycamptothecin) extracted from the incubates. Conjugates: hMN-14-[CL1-SN-38 (7)], square; hMN-14-[CL2-SN-38 (16)], triangle; hMN-14-[CL2-SN-38-10-CO2Et) (17)], inverted triangle; hMN-14-[CL3-SN-38 (21)], diamond; hMN-14-[CL4-SN-38 (25)], X; and hMN-14-[CL5-SN-38 (28)], circle. With hMN-14-21 conjugate, only buffer-stability was determined. The plots were generated using the Prism™ software and the equation for one-phase exponential association [Y = Ymax*(1-exp(-K*X))]. The half-lives, determined as 0.69/K where K is rate constant, are given in Table 2.
Table 2.
In vitro stability of hMN-14-SN-38 conjugates at 37 °C
| Conjugate | Half-life |
|
|---|---|---|
| PBS | human serum | |
| hMN-14-7 | 8.6 h | 10.8 h |
| hMN-14-16 | 30.1 h | 36.2 h |
| hMN-14-17 | 98.4 h | 65.9 h |
| hMN-14-21 | 48.8 h | n/da |
| hMN-14-25 | 27.6 h | 32.2 h |
| hMN-14-28 | 18.7 h | 14.4 h |
n/d, not determined
In vitro bindings and cytotoxicities
Table 3 details binding data obtained using five SN-38 conjugates of hMN-14, and LoVo human colon adenocarcinoma cell line. The cell bindings (Kd) are similar to the unmodified mAb in these determinations. Cell-bindings of the conjugates of 7, 16, and 21 and the humanized anti-EGP-1 mAb, hRS7, in the pancreatic adenocarcinoma cell line, CaPan-1, were likewise similar to that of unmodified hRS7 (not shown). Table 4 shows the in vitro cytotoxicities of the hMN-14 conjugates. The IC50 for the conjugate of 7 is not significantly different from that of SN-38. The IC50 values for conjugates of 16, 17, and 28 are somewhat higher than that of SN-38 (5–6 nM vs. 2–3 nM for SN-38), but are still in a single digit nM range similar to that for the free drug. The IC50 value for the conjugate of 21 was the highest (IC50: 9.5 nM), in this cell line, among the SN-38 derivatives tested.
Table 3.
In vitro binding on LoVo human colon adenocarcinoma cell line
| Expt. | Material | SN-38 | Kd | 95% C.I.b |
|---|---|---|---|---|
| No. | MSRa | (nM) | ||
| 1 | hMN-14 | - | 1.4 | 1.2–1.7 |
| hMN-14-7 | 7.1 | 1.4 | 0.6–2.2 | |
| hMN-14-16 | 4.0 | 1.4 | 1.2–1.7 | |
| hMN-14-21 | 5.5 | 1.5 | 1.1–1.9 | |
| 2 | hMN-14 | - | 2.1 | 1.1–3.0 |
| hMN-14-17 | 6.2 | 2.3 | 1.2–3.4 | |
| hMN-14-28 | 6.9 | 2.4 | 1.8–3.7 | |
MSR, molar substitution ratio
C.I., confidence interval
Table 4.
In vitro cytotoxicity on LoVo human colon adenocarcinoma cell line (96 h exposure)
| Expt. | Material | SN-38 | IC50 | 95% C.I.b |
|---|---|---|---|---|
| No. | MSRa | (nM) | ||
| 1 | Free SN-38 | - | 3.2 | 2.6–4.0 |
| hMN-14-7 | 7.1 | 4.1 | 3.4–5.0 | |
| hMN-14-16 | 4.0 | 5.3 | 4.7–5.9 | |
| hMN-14-21 | 5.5 | 9.5 | 8.4–10.8 | |
| 2 | Free SN-38 | - | 2.4 | 1.9–3.1 |
| hMN-14-17 | 6.2 | 5.2 | 4.6–5.9 | |
| hMN-14-28 | 6.9 | 5.8 | 5.1–6.6 | |
MSR, molar substitution ratio
C.I., confidence interval
The choice of the bifunctional SN-38 derivative
As with the CL-SN-38 conjugate, the CL1-SN-38 and CL2-SN-38 conjugates of hMN-14 mAb exhibited significant and selective efficacies in the lung metastatic model of human colon carcinoma in nude mouse;28 therapy with the specific CL1-SN-38 conjugate increased the median survival by 1.7-fold versus treatment with a non-targeting control conjugate, while the specific CL2-SN-38 conjugate increased the median survival 3-fold versus treatment with the control conjugate. Specific tumor growth controls were also observed using CL2-SN-38 conjugates of other mAbs in other tumor models.28 In vitro stability is one criterion to consider in the choice of the specific conjugate, but therapeutic efficacy depends on other factors. For instance, the in vitro cytotoxicity of hMN-14-CL3-SN-38 was inferior to that of hMN-14-CL1-SN-38 conjugate (Table 4), despite the stability advantage for the former. This may be due to the generation of SN-38-20-O-glycinate from hMN-14-21 conjugate after intracellular processing, and the opportunity for intramolecular lactone ring opening before ester hydrolysis.
In ongoing experiments, the conjugates of 16 and 17 are seen to be similar in therapeutic efficacies, and a choice between the two must await more detailed comparisons.
