Abstract
Objectives. I examined genetic influences on smoking among adolescents and differences in the heritability of smoking across states in the United States.
Methods. With data from the National Longitudinal Study of Adolescent Health (participants aged 12–21 years), I used a multilevel twin- and sibling-pair (N = 2060 pairs) regression model.
Results. Daily smoking (hereditability estimate [h2] = 0.54) and smoking onset (h2 = 0.42) were both highly heritable. Whereas the genetic influences on smoking onset were consistent across states, there was significant variation in these influences on daily smoking. Genetic influences on daily smoking were lower in states with relatively high taxes on cigarettes and in those with greater controls on the vending machines and cigarette advertising. Genetic influences were also negatively associated with rates of smoking among youths.
Conclusions. At the state level, gene–environment interaction models are best characterized by the model of social control. State policies may influence genetic tendencies to smoke regularly, but they have not affected the genetic contributions to cigarette onset or experimentation. Future tobacco-control policies may emphasize the heritable endophenotypes that increase the likelihood that adolescents will initiate smoking.
There is consistent evidence that genetic and social factors equally influence smoking behaviors.1–11 However, little work has jointly considered the simultaneous effects of genetic and social factors on cigarette usage among adolescents and young adults. In work that does consider gene–environment interactions, the emphasis is typically on the social and demographic characteristics of individuals or families9,12–16 rather than on the composition of larger social contexts at the national, regional, state, county, or neighborhood level, even though these social environments are more relevant to existing theory on gene–environment interactions.17
The proportion of variance that is caused by genetic influences, called a heritability estimate, (h2), is believed to be just higher than 50% for smoking.1–5 Heritability estimates are obtained by comparing the concordance of smoking among siblings and twins, such as the values presented in Table 1. These results report the number of sibling or twin pairs from the National Longitudinal Study of Adolescent Health study (Add Health) in which neither, one, or both members of the pair are smokers. Evidence for genetic influences can be seen for both measures of smoking in which the correlation among monozygotic (identical) twins is significantly higher compared to the correlation among dizygotic (fraternal) twins. Genetic influences are formally characterized in Table 2 using quantitative genetic methods. According to these results, 42% of the variance of smoking onset and 54% of the variance of daily smoking is because of additive genetic effects. These estimates are in line with other research in this area.2,12,18 Estimates for the heritability of daily smoking range from 40% to 70%,9,19 and the literature on gene–environment interactions provides insights about the social contexts that may enhance heritability and those in which genetic influences should be muted.17
TABLE 1.
Descriptive Statistics for Smoking Onset and Daily Smoking Status Among Twin and Sibling Pairs (N = 2060): National Longitudinal Study of Adolescent Health, Wave 2, September 1994–April 1995
| Smoking Onset |
Daily Smoking |
||||||||
| Total No. | Neither, No. | One, No. | Both, No. | r | Neither, No. | One, No. | Both, No. | r | |
| Monozygotic twins | 248 | 113 | 68 | 67 | 0.63 | 186 | 31 | 31 | 0.83 |
| Dizygotic twins | 378 | 159 | 132 | 87 | 0.42 | 280 | 65 | 33 | 0.65 |
| Full siblings | 1066 | 396 | 384 | 286 | 0.42 | 716 | 248 | 102 | 0.51 |
| Half siblings | 368 | 118 | 146 | 104 | 0.32 | 240 | 92 | 36 | 0.47 |
TABLE 2.
Quantitative Genetic Parameter Estimates for Smoking Onset and Daily Smoking Status Among Twin and Sibling Pairs (N = 2060): National Longitudinal Study of Adolescent Health, Wave 2, September 1994–April 1995
| Smoking Onset, Variance (95% CI) | Daily Smoking, Variance (95% CI) | |
| Heritability | 0.42 (0.15, 0.66) | 0.54 (0.29, 0.74) |
| Shared environment | 0.21 (0.06, 0.36) | 0.29 (0.14, 0.44) |
| Nonshared environment | 0.37 (0.10, 0.19) | 0.17 (0.09, 0.29) |
Note. Heritability estimates and 95% confidence intervals (in parentheses) were calculated by using Mx version 1.7.03 (Medical College of Virginia, Richmond, VA). This freely available structural equation modeling package contains a number of standard procedures to decompose phenotypic variance into genetic and environmental components. A modified version of the script ctVCut2c.mx was used to estimate the parameters presented.
Shanahan and Hofer17 reviewed the literature on gene–environment interactions and pointed out that genetic influences on a variety of different behaviors are attenuated by social control. The institutional perspective emphasizes limits on the locations in which smoking is permitted, limits on the sale of tobacco products, educational programs, and taxes. These policies influence smoking in general, but they may be particularly effective in reducing the phenotypic expression of genetic factors that predispose people to initiate smoking or, following initiation, to become daily smokers.20 The other control model emphasizes antismoking norms and values that limit genetic influences on smoking.5,21
There are also reasons to believe that environmental characteristics may increase the relative influence of genes on the risk of smoking. According to the social trigger model, latent (e.g., genetic) tendencies to smoke are most likely to differentiate between individuals within environments in which there are social pressures to smoke cigarettes. Therefore, the heritability of smoking should increase with increased prevalence of smoking, decreased social sanctions against smoking, and increased expectations of tobacco use. Boardman et al.22 showed that the heritability of daily smoking is significantly higher within schools in which the most popular students smoke the most compared with schools in which there were less clear prosmoking norms. Finally, according to the social distinction model, genetic vulnerability to tobacco use may manifest more clearly within environments in which smoking is less common. Raine23 called this the “social push perspective” and suggested that we should examine genetic associations in benign environments or those that lack social factors that encourage smoking.
The literature on gene–environment interactions may shed new light on tobacco-control policies. Specifically, policies may influence the smoking rates of a state, but they may do little to influence the genetic factors that encourage individuals to either begin smoking or to smoke regularly. If this is the case, then focused policies aimed at the biophysical causes of cigarette consumption can be developed. The influence of social controls may be very different for different endophenotypes—such as personality characteristics or nicotine metabolism—that are linked with cigarette initiation or daily use. Therefore, the examination of the genetic influences on smoking across states may provide a new perspective on the nature, scope, and effectiveness of tobacco-control policies.
I build upon existing research on gene–environment interactions by examining the ways in which institutional and social characteristics of states moderate genetic predispositions toward cigarette use. My main assertion is that heritability estimates denote averages and that genetic tendencies to smoke should vary across discrete social environments; we should only expect consistent genetic associations within comparable social environments.
METHODS
I drew all data in these analyses from wave 2 of Add Health, a nationally representative, longitudinal survey of adolescents obtained from an initial in-school survey of middle and high school students conducted from September 1994 to April 1995.24,25 In total, 90 118 adolescents who attended 80 high schools and 54 middle schools (both public and private) took part in the initial interview. During the months of April through December 1995, a sample of the in-school respondents (stratified by gender and grade) was selected to participate in an in-home face-to-face interview (wave 1); participation rates were quite high (80%). Roughly 1 year later, respondents were interviewed again in their homes; participation rates for wave 2 were similarly high (85%).
The 2 dependent variables in the analyses were taken from wave 2 of the study, in which respondents were asked if they had “ever smoked an entire cigarette” and, if so, had they ever smoked “at least 1 cigarette a day for 30 days.” I used the same definitions as previous work in this area20 to refer to these outcomes as smoking onset and daily smoking, respectively. Overall, 45% of the respondents reported smoking onset and 21% reported daily smoking.
I relied on 6 state-level measures to characterize social and institutional influences on smoking. The first was the percentage of adults who reported regular smoking (mean = 23.33; SD = 2.90), data for which were taken from the Behavioral Risk Factor Surveillance System (BRFSS) for 1992 and 1993. Current smokers were those who reported ever smoking at least 100 cigarettes and who currently smoked. Irregular smokers (current smokers who stated that they did not smoke regularly when asked to report the average number of cigarettes they smoked each day) were included in the BRFSS estimates. Data for the state of Wyoming were from the 1992 to 1993 Current Population Survey. The Current Population Survey defined current smokers as those who reported ever smoking at least 100 cigarettes and who currently smoked every day or on some days. The second measure was the percentage of 9th through 12th graders who reported frequent smoking (mean = 14.15; SD = 10.30); data was obtained from the Youth Risk Behavior Surveillance study.26 Youths who reported having smoked a cigarette on at least 20 of the past 30 days were defined as frequent smokers. The third measure, excise tax per pack of cigarettes (mean = 32.14; SD = 17.46), was derived with data from the Centers for Disease Control and Prevention State Tobacco Activities Tracking and Evaluation System,27 which tracks enacted state legislation. These data reflect legislation active as of December 1, 1995.
Data for the fourth measure, cigarette restrictions (mean = 1.26; SD = 0.73), were obtained from LaVonne and Gardiner.28 This measure is the sum of the following 2 characteristics: restrictions on the location of vending machines that sell cigarettes and prohibition of billboard advertising for tobacco products within 500 feet of schools. The fifth measure, full-time staff equivalent for tobacco control (mean = 0.31; SD = 0.37), captures the number of staff that are dedicated to tobacco control, excluding nonprofit organizations, for the year 1994.28 The final measure, tobacco control expenditures (mean = 11.26; SD = 1.07) describes the total amount spent (in logged dollars) on tobacco control in each state.28 The estimates drew on a number of sources and were meant to represent a “snapshot of programs as of late 1995.”28(p2) The state-level tobacco-control expenditures relied on responses from the coordinators of the American Stop Smoking Intervention Study (National Cancer Institute), Initiatives to Mobilize for the Prevention and Control of Tobacco Use program (Centers for Disease Control and Prevention), and SmokeLess (Robert Wood Johnson Foundation). Three additional contacts for state-level representatives from the American Cancer Society, the American Heart Association, and the American Lung Association were included in the data collection. The estimate for each state was calculated by response from at least 3 organizations per state including at least 1 government agency and 1 nonprofit agency. These estimates were then verified by comparing the response to the Attachment 6 (“Tobacco sales to minors and law enforcement”) submitted to the Center for Substance Abuse Prevention.