Conclusion
The versatile click cycloaddition approach provided a facile access to a series of bifunctional SN-38 derivatives suitable for antibody conjugation. The provision of a short PEG spacer in the cross-linker facilitated aqueous solubility. Several antibody conjugates could be made with mean drug substitutions in the range of 5–7. Detailed stability determinations in biological media indicated a correlation between conjugate stability and the apparent hydrophobicity of the cross-linker-attached SN-38. The conjugates were found to maintain antigen bindings in in vitro assays involving specific cell lines, and were found cytotoxic in a single-digit nanomolar range. Based on extensive preclinical studies, it is our intent to further focus on the conjugates of CL2-SN-38 and CL2-SN-38(Et).
Experimental section
General procedures
All chemicals were purchased from Sigma-Aldrich company (St. Louis, Mo) unless otherwise stated. Succinimidyl 6-maleimidocaproate and SMCC were purchased from Molecular Biosciences (Boulder, CO). The PEG derivatives were obtained from EMD chemicals (San Diego, CA) and BioVectra DCL (Charlottetown, PE, Canada). SN-38 was purchased from SINOVA (Bethesda, MD). All nonaqueous reactions were performed using anhydrous solvents, unless otherwise stated, under positive pressure of argon. In reaction work up, organic extracts were dried with anhydrous sodium sulfate. 1H NMR spectra at 500 MHz and 13C NMR spectra at 125.7 MHz were recorded on a Varian INOVA NMR spectrometer (Varian Inc., Palo Alto, CA) at Rutgers University Chemistry Department (Newark, NJ). Chemical shifts were referenced to tetramethylsilane at 0 ppm. Exact mass determinations by high-resolution mass spectra were performed at Scripps Center for Mass Spectrometry (La Jolla, CA) using Agilent LC/MSD TOF instrument, a flow rate of 0.3 mL/min of methanolic sample solution, and a capillary voltage of 3500 V. Purifications were performed by flash chromatography on 230–400 mesh silica gel according to well-established procedures using dichloromethane-methanol gradient elutions (usually 0–8 % methanol, with up to 18% methanol in final preparations that generate TFA or DCA salt of lysine side chain amino group), unless otherwise stated. Analytical HPLC was carried out on a Waters Nova-pak HR C18 column (6 µm, 60 Å, 7.8×300 mm), fitted with a guard column and an in-line dual absorbance detector set at 254 nm and 360 nm, and with gradient elution carried out by method 1 or method 2 using buffers A and B as follows: 100% A changing to 100% B over 10 min (linear) at a flow rate of 3 mL/min, changing to 100% B at a flow rate of 4.5 mL/min at 10.1 min and remaining isocratic at this flow rate for 5 min (the isocratic elution with 100 % B was performed for 10 or 15 min for relatively lipophilic intermediates). Method 1: Buffer A was 0.3 % aq. ammonium acetate, pH 4.43; buffer B is 9:1 v/v acetonitrile: buffer A. Method 2: Buffer A was 0.3 % aq. ammonium acetate, pH 4.43; buffer B is 9:1 v/v methanol: buffer A. Analytical samples of some of the intermediates were procured by HPLC using method 1. Monoclonal antibodies were obtained from Immunomedics’ production laboratories. Size-exclusion HPLCs of antibody conjugates were carried out on an analytical Bio-Sil 250 size-exclusion column, in series with a guard column (both from Bio-Rad laboratories, Hercules, CA), using 0.2 M sodium phosphate buffer (pH 6.8) as the mobile phase at a flow rate of 1 mL/min with in-line UV (280 or 360 nm) detection. Cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA).
In TFA-mediated deprotection of ‘BOC’ and ‘MMT’ groups in SN-38 derivatives, a TFA deprotection mixture consisting of 2 mL of TFA, 0.5 mL of dichloromethane, 0.12 mL of anisole, and 0.06 mL of water was used, and is simply referred to as ‘treatment with TFA’ in the relevant experiments. In a similar manner, in instances where only ‘MMT’ needed to be removed, the milder DCA-mediated deprotection was carried out using a mixture of 0.5 mL of DCA, 5 mL dichloromethane, 0.05 mL of anisole, and 0.025 mL of water; and the deprotection is referred to as ‘treatment with DCA’.
10-O-tert-Butoxycarbonyl-SN-38–20-O-chloroformate (1b)
In a typical example, 1a18 (0.358 g, 0.073 mmol), DMAP (0.266 g, 0.218 mmol) and triphosgene (0.0095g, 0.032 mmol) were taken in an eppendorf vial and the reaction was initiated by adding dichloromethane (1.5 mL). The mixture turned to clear light yellow solution within a minute. The formation of the chloroformate was monitored by quenching an aliquot with anhydrous methanol and comparing the TLC mobility with that of 1a. The formation of the chloroformate was complete within a few minutes, and the product was used as such after 7 min from the start-up of the reaction. In larger-scale runs, the reagents were scaled-up proportionally. 20-Chloroformates derived from other 10-substituted SN-38 derivatives were prepared similarly. In all instances, the chloroformates were reacted in situ with relevant cross-linkers.
MC-Phe-Lys-PABOCO-20-O–SN-38 (3; CL-SN-38)
In one run, the chloroformate (1b), generated from BOC-SN-38 (1a; 0.0227 g; 0.046 mmol), was reacted in situ with the known reagent, MC-Phe-Lys(MMT)-PABOH,30 (0.0487 g; 0.056 mmol) for a short duration, typically under 5 min. The reaction mixture was then purified by flash chromatography using methanol-dichloromethane gradient to obtain the carbonate, 2 (off-white solid; 0.022 g; 34.5%). In one run, 2 (0.0316 g; 0.0229 mmol) was treated with TFA for a few min, typically < 5 min, and the product was recovered by precipitation with diethyl ether. TFA-treatment was repeated two times additionally, each for a short duration of less than five min, and the title product was obtained as the TFA salt in 91.7% purity (cream solid; 0.0215 g; 83.7%). This level of purity was judged adequate for exploratory mAb conjugations. Analytical sample of 3 was prepared by HPLC under the condition described in general methods.
SN-38–20-O-glycinate HCl salt (1d)
BOC-SN-38 (1a; 0.322 g; 0.65 mmol) was dissolved in dichloromethane (10 mL) and reacted, under stirring, with BOC-glycine (0.23 g, 1.31 mmol), diisopropylcarbodiimide (0.202 mL; 0.165 g; 1.31 mmol) and DMAP (0.096 g, 0.79 mmol) overnight at ambient temperature. Product 1c was isolated by flash chromatography (pale yellow powder; yield: 0.48, 94 %). TFA deprotection of 1c (0.4 g), followed by precipitation in ethyl ether, yielded the TFA salt, which was dissolved in ethanol (5 mL) and a drop of concentrated HCl, and the solvent was evaporated to obtain 1d (yellow powder; yield: quantitative).
10-O-Ethoxycarbonyl-SN-38 (1e)
To a stirred suspension of SN-38 (0.52 g, 1.327 mmol) in DMF (5 mL), at 4 °C, was added ethylchloroformate (0.252 mL, 0.287 g, 2.645 mmol) and DIEA (0.462 mL, 0.342 g, 2.65 mmol); the cooling bath was removed and the clear solution was stirred at room temperature for 30 min. The product was purified by flash chromatography (off-white solid; yield: 0.586 g, 95%).
10-O-tert-Butyldimethylsilyl-SN-38 (1g)
SN-38 (0.63 g, 1.607 mmol) and tert-butyldimethylsilyl chloride (0.58 g, 3.85 mmol) were dissolved in DMF (15 mL), and DIEA (0.84 mL, 0.622 g, 4.81 mmol) was added. The reaction mixture was stirred for 2 h, solvent and DIEA were removed, and the residue was purified by flash chromatography to obtain 1g (yellow powder, yield: 0.79 g, 97%). Anal. (C28H34N2O5Si) C, H, N.
4-(N-Maleimidomethyl)-N-(2-propynyl)cyclohexane-1-carboxamide (4)
To SMCC (1.131 g, 3.383 mmol), taken in dichloromethane (25 mL), was added propargylamine (0.25 mL; 0.2 g; 3.63 mmol) and DIEA (0.59 mL, 0.4366g, 3.378 mmol), and stirred for 1 h at ambient temperature under argon. The reaction mixture was diluted with dichloromethane (100 mL), washed with 1N HCl and brine (100 mL each), and dried. The crude product was purified by flash chromatography using dichloromethane-methanol gradient elution (100:0 − 98:2) to obtain the title compound (colorless powder; yield: 0.844 g, 91%). Anal. calcd for C15H18N2O3: C, 65.68; H, 6.61; N, 10.21. Found: C, 65.13; H, 6.72; N, 10.04
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-Gly-20-O-SN-38]heptaethyleneglycol (6)
O-(2-Azidoethyl)-O’-(N-diglycolyl-2-aminoethyl)heptaethyleneglycol (5; 0.6 g, 1.083 mmol) was activated with DCC (0.228 g; 1.105 mmol), NHS (0.127 g, 1.103 mmol), and catalytic amount of DMAP (0.01 g, 0.008 mmol) in dichloromethane (20 mL) for 30 min at ambient temperature. To this was added solid SN-38-20-O-glycinate (1d; 0.7g, 1.264 mmol) and DIEA (0.22 mL, 1.26 mmol). After stirring for 1 h, the precipitated urea was filtered and the filtrate was concentrated and purified by flash chromatography to obtain 6 (oil; yield: 1.4 g).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Gly-20-O-SN-38]heptaethyleneglycol (7; CL1-SN38)
A solution of the azide precursor 6 (0.208 g; 0.211 mmol) in DMSO (0.5 mL) was added to the acetylenic reagent 5 (0.173 g; 0.631 mmol) in DMSO (1.5 mL), followed by 1 mL of water, 0.05 M aqueous cupric sulfate (0.21 mL; 0.011 mmol) and 0.5 M aqueous sodium ascorbate (0.21 mL, 0.105 mmol). The somewhat cloudy solution was stirred at ambient temperature for 1 h. Solvents were removed by bulb-to-bulb distillation, and the title product was purified by flash chromatography (gum; yield: 0.173 g, 65%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT) PABOH]heptaethyleneglycol (8)