The Add Health study oversampled twin pairs identified in the in-school survey, which enabled quantitative genetic analysis.29 Respondents who reported during wave 1 that they had a full sibling or a twin were included in the pairs roster; of the 3139 pairs who were asked, 83% (n = 2612) agreed to take part in the study. I used several restrictions in the analyses: (1) of the 37 states with tobacco-control information (see Kandel et al.20 for a more detailed discussion of these data), I only used those states with at least 9 sibling or twin pairs to ensure proper power of multilevel parameter estimates30; (2) I dropped pairs if either member had missing smoking data; and (3) I dropped pairs whose members resided in different states. The final sample contained 2060 pairs nested within 31 states.
Based on 11 genetic markers used to confirm the reported zygosity of the twin and sibling pairs, 34 pairs were reassigned zygosity status as a result of this test. This criterion of zygosity yielded 248 identical twin, 378 fraternal twin, 1066 full-sibling, and 368 half-sibling pairs. The average age for the sample was 16.42 years (SD = 1.79), and boys and girls were equally represented (51.4% girls). The average number of pairs per state was 67.8 (SD = 12.6; minimum = 9; 1st quartile = 30; median = 45; 3rd quartile = 88; maximum = 320).
Estimating Heritability Across States
I used a multilevel sibling and twin regression approach. The single-level model presented in equation 1 is often called the DeFries–Fulker31 model. It predicts the status of the first sibling (y1) of the ith pair as a function of the second sibling's status on the same outcome (y2); a measure of genetic similarity—the average proportion of alleles shared by descent for each pair type—(g); and an interaction between genetic similarity and the second siblings' score (giy2i):
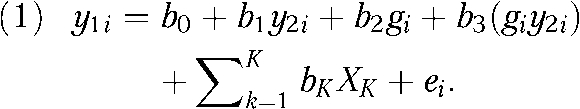 |
A positive and significant value for b1 suggests that siblings have similar values for the dependent variable. I used the interaction term for b3 to test the extent to which sibling resemblance is a function of their genetic similarity. Because identical twins share all of their genes, their genetic similarity score was g = 1; fraternal twins and full siblings received a score of g = 0.5, and half-siblings a score of g = 0.25. A positive and significant value for b3 indicates that the similarity for smoking among pairs is conditional upon their genetic similarity. If the distribution of the dependent variable is standard and normal, then the parameter estimates for b1 and b3 describe the relative contribution of shared environment (c2) and heritability (h2), respectively, and the remaining proportion is because of nonshared environmental characteristics (e2). This model is quite flexible, can be extended to include K covariates, and is well suited to complex sampling designs.
Rowe et al.32 extended this model to a multilevel framework in which sibling and twin pairs are nested within schools. They estimated an average heritability of 0.38 for aggression, and they showed significant variation about this average across schools. There have been other multilevel extensions of behavioral genetic analyses33 but this model is useful because it estimates environmental moderation in the heritability estimate (gene–environment interactions) without the inclusion of an a priori moderator. This model, used in previous research,22 is expressed as a multilevel logit specification in equation 2:
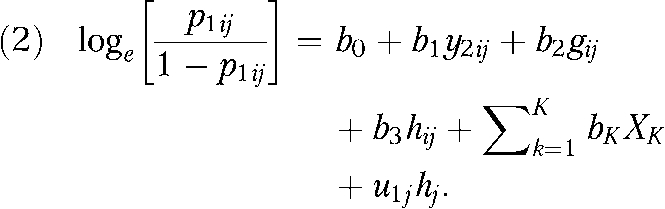 |
The estimate u1 j specifies an offset to the heritability estimate for sibling and twin pairs who currently reside in the j th state. Although the interpretation of the value of the “heritability” estimate is not consistent with the standard normal model, the role of the link function makes it possible to estimate similar models for continuous, count, overdispersed count, and multinomial distributions. That is, this model will not provide traditional behavioral genetic parameter estimates similar to those presented in Table 1. However, it is still possible to estimate state-level influences on the magnitude of genetic influences by using the multilevel design.
The variance of this estimate 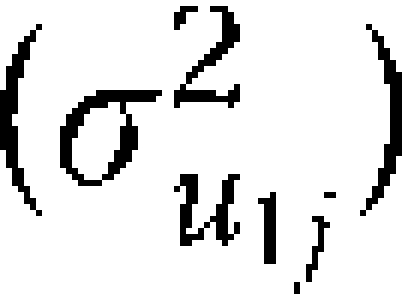 is the primary parameter estimate from this model, and significance of this estimate provides evidence for state-level moderation in heritability (gene–environment interactions). After the baseline multilevel regressions are calculated, the latent state-level heritability estimates are regressed on 6 state-level factors that are hypothesized to influence the heritability of smoking. Together with changes in the likelihood function, standard statistical criteria can be used to assess the contribution to overall model fit.
is the primary parameter estimate from this model, and significance of this estimate provides evidence for state-level moderation in heritability (gene–environment interactions). After the baseline multilevel regressions are calculated, the latent state-level heritability estimates are regressed on 6 state-level factors that are hypothesized to influence the heritability of smoking. Together with changes in the likelihood function, standard statistical criteria can be used to assess the contribution to overall model fit.