O-(2-Azidoethyl)-O’-(N-diglycolyl-2-aminoethyl)heptaethyleneglycol (5; 0.430 g, 0.776 mmol) and the known intermediate Phe-Lys(MMT)-PABOH (0.52 g, 0.776 mmol) were dissolved in dichloromethane (20 mL) and cooled in ice bath. EEDQ (0.211 g, 0.853 mmol) was then added, and the clear solution was stirred under argon overnight, with the bath temperature rising to ambient temperature. Solvent removal followed by flash chromatography using the general condition or ethyl acetate-methanol (100:0 − 90:10) gradient elution furnished the title product (gum; yield: 0.56 g, 60%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT) PABOCO–20-O-SN-38-10-O-tert-butoxycarbonyl]heptaethyleneglycol (9)
Reagent 8 (0.341 g; 0.283 mmol) was coupled to 1b; the latter was generated from 1a (0.12 g; 0.244 mmol) according to the general procedure for chloroformate formation. The product was isolated by flash chromatography (gum; yield: 0.294 g, 70%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT) PABOCO–20-O-SN-38-10-O-tert-butyldimethylsilyl]heptaethyleneglycol (11)
The TBDMS derivative 1g (0.540 g, 1.065 mmol) was converted to the corresponding 20-O-chloroformate, 1h, according to the general procedure described previously, which was reacted in situ with the cross-linker 8 (1.20 g, 0.994 mmol) for 20 min. Flash chromatography furnished the title product (pale yellow foam; yield: 1.339 g, 77.5%).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT)-PABOCO-20-O-SN-38-10-O-tert-butoxycarbonyl]heptaethyleneglycol (13)
The azide, 9 (0.255 g, 0.148 mmol) and the acetylenic reagent, 4 (0.101 g, 0.369 mmol) were mixed in DMSO (3 mL) and water (1 mL). Solid cuprous bromide (0.042 g, 0.294 mmol) was added to the clear solution, and the heterogeneous mixture was stirred for 30 min. Solvents were removed, and the crude product was purified by flash chromatography to obtain 13 (yellowish gum; yield: 0.233 g, 79%).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT)-PABOCO-20-O-SN-38]heptaethyleneglycol (15)
The azide 11 (1.339 g, 0.77 mmol) was dissolved in dichloromethane (50 mL), and treated with 1 M solution of tetrabutylammonium fluoride in THF (1.9 mL, 1.9 mmol) and acetic acid (0.231 mL, 3.8 mmol). The reaction mixture was stirred for 20 min, diluted with dichloromethane (200 mL), washed twice with half-saturated sodium chloride, and dried. Solvent removal furnished the desilylated product 12 (gum), which was used as such in the next step. Intermediate 12 and the acetylenic reagent 4 (0.52 g, 1.90 mmol) were dissolved in DMSO (8 mL) and water (2 mL); to this was added cuprous bromide (0.22 g, 1.54 mmol). The reaction mixture was stirred for 80 min, and then diluted with dichloromethane. The organic extract was washed twice with half-saturated aq sodium chloride (100 mL) and dried. The title product was obtained by flash chromatography (gum; yield: 0.84 g; 58% in 2 steps).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys-PABOCO-20-O-SN-38]heptaethyleneglycol (16; CL2-SN-38)
The product 13 (0.14 g; 0.07 mmol) was subjected to TFA treatment for < 5 min, and purified on a short column of silica gel (230–400 mesh) using 5–18 % methanol-dichloromethane gradient elution. The product, 16, ‘CL2-SN-38’, was isolated as the TFA salt (gum; yield: 0.097 g; 80%). The same product was also obtained from 15 (0.584 g; 0.307 mmol) by deprotection with DCA, followed by ether precipitation (yellowish green glassy solid; yield: 0.58 g, 95%).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys-PABOCO-20-O-SN-38-10-O-ethoxycarbonyl]heptaethyleneglycol (17)
This product was prepared from 1e in a sequence of 3 steps of carbonate formation, click chemistry, and MMT deprotection under conditions used for preparing 16, except that the final deprotection of MMT group was accomplished by exposure to DCA. The bis carbonate 10 (gum; yield: 88%) was subjected to click cycloaddition with 4 to obtain 14 (gum; yield: 76%). Deprotection of 14 (0.111g) with DCA furnished the title compound, 17, after precipitation of crude reaction product in ethyl ether, followed by flash chromatography (glassy solid; yield: 0.0883 g, 86%).
Activated carbonate, 18
The azido compound 8 (0.27 g; 0.22 mmol) was activated with bis(nitrophenyl)carbonate (0.204 g; 0.67 mmol) and DIEA (0.039 mL, 0.22 mmol) in dichloromethane (10 mL) for 3 days at ambient temperature. Flash chromatography furnished the activated carbonate, 18 (gum; yield: 0.25 g, 69%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys(MMT)-PABOCO-Gly–-20-O-SN-38]heptaethyleneglycol(19)
SN-38-20-O-glycinate 1d (0.028 g; 0.058 mmol) was coupled to 18 (0.08 g; 0.058 mmol) in DMF (1 mL) and DIEA (0.025 mL; 0.14 mmol). After 4 h of stirring, solvent was removed and the crude product was purified by flash chromatography to obtain 19 (foam; yield: 0.052 g, 54%).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-Phe-Lys-PABOCO-Gly-20-O-SN-38]heptaethyleneglycol (21; CL3-SN-38)
The azide 19 (0.05 g, 0.03 mmol) and the acetylenic reagent 4 (0.024 g, 0.087 mmol) were mixed in 2 mL of 1:1 DMSO-water. Solid cuprous bromide (0.0045 g, 0.031 mmol) was added, and the heterogeneous mixture was stirred for 1 h. The crude product was precipitated by dilution with water, and purified by flash chromatography to obtain 20 as gummy material (yield: 0.041 g, 76%). The product was subjected to short-duration treatment with TFA and purified by precipitation with ethyl ether to obtain the title product, 21 (glassy solid; yield: 0.031 g, 90%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-PABOH]heptaethyleneglycol (22)
O-(2-azidoethyl)-O’-(N-diglycolyl-2-aminoethyl)heptaethyleneglycol (5; 0.207 g, 0.374 mmol), p-aminobezyl alcohol (0.055 g, 0.447 mmol) and EEDQ (0.137 g, 0.554 mmol) were dissolved in dichloromethane (4 mL), and stirred at room temperature overnight. Solvent was removed and the residue was wahed with ethyl ether. The crude product was purified by flash chromatography (gum; yield: 0.207 g, 83%).
O-(2-Azidoethyl)-O’-[(N-diglycolyl-2-aminoethyl)-PABOCO–20-O-SN-38-10-O-tert-butoxycarbonyl]heptaethyleneglycol (23)
Product 22 (0.190 g, 0.288 mmol) was reacted with 1b; the latter was generated from 1a (0.122 g, 0.248 mmol) according to the general procedure. The title product was obtained by flash chromatography (gum; yield: 0.14 g, 48% yield).
O-{2-[(1,2,3-Triazolyl)-4-(4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-PABOCO-20-O-SN-38-10-O-tert-butoxycarbonyl]heptaethyleneglycol (24)
The azide precursor 23 (0.14 g; 0.119 mmol) and the acetylenic reagent 4 (0.1 g, 0.364 mmol) were mixed in DMSO (3 mL) and water (1 mL). Solid cuprous bromide (0.05 g, 0.348 mmol) was added, and the heterogeneous mixture was stirred for 10 min. More water (0.5 mL) was added, and the reaction was continued for 30 min. Solvents were removed and the crude product was purified by flash chromatography (gum; 0.135 g, 84% yield).
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-[(N-diglycolyl-2-aminoethyl)-PABOCO-20-O-SN-38]heptaethyleneglycol (25; CL4-SN-38)
Product 24 (0.143 g) was treated with TFA for < 5 min. After removal of TFA and solvent, the product was purified by flash chromatography (glassy solid; yield, as TFA salt: 0.1 g, 75%).
O-(2-Azidoethyl)-O’-(10-O-tert-butoxycarbonyl-SN-38-20-O-carbonyl)heptaethyleneglycol (26)
The chloroformate 1b, prepared from 1a (0.213 g; 0.433 mmol) was reacted with O-(2-azidoethyl)heptaethyleneglycol (0.205 g, 0.518 mmol) and the reaction mixture was purified by flash chromatography to obtain the azido derivative (yellow glassy solid; yield: 0.312 g, 79%). Analysis: calc. for C44H59N5O16.3H2O: C, 54.55%; H, 6.72%; N, 7.23%; found: C, 54.24; H, 6.12; N, 6.98%.
O-{2-[(1,2,3-Triazolyl)-4-[4-(N-maleimidomethyl)cyclohexane-1-carboxamidomethyl]]ethyl}-O’-(SN-38-20-O-carbonyl)heptaethyleneglycol (28; CL5-SN-38)
The azido derivative 26 (0.105 g, 0.115 mmol) was reacted with reagent 4 (0.09 g, 0.328 mmol) and cuprous bromide (0.047 g, 0.328 mmol) in a mixture of 1:1 v/v DMSO and water (2 mL) for 30 min. DMSO was removed by bulb-bulb distillation, and the crude product was subjected to flash chromatography to obtain 27 (glassy solid; yield: 0.110 g, 81%). The latter intermediate (0.05 g) was subjected to TFA deprotection, and the crude product was purified by flash chromatography to furnish the title product (glassy solid; yield: 0.04 g, 87%).