Focusing on state-level variation helps to rule out problems concerning both selection and level of aggregation that often hinder research on gene–environment interactions. If the selection of environment is a function of an individual's genes, then correlation between genes and environments (rGE) can have serious implications for the interpretation of gene–environment interaction parameter estimates.34,35 Although it is certainly possible, it is unlikely that families select a particular state because of institutional or social characteristics of smoking as a function of their genetic characteristics. Thus, the gene–environment interactions parameterization described here is potentially less sensitive to this issue.
RESULTS
Table 3 presents parameter estimates from 2 multilevel logistic regression models of twin and sibling pairs. For both smoking onset (b = 1.085) and daily smoking (b = 1.609), individuals were significantly more likely to use cigarettes if their sibling was also using cigarettes. Importantly, the positive and significant interaction terms (b = 1.520; P < .023, and b = 2.186; P < .017) indicate that the sibling association was significantly higher for siblings who were more alike one another genetically. According to these results, the average rate of smoking onset varied significantly across states (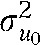 = 0.114; P < .001); however, the measure of genetic influence on smoking onset did not (
= 0.114; P < .001); however, the measure of genetic influence on smoking onset did not (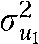 = 0.025; P < .798). With respect to daily smoking, there was evidence that both the rate of smoking (
= 0.025; P < .798). With respect to daily smoking, there was evidence that both the rate of smoking (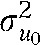 = 0.103; P < .004) and the genetic influences on daily smoking (
= 0.103; P < .004) and the genetic influences on daily smoking (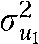 = 3.432; P < .056) varied across states. Thus, on average, approximately half of the variance in daily smoking was genetically influenced (the estimates from Table 2), but there was significant variability in this estimate across states. Although age and gender were included as statistical controls, gender did not appear to be associated with either smoking initiation or daily smoking. Age was not associated with smoking initiation, but it was positively associated with the onset of daily smoking (b = 0.186; P < .001).
= 3.432; P < .056) varied across states. Thus, on average, approximately half of the variance in daily smoking was genetically influenced (the estimates from Table 2), but there was significant variability in this estimate across states. Although age and gender were included as statistical controls, gender did not appear to be associated with either smoking initiation or daily smoking. Age was not associated with smoking initiation, but it was positively associated with the onset of daily smoking (b = 0.186; P < .001).
TABLE 3.
Multilevel Logistic Regression Estimates for State-Level Moderation of Smoking Heritability Among Sibling and Twin Pairs (N = 2060 Sibling Pairs): National Longitudinal Study of Adolescent Health, Wave 2, September 1994–April 1995
| Smoking Onset |
Daily Smoking |
|||
| b (SE) | P | b (SE) | P | |
| Intercept | −0.442 (0.632) | .484 | −4.825 (0.481) | <.001 |
| Girlsa | 0.042 (0.117) | .718 | 0.102 (0.127) | .424 |
| Age at wave 2 | −0.015 (0.040) | .697 | 0.186 (0.029) | <.001 |
| Smoking at wave 2b | 1.085 (0.133) | <.001 | 1.609 (0.209) | <.001 |
| Genetic similarity | −1.075 (0.400) | .007 | −1.034 (0.392) | .008 |
| Genetic similarity × smoking status | 1.520 (0.670) | .023 | 2.186 (0.917) | .017 |
| State-level variance estimates | ||||
| Intercept | 0.114 (0.024) | <.001 | 0.103 (0.033) | .004 |
| Slope | 0.025 (0.097) | .798 | 3.432 (1.732) | .056 |
| Covariance (intercept, slope) | −0.054 (0.106) | .614 | −0.594 (0.179) | .002 |
| Model descriptors | ||||
| Log likelihood, base model | −2703.189 | −2047.796 | ||
| Log likelihood, current model | −2669.619 | −2023.412 | ||
| Likelihood ratio | 67.141 | 48.768 | ||
| df | 3.000 | 3.000 | ||
| P | <.001 | <.001 | ||
Note. Entries represent parameter estimates and standard errors obtained from a multilevel logistic regression model of paired data (level 1) nested within states (level 2). Significance levels are reported as 2-tailed. All data are weighted to reflect the complex design of the National Longitudinal Study of Adolescent Health. All models were estimated by using the GLLAMM command in STATA 9.2 (StataCorp, College Station, TX).
Boys were the reference group.
Nonsmoking at wave 2 was the reference group.