Conjugation of maleimide-containing SN-38 substrates to mildly reduced antibodies
The anti-CEACAM5 humanized mAb, hMN-14 (labetuzumab), the anti-CD22 humanized mAb, hLL2 (epratuzumab), the anti-CD20 humanized mAb, hA20 (veltuzumab), the anti-EGP-1 humanized mAb, hRS7, and anti-MUC1 humanized mAb, hPAM4, were used in these studies. Each antibody was reduced with 64-fold excess of DTT in 40 mM PBS, pH 7.4, containing 5.4 mM EDTA, at 37 °C (bath) for 45 min. The reduced product was purified on centrifuged size-exclusion column and buffer-exchanged with 75 mM sodium acetate-1 mM EDTA, pH 6.5. The thiol content was determined by Ellman’s assay, and was in the 7–8 SH/IgG range. Alternatively, the antibodies were reduced with TCEP in 75 mM sodium acetate buffer, pH 6.5, followed by in situ conjugation. The reduced mAb was reacted with ~10-to-15-fold molar excess of bifunctional SN-38 using DMSO (10% v/v) as co-solvent, and incubated for 20 min at ambient temperature. The conjugate was purified by centrifugal SEC, passage through a Biobead™ hydrophobic column, and finally by ultrafiltration-diafiltration. The product was assayed for SN-38 concentration by absorbance at 366 nm that was correlated with standards, while the protein concentration was deduced from absorbance at 280 nm and corrected for spillover of SN-38 absorbance at this wavelength. From these, average SN-38/mAb molar substitution ratios were calculated. The purified conjugates were formulated with the addition of excipients (25 mM trehalose and 0.01% polysorbate 80), lyophilized, and stored in a −20 °C freezer.
In vitro hydrolytic and serum stabilities
Solutions of the conjugates (10 mg/mL; 0.027 mL), freshly reconstituted from lyophilizates, were added to 0.873 mL of 40 mM PBS, pH 7.4, or human serum and incubated at 37 °C. At periodic intervals, aliquots were withdrawn, and a known amount of 10-hydroxycamptothecin was added as an internal standard. After precipitating the protein with acetonitrile, the dissociated SN-38 was extracted with chloroform. Fixed volumes of the extracts were analyzed by reversed-phase HPLC, quantifying for SN-38 by fluorescence detection of HPLC peaks. SN-38/internal standard peak ratios were correlated with SN-38 standard curve, the latter generated by plotting SN-38/internal standard peak ratios as a function of SN-38 concentration. The kinetic plots for SN-38 release were generated with the standard Prism® software. Equation for one phase exponential association, with plots starting at zero and ascending to Ymax with a rate constant K, was used to calculate half-lives as 0.69/K.
Binding Study
LumiGLO Chemiluminescent Substrate System (KPL, Gaithersberg, MD) was used to detect the binding of hMN-14-immunoconjugate to LoVo cells. Briefly, cells were plated into a 96-well plate (1×105 cells/well) and incubated overnight. The media in the wells were replaced with fresh media (50 µL/well). Each immunoconjugate was serially diluted, and additions of 50 µL aliquots into triplicate wells yielded a concentration range of 20 µg/mL–0.0098 µg/mL. Plates were incubated for 1.5 h at 4 °C, centrifuged for 5 min at 600 g, and the media was poured out and the plate was blotted dry. The cells were washed once with ice-cold media (200 µL/well) and centrifuged and blotted dry as before. Each well then received 100 µL of HRP-conjugated goat-anti-human secondary antibody (Jackson Immunoresearch, West Grove, PA) at a 1:20,000 dilution in media. Control wells received only cells plus secondary antibody. The plate was incubated for 1 h at 4 °C in the dark, and was centrifuged and blotted dry as before. The cells were washed twice with ice-cold media and once with ice-cold D-PBS. LumiGLO reagent (100 µL/well) was added to all wells and the plate was read for luminescence using an Envision plate reader (Perkin Elmer, Boston MA). Data were analyzed by non-linear regression to determine the equilibrium dissociation constant (Kd).
Growth Inhibition
In vitro cytotoxicities of the immunoconjugates were determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium dye reduction assay (MTS dye reduction assay; Promega, Madison, WI). Briefly, LoVo cells were harvested with Cell Dissociation Buffer (Invitrogen Corp., Grand Island, NY) and plated into 96-well plates (2×103 cells per well). Each lyophilized vial of immunoconjugate was reconstituted with sterile media to make a final concentration of 1500 nM in SN-38 equivalents. From these stock vials various 1:5 dilutions of each were made such that adding 20 µL per well would yield final concentrations ranging from 250 to 0.004 nM of SN-38 equivalents. Free SN-38, used as positive control, was likewise diluted from 250 to 0.004 nM in 1:5 serial dilutions. Unconjugated hMN-14 IgG (negative control) was diluted to match the same protein dose as the immunoconjugates (6 µg/mL to 0.015 ng/mL). After all the reagents were added, the plates were placed in a 37 °C/5% CO2 incubator for 96 h. MTS dye was the added to the plates (20 µL/well). The plates were further incubated for 4 h, and then read at 490 nm. Dose-response curves were generated from the mean of triplicate determinations and IC50 values were calculated using PrismPad® software (Advanced Graphics Software, Encinitas, CA).
Supplementary Material
H-1 and C-13 NMR data and HRMS data for target compounds, a table listing the purity based on HPLC as well as HPLC traces for target compounds, and elemental analysis for 1g.
Acknowledgment
This work was supported in part by SBIR grant CA114802-01A1 (PI: SVG) from the National Cancer Institute of the NIH. We thank Agatha Sheerin, Roberto Arrojo, and Fatma Tat for expert technical support.