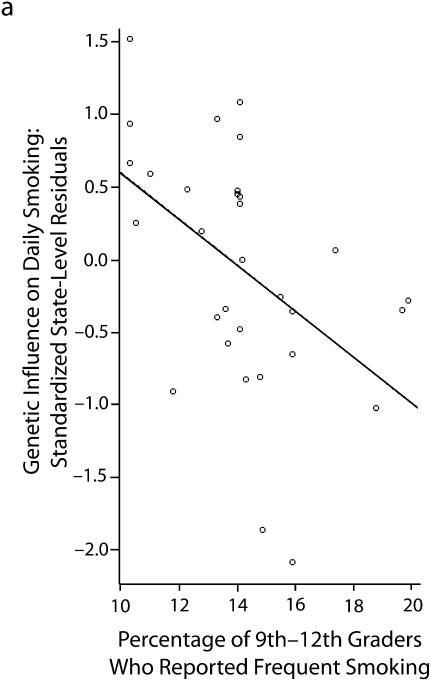
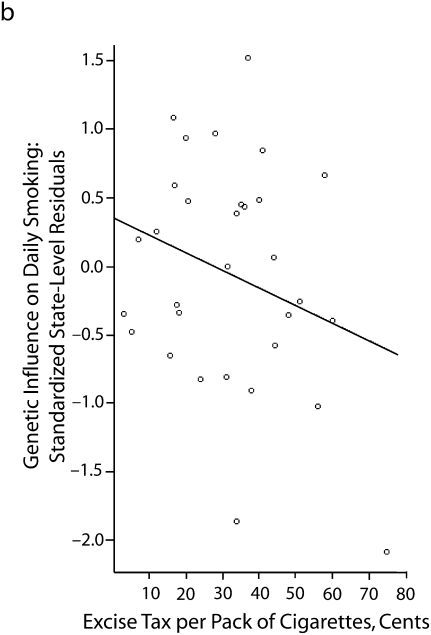
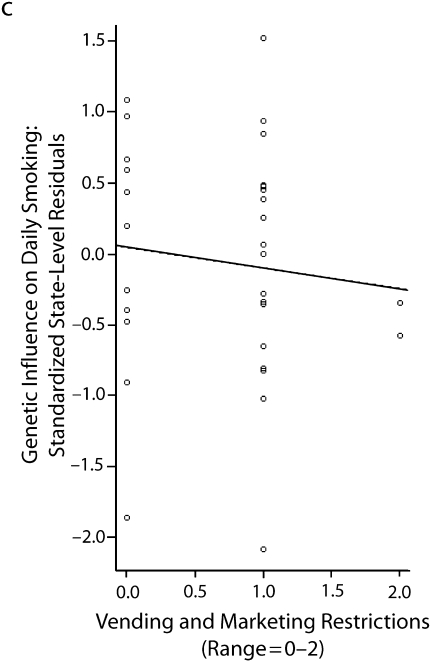
In 6 subsequent multilevel sibling and twin models, the latent slope for the heritability (i.e., the state-specific heritability estimates) of daily smoking was regressed on state-level factors. These models are summarized in Table 4. Of the 6 factors examined, 3 factors emerged as significant predictors. Marketing and vending machine restrictions appeared to slightly reduce the genetic influences on regular smoking. Similarly, state-level excise taxes on cigarettes also appeared to reduce genetic influences on regular smoking. Both findings support the social control gene–environment interactions. Interestingly, these results also showed that state-level smoking by youths was negatively associated with the genetic influences on regular smoking. As discussed earlier, the social distinction model anticipated this association. These associations are illustrated in Figure 1, in which the estimates on the y-axis are the standardized state-level residuals for daily smoking obtained from the multilevel model in Table 3.
TABLE 4.
State-Level Characteristics Associated With State-Level Variance in the Heritability of Daily Smoking: National Longitudinal Study of Adolescent Health, Wave 2, September 1994–April 1995
| Association |
Model Fit |
||||
| b (SE) | t | P | χ2 | P | |
| Marketing and vending machine restrictions | −1.983 (1.384) | −1.432 | .082 | 3.204 | .073 |
| Full-time staff equivalent for tobacco control | 0.978 (2.129) | 0.459 | .325 | 0.036 | .850 |
| Prevention budget (logged) | −0.306 (0.647) | −0.473 | .320 | 0.104 | .747 |
| Excise tax per pack of cigarettes | −0.533 (0.317) | −1.681 | .052 | 42.182 | <.001 |
| Adult smoking prevalence | 0.035 (0.219) | 0.161 | .437 | 0.004 | .951 |
| Youth smoking prevalence | −0.543 (0.242) | −2.244 | .017 | 102.673 | <.001 |
Note. Parameter estimates describe the association between each state-level characteristic and the latent state-level heritability estimate. These models were estimated by using the GEQS command in the GLLAMM procedure available in STATA version 9.2 (StataCorp, College Station, TX). Parameter estimates were weighted to reflect nonrandom sampling in the National Longitudinal Study of Adolescent Health.
FIGURE 1.
The association between state-level factors and state-level differences in the genetic influences on daily smoking among US adolescents in 9th to 12th grade, by (a) smoking prevalence, (b) excise tax per pack of cigarettes, and (c) vending machine and marketing restrictions: National Longitudinal Study of Adolescent Health, Wave 2, September 1994–April 1995.
Note. There were 2060 sibling pairs in the sample and information on respondents from 31 states. The circles represent state-specific estimates (obtained from the second column of Table 3). Standardized empirical Bayes estimates (i.e., state-level offsets to the heritability estimate) indicate the relative influence of genetic factors on daily smoking.
DISCUSSION
My findings contribute to the public health literature in several ways. First, the results are consistent with the notion that parameter estimates describing genetic influences on daily smoking should be considered averages; even across the 31 states I studied, there was a great deal of variability in the heritability of daily smoking. To date, dispersion around the average heritability has been conceptualized as an empirical standard error used in a meta-analytic approach—that is, it is used to validate statistical inference.6 However, as is shown here, the variation of heritability estimates across different social settings should be anticipated on the basis of existing theory on gene–environment interactions.
Second, it is equally important to highlight that there was no variation in the heritability estimate for smoking onset. Although there were only moderate differences in the relative magnitude of genetic influences for daily smoking and smoking onset, there were important differences in the environmental moderation of these respective genetic influences. As others have shown36 this is additional evidence that the genes responsible for smoking onset may be different from those associated with daily smoking. That is, initiation of smoking is more heavily influenced by personality characteristics such as novelty seeking or impulsivity, whereas regular tobacco use has more to do with nicotine metabolism.13,37,38 This gene–environment interaction finding is also in line with other work,22 and it reinforces the idea that the social moderation perspective is not necessarily generalizable; rather, broad social and institutional forces that affect genetic tendencies are limited to specific health-related behaviors.
This finding is highly relevant to the field of public health precisely because the heritability of smoking onset did not vary across states. Despite the influence of state-level policies on cigarette use among adolescents,20 these policies do not appear to influence the genetic factors that may lead individuals to initiate smoking. Most antismoking policy research addresses onset and highlights the role of immediate social influences and refusal skills, which have been shown to reduce onset by up to 30%.39–41 However, these policies may only be effective among those for whom smoking initiation does not have an important genetic cause. The ineffectiveness of these policies to affect genetic vulnerabilities suggests that the current policies may not necessarily address the key endophenotype that has an established genetic base such as impulsivity.42
Third, these findings contribute to the gene–environment interaction literature. When the environment is characterized as a state of residence, the results suggest that social control and social distinction are relevant gene–environment interaction models but that social triggering does not appear to be operating at this level. The triggering model tends to operate through more immediate social normative forces, which is why work showing triggering effects22 tends to focus on more proximate social contexts such as school environments. Moreover, the triggering model hypothesizes that the social environment causes genetic expression, which is different from the social distinction model in which the social environment simply clarifies genetic influences. In other words, if an individual with genetic tendencies to smoke cigarettes lacks social factors that “push” him to smoke, then biological factors may better explain his smoking. Or, as Raine makes clear, proper specification of the environment simply minimizes the “noise” in the study, which allows for “biology to shine through”23(p14) —a perspective that is evident in other quantitative genetic studies.43
By contrast, the social control model emphasizes both social norms (the meso level) and institutional constraints (the macro level). Therefore, it is reasonable that observed reduction in the heritability of smoking was caused, in part, by state-level prices per pack of cigarettes. As shown elsewhere, smoking by youths is reduced by increases in the real price of cigarettes,44 and adolescents are more sensitive to price increases than are adults: a 10% increase in the price of cigarettes will reduce demand by 4% among adults but by 6% among youths.45 My results add to this research because they suggest that real prices also serve as an immediate form of social control that places very real limits on the ability of latent characteristics to manifest. The invariance of smoking onset (and experimentation) is not evident in the regular consumption of cigarettes suggesting that the real price of cigarettes causes the genetic influences on cigarette consumption to operate differently.
Limitations
There are at least 5 limitations that should be considered when interpreting my results. The most important limitation is the potential violation of the equal environment assumption. My models assume that sibling pairs are treated similarly and raised in similar environments, regardless of their genetic similarity. This assumption has been tested explicitly with identical and fraternal twin pairs, and the results are mixed.46,47 This assumption is even further complicated when the pair design is extended to full and half siblings, which include opposite-gender pairs and those who differ in age. To adjust for this concern, I estimated similar models in which I adjusted for the proportion of friends that the sibling pair had in common, and this adjustment did not affect the parameter estimates (results not shown). This 1 control cannot possibly capture the environmental similarities of identical twins, fraternal twins, full siblings, and half siblings; however, the parameter estimates calculated in this study are similar to those from studies that used only twin pairs. Nevertheless, heritability estimates certainly contain influences that are environmental and should be interpreted accordingly.
Second, there are only 2 published studies that have used similar techniques.22,32 Although both nonlinear mixed models and sibling–twin regression models are well established, their combination denotes a relatively new approach. Thus, as with most new analyses, these findings should be considered unique until comparable results are obtained with similar methods from other studies.
Third, the sample sizes for some states were relatively small, and state-specific heritability estimates have relatively wide error intervals. I employed a variety of weighting strategies to test the influence of states with small sample sizes on the state-level estimates; none of the methods significantly affected the substantive conclusions. Nevertheless, it is important to recognize that the state-level influences are limited to a median of 45 pairs per state. Moreover, I included only 31 states in these analyses, and, although this is a random sample of adolescents in the United States, it is important to note that 19 states were not included in these analyses. The use of 31 states is also important because it reduced the number of level 2 observations and reduced the power to detect level 2 moderation. Therefore, the risk of a type II error is possible with respect to the null results for smoking onset.