Abbreviations
- AcOH
acetic acid
- BOC
tert-butoxycarbonyl
- CLn-SN-38 (n = none or 1–5)
cross-linker-attached SN-38 versions
- CEACAM5
carcinoembryonic antigen-related cell adhesion molecule 5
- CPT
camptothecin
- DCA
dichloroacetic acid
- DCC
dicyclohexylcarbodiimide
- DIEA
diisopropylethylamine
- DMAP
4-dimethylaminopyridine
- DMF
dimethylformamide
- DMSO
dimethylsulfoxide
- D-PBS
Dulbecco’s phosphate buffered saline
- EEDQ
2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline
- EGP-1
epithelial glycoprotein-1
- Fmoc
fluorenylmethoxycarbonyl
- HPMA
hydroxypropyl methacrylamide
- mAb
monoclonal antibody
- MC
maleimidocaproyl
- MMT
monomethoxytrityl
- NHS
N-hydroxysuccinimide
- PAB
p-aminobenzyl
- PEG
polyethylene glycol
- s.c.
subcutaneous
- SE-HPLC
size-exclusion HPLC
- SMCC
succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
- TBAF
tetrabutylammonium fluoride
- TBDMS
tert -butyldimethylsilyl chloride
- TCEP
tris (2-carboxyethyl) phosphine
- TFA
trifluoroacetic acid
- THF
tetrahydrofuran
- tR
retention time
References
- 1.Potmesil M. Camptothecins: from bench research to hospital wards. Cancer Res. 1994;54:1431–1439. [PubMed] [Google Scholar]
- 2.Sawada S, Okajima S, Aiyama R, Nokata K, Furuta T, Yokokura T, Sugino E, Yamaguchi K, Miyasaka T. Synthesis and antitumor activity of 20(S)-camptothecin derivatives: carbamate-linked, water-soluble derivatives of 7-ethyl-10-hydroxycamptothecin. Chem. Pharm. Bull. (Tokyo) 1991;39:1446–1450. doi: 10.1248/cpb.39.1446. [DOI] [PubMed] [Google Scholar]
- 3.Rivory LP, Bowles MR, Robert J, Pond SM. Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochem. Pharmacol. 1996;52:1103–1111. doi: 10.1016/0006-2952(96)00457-1. [DOI] [PubMed] [Google Scholar]
- 4.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 5.Rougier P, Bugat R. CPT-11 in the treatment of colorectal cancer: Clinical efficacy and safety profile. Seminars Oncol. (suppl. 3) 1996;23:34–41. [PubMed] [Google Scholar]
- 6.Saijo N. Preclinical and clinical trials of topoisomerase inhibitors. Ann. N.Y. Acad. Sci. 2000;922:92–99. doi: 10.1111/j.1749-6632.2000.tb07028.x. [DOI] [PubMed] [Google Scholar]
- 7.Satoh T, Hosokawa M, Atsumi R, Suzuki W, Hakusui H, Nagai E. Metabolic activation of CPT-11, 7-ethyl-10-[4-(1-piperidino)-1- piperidino]carbonyloxycamptothecin, a novel antitumor agent, by carboxylesterase. Biol. Pharm. Bull. 1994;17:662–664. doi: 10.1248/bpb.17.662. [DOI] [PubMed] [Google Scholar]
- 8.Sparreboom A, de Jonge MJ, de BP, Brouwer E, Nooter K, Loos WJ, van Alphen RJ, Mathijssen RH, Stoter G, Verweij J. Irinotecan (CPT-11) metabolism and disposition in cancer patients. Clin. Cancer Res. 1998;4:2747–2754. [PubMed] [Google Scholar]
- 9.Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 1994;54:3723–3725. [PubMed] [Google Scholar]
- 10.Rivory LP, Riou JF, Haaz MC, Sable S, Vuilhorgne M, Commercon A, Pond SM, Robert J. Identification and properties of a major plasma metabolite of irinotecan (CPT-11) isolated from the plasma of patients. Cancer Res. 1996;56:3689–3694. [PubMed] [Google Scholar]
- 11.Burke TG, Mi Z. The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J. Med. Chem. 1994;37:40–46. doi: 10.1021/jm00027a005. [DOI] [PubMed] [Google Scholar]
- 12.Giovanella BC, Harris N, Mendoza J, Cao Z, Liehr J, Stehlin JS. Dependence of anticancer activity of camptothecins on maintaining their lactone function. Ann. N. Y. Acad. Sci. 2000;922:27–35. doi: 10.1111/j.1749-6632.2000.tb07022.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Lee C, Sai P, Choe YH, Boro M, Pendri A, Guan S, Greenwald RB. 20-O-acylcamptothecin derivatives: evidence for lactone stabilization. J. Org. Chem. 2000;65:4601–4606. doi: 10.1021/jo000221n. [DOI] [PubMed] [Google Scholar]
- 14.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin. Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- 15.Greenwald RB, Pendri A, Conover C, Gilbert C, Yang R, Xia J. Drug delivery systems. 2. Camptothecin 20-O-poly(ethylene glycol) ester transport forms. J. Med. Chem. 1996;39:1938–1940. doi: 10.1021/jm9600555. [DOI] [PubMed] [Google Scholar]
- 16.Singer JW, De VP, Bhatt R, Tulinsky J, Klein P, Li C, Milas L, Lewis RA, Wallace S. Conjugation of camptothecins to poly-(L-glutamic acid) Ann. N. Y. Acad. Sci. 2000;922:136–150. doi: 10.1111/j.1749-6632.2000.tb07032.x. [DOI] [PubMed] [Google Scholar]