Fourth, only a limited number of characteristics were included as controls in the analyses. Although there are clear social differences in the prevalence of smoking onset and daily smoking,7,19 these factors should not affect the heritability estimates. Nevertheless, readers should consider that gender and age are the only sociodemographic covariates included in the multivariate models.
Finally, although the initiation of smoking during adolescence is an important public health concern, the age ranges here do not permit more detailed descriptions of progression and dependence. The requisite sample sizes for nesting sibling pairs within states were only obtained from the first 2 waves of data collection; thus, I could not take advantage of the third wave of data collection. It would be useful to see if these smoking patterns continue into adulthood.
Conclusions
My findings reinforce the idea that genetic and environmental influences may not operate in an additive manner. The extension of multilevel modeling techniques to behavioral genetic analysis is especially important given the recent interest in molecular studies such as genome-wide associations. The multilevel concept and method provide a parsimonious way to incorporate environmental moderation into molecular studies that may shed new light on the independent and interactive effects of environmental and genetic factors to a range of important health outcomes.
Acknowledgments
This study is part of a larger study funded by the National Institute of Child Health and Human Development (grant K01 HD 50336). Research funds were also provided by the National Institutes of Health, National Institute of Child Health and Human Development–funded University of Colorado Population Center (grant R21 HD 051146-01). This research involved data from the National Longitudinal Study of Adolescent Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant P01-HD31921), with cooperative funding from 17 other agencies. No direct support was received from grant P01-HD31921 for this analysis. Persons interested in obtaining data files from the National Longitudinal Study of Adolescent Health should contact Add Health, Carolina Population Center, 123 W Franklin St, Chapel Hill, NC 27516-2524 (addhealth@unc.edu).
Special acknowledgment is due to Ronald R. Rindfuss and Barbara Entwisle for assistance in the original study design.
Human Participant Protection
The use of the contextual data information and the sibling pair data from the National Longitudinal Study of Adolescent Health study was reviewed by the institutional review board of the University of Colorado and was found to be exempt.
References
- 1.Batra V, Patkar AA, Berrettini WH, Weinstein SP, Leone FT. The genetic determinants of smoking. Chest 2003;123:1730–1739 [DOI] [PubMed] [Google Scholar]
- 2.Heath AC, Madden PAF. Genetic influences on smoking behavior. In: Turner JR, Cardon LR, Hewitt JK, eds. Behavior Genetic Approaches in Behavioral Medicine New York, NY: Plenum Press; 1995:45–66 [Google Scholar]
- 3.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res 1999;1:S51–S57 [DOI] [PubMed] [Google Scholar]
- 4.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking: a study of male twins. N Engl J Med 1992;327:829–833 [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Arch Gen Psychiatry 2000;57:886–892 [DOI] [PubMed] [Google Scholar]
- 6.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003;98:23–31 [DOI] [PubMed] [Google Scholar]
- 7.Hu M-C, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health 2006;96:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander C, Piazza M, Mekos D, Valente T. Peers, schools, and adolescent cigarette smoking. J Adolesc Health 2001;29:22–30 [DOI] [PubMed] [Google Scholar]
- 9.Slomkowski C, Rende R, Novak S, Lloyd-Richardson E, Niaru R. Sibling effects on smoking in adolescence: evidence for social influence from genetically informative design. Addiction 2005;100:430–438 [DOI] [PubMed] [Google Scholar]
- 10.Ellickson PL, Bird CE, Orlando M, Klein DJ, McCaffrey DF. Social context and adolescent health behavior: does school-level smoking prevalence affect students' subsequent smoking behavior? J Health Soc Behav 2003;44:525–535 [PubMed] [Google Scholar]
- 11.Eitle DJ, Eitle TM. School and county characteristics as predictors of school rates of drug, alcohol, and tobacco offenses. J Health Soc Behav 2004;45:408–421 [DOI] [PubMed] [Google Scholar]
- 12.Heath AC, Kirk KM, Meyer JM, Martin NG. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav Genet 1999;29:395–407 [DOI] [PubMed] [Google Scholar]
- 13.Lerman C, Caporaso NE, Audrain J, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol 1999;18:14–20 [DOI] [PubMed] [Google Scholar]
- 14.Timberlake DS, Rhee SH, Haberstick BC, et al. The moderating effects of religiosity on the genetic and environmental determinants of smoking initiation. Nicotine Tob Res. 2006;8:123–133 [DOI] [PubMed] [Google Scholar]
- 15.Johnston TD, Edwards L. Genes, interactions, and the development of behavior. Psychol Rev 2002;109:26–34 [DOI] [PubMed] [Google Scholar]
- 16.Ryff CD, Singer BH. Social environments and the genetics of aging: advancing knowledge of protective health mechanisms. J Gerontol B Psychol Sci Soc Sci 2005;60:12–23 [DOI] [PubMed] [Google Scholar]
- 17.Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. J Gerontol B Psychol Sci Soc Sci 2005;60:65–76 [DOI] [PubMed] [Google Scholar]
- 18.Rende R. Genes, environment, and addictive behavior: etiology of individual-differences and extreme cases. Addiction 1993;88:1183–1188 [DOI] [PubMed] [Google Scholar]
- 19.Maes HH, Woodard CE, Murrelle L, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol 1999;60:293–305 [DOI] [PubMed] [Google Scholar]
- 20.Kandel DB, Kiros GE, Schaffran C, Hu MC. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health 2004;94:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timberlake DS, Haberstick BC, Lessem JM, et al. An association between the DAT1 polymorphism and smoking behavior in young adults from the National Longitudinal Study of Adolescent Health. Health Psychol 2006;25:190–197 [DOI] [PubMed] [Google Scholar]
- 22.Boardman JD, Saint Onge JM, Haberstick BC, Timberlake DS, Hewitt JK. Do schools moderate the genetic determinants of smoking? Behav Genet 2008;38:234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J Abnorm Child Psychol 2002;30:311–326 [DOI] [PubMed] [Google Scholar]
- 24.Udry JR. The National Longitudinal Study of Adolescent Health (Add Health), Waves I & II, 1994–1996; Wave III, 2001–2002 [machine-readable data file and documentation]. Chapel Hill: Carolina Population Center, University of North Carolina at Chapel Hill; 2003.