- 17.Zamai M, VandeVen M, Farao M, Gratton E, Ghiglieri A, Castelli MG, Fontana E, D' Argy R, Fiorino A, Pesenti E, Suarato A, Caiolfa VR. Camptothecin poly[n-(2-hydroxypropyl) methacrylamide] copolymers in antitopoisomerase-I tumor therapy: intratumor release and antitumor efficacy. Mol. Cancer Ther. 2003;2:29–40. [PubMed] [Google Scholar]
- 18.Zhao H, Rubio B, Sapra P, Wu D, Reddy P, Sai P, Martinez A, Gao Y, Lozanguiez Y, Longley C, Greenberger LM, Horak ID. Novel prodrugs of SN-38 using multiarm poly(ethylene glycol) linkers. Bioconjug. Chem. 2008;19:849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, Ahmad I. Development and characterization of a novel liposome-based formulation of SN-38. Int. J. Pharm. 2004;270:93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Okuno S, Harada M, Yano T, Yano S, Kiuchi S, Tsuda N, Sakamura Y, Imai J, Kawaguchi T, Tsujihara K. Complete regression of xenografted human carcinomas by camptothecin analogue-carboxymethyl dextran conjugate (T-0128) Cancer Res. 2000;60:2988–2995. [PubMed] [Google Scholar]
- 21.Meyer-Losic F, Nicolazzi C, Quinonero J, Ribes F, Michel M, Dubois V, de CC, Boukaissi M, Chene AS, Tranchant I, Arranz V, Zoubaa I, Fruchart JS, Ravel D, Kearsey J. DTS-108, a novel peptidic prodrug of SN-38: in vivo efficacy and toxicokinetic studies. Clin. Cancer Res. 2008;14:2145–2153. doi: 10.1158/1078-0432.CCR-07-4580. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu Y, Nagata H, Kikuchi Y, Umezawa S, Hasumi K, Yokokura T. Cytotoxic agents active against mucinous adenocarcinoma of the ovary. Oncol. Rep. 1998;5:99–101. doi: 10.3892/or.5.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J. Clin. 2006;56:226–243. doi: 10.3322/canjclin.56.4.226. [DOI] [PubMed] [Google Scholar]
- 24.Govindan SV, Griffiths GL, Hansen HJ, Horak ID, Goldenberg DM. Cancer therapy with radiolabeled and drug/toxin-conjugated antibodies. Technol. Cancer Res. Treat. 2005;4:375–391. doi: 10.1177/153303460500400406. [DOI] [PubMed] [Google Scholar]
- 25.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 26.Chari RV. Targeted cancer therapy: confering specificity to cytotoxic drugs. Acc. Chem. Res. 2008;41:98–107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- 27.Govindan SV, Cardillo TM, D’Souza CA, Nair A, Sheerin A, Singh S, Hansen HJ, Goldenberg DM. Therapy of human colonic and lung cancer xenograftswith SN-38 conjugates of anti-CEACAM5 and anti-EGP-1 humanized monoclonal antibodies. Proceedings of the 2007 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; October 22–26, 2007; San Francisco, CA. (abstract C287) [Google Scholar]
- 28.Govindan SV, Moon S-J, Cardillo TM, Gold DV, Hansen HJ, Goldenberg DM. Antibody-targeted chemotherapy of human colonic, lung, and pancreatic cancer xenografts with conjugates of the topoisomerase I inhibitor, SN-38. Proceedings of the 6th International Symposium on Targeted Anticancer Therapies; March 20–22, 2008; Bethesda, MD. (abstract B06) [Google Scholar]
- 29.Walker MA, Dubowchik GM, Hofstead SJ, Trail PA, Firestone RA. Synthesis of an immunoconjugate of camptothecin. Bioorg. Med. Chem. Lett. 2002;12:217–219. doi: 10.1016/s0960-894x(01)00707-7. [DOI] [PubMed] [Google Scholar]
- 30.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug. Chem. 2002;13:855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 31.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov. Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 32.He X, Lu W, Jiang X, Cai J, Zhang X, Ding J. Synthesis and biological evaluation of bis and monocarbonate prodrugs of 10-hydroxycamptothecins. Bioorg. Med. Chem. 2004;12:4003–4008. doi: 10.1016/j.bmc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 34.de Bruijn P, de Jonge MJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Femtomole quantitation of 7-ethyl-10-hydroxycamptothecine (SN-38) in plasma samples by reversed-phase high-performance liquid chromatography. Anal. Biochem. 1999;269:174–178. doi: 10.1006/abio.1999.4001. [DOI] [PubMed] [Google Scholar]
- 35.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 36.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou HR, Upeslacis J, Shochat D, Mountain A, Flowers DA, Bernstein I. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H-1 and C-13 NMR data and HRMS data for target compounds, a table listing the purity based on HPLC as well as HPLC traces for target compounds, and elemental analysis for 1g.