- 25.Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. The National Longitudinal Study of Adolescent Health: research design. Available at: http://www.cpc.unc.edu/projects/addhealth/design. Accessed June 30, 2006
- 26.Kann L, Warren CW, Harris WA, et al. Youth risk behavior surveillance—United States, 1993. MMWR CDC Surveill Summ 1995;44:1–56 [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention State Tobacco Activities Tracking and Evaluation System. Available at: http://apps.nccd.cdc.gov/statesystem. Accessed November 7, 2008
- 28.LaVonne AD, Gardiner JA. Reducing Youth Access to Tobacco: A Partial Inventory of State Initiatives. Chicago: University of Illinois at Chicago; 1996 [Google Scholar]
- 29.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics New York, NY: Pearson-Prentice Hall; 1996 [Google Scholar]
- 30.Snijders TAB. Power and sample size in multilevel linear models. In: Everitt BS, Howell DC, eds. Encyclopedia of Statistics in Behavioral Science Vol. 3 Chichester, England: Wiley; 2005:1570–1573 [Google Scholar]
- 31.DeFries JC, Fulker DW. Multiple-regression analysis of twin data. Behav Genet 1985;15:467–473 [DOI] [PubMed] [Google Scholar]
- 32.Rowe DC, Almeida DM, Jacobson KC. School context and genetic influences on aggression in adolescence. Psychol Sci 1999;10:277–280 [Google Scholar]
- 33.Guo G, Wang JM. The mixed or multilevel model for behavior genetic analysis. Behav Genet 2002;32,37–49 [DOI] [PubMed] [Google Scholar]
- 34.Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry 2007;12:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleveland HH, Wiebe RP. The moderation of genetic and shared-environmental influences on adolescent drinking by levels of parental drinking. J Stud Alcohol 2003;64:182–194 [DOI] [PubMed] [Google Scholar]
- 36.Madden PAF, Heath AC, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behav Genet 1999;29:423–431 [DOI] [PubMed] [Google Scholar]
- 37.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature 1998;393:750. [DOI] [PubMed] [Google Scholar]
- 38.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet 2000;64:383–390 [DOI] [PubMed] [Google Scholar]
- 39.Lantz PM, Jacobson PD, Warner KE, et al. Investing in youth tobacco control: a review of smoking prevention and control strategies. Tob Control 2000;9:47–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine Growing Up Tobacco Free: Preventing Nicotine Addiction in Children and Youth. Washington, DC: National Academy Press; 1994 [PubMed] [Google Scholar]
- 41.Rooney BL, Murray DM. A meta-analysis of smoking prevention programs after adjustment for errors in the unit of analysis. Health Educ Q 1996;23:48–64 [DOI] [PubMed] [Google Scholar]
- 42.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 2005;8:1450–1457 [DOI] [PubMed] [Google Scholar]
- 43.Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behav Genet 2005;35:115–120 [DOI] [PubMed] [Google Scholar]
- 44.Tauras JA, Chaloupka FJ, Farrelly MC, et al. State tobacco control spending and youth smoking. Am J Public Health 2005;95:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel M, Biener L, Rigotti NA. The effect of local tobacco sales laws on adolescent smoking onset. Prev Med 1999;29:334–342 [DOI] [PubMed] [Google Scholar]
- 46.Kessler RC, Gilman SE, Thornton LM, Kendler KS. Health, well-being, and social responsibility in the MIDUS twin and sibling subsamples. : Brim OG, Ryff CD, Kessler RC, eds. How Healthy Are We?: A National Study of Well-Being at Midlife Chicago, IL: University of Chicago Press; 2004:124–152 [Google Scholar]
- 47.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal environment assumption in twin studies of psychiatric illness. Behav Genet 1993;23:21–27 [DOI] [PubMed] [Google Scholar]