Abstract
Objectives. We examined the prevalence of frailty among Mexican American older adults and explored the correlates associated with becoming frail to determine their affect on disability and morbidity in this population.
Methods. We studied the trajectory of frailty over 10 years in 2049 Mexican Americans participating in the Hispanic Established Populations Epidemiologic Studies of the Elderly. We constructed a frailty index based on weight loss, exhaustion, grip strength, walking speed, and physical activity and collected data on sociodemographic and health status, comorbidities, and functional measures of performance.
Results. The sample was 58% female, with a mean age of 74.43 years (SD = 6.04) at baseline. Fifty-five percent of participants at baseline and 75% of the surviving sample at follow-up (n = 777) were classified as prefrail or frail. Of persons identified as frail at baseline, 84% died by the end of follow-up. Baseline age, diabetes, arthritis, smoking status, body mass index, cognition, negative affect, and number of comorbid conditions were predictors of frailty at follow-up (R2 = 0.29; P < .05).
Conclusions. Further research into ways to reduce the number of Mexican American older adults who become frail and disabled and therefore lose their independence is needed. Future studies should continue to examine the trajectory of frailty as a dynamic process that includes psychosocial and cognitive components.
Frailty has been identified as a precursor to disability, institutionalization, and mortality in older adults.1,2 Research on frailty among minority older adults and underserved populations is sparse despite evidence of cultural and physiological differences among racial and ethnic groups.3,4 We know, for example, that Hispanics have a high incidence of diabetes and obesity and poor access to primary care compared with non-Hispanic Whites.4 These factors potentially affect the development of frailty in this population in ways that are currently unknown.
We sought to systematically examine who becomes frail in a large well-defined sample of Mexican American older adults studied over 10 years. Our primary aim was to determine, by an established definition, the change in prevalence of frailty. We selected the index proposed by Fried and Walston5 to define frailty because the index (1) is comprehensive and multifactorial, (2) provides a measure of frailty that can be used in clinics and the community, and (3) is the most widely cited and discussed definition in the aging literature.6,7 We acknowledge, however, that definitions of frailty are still under discussion among researchers in this field.2,8,9
Our secondary aim was to explore the correlates associated with becoming frail among Mexican American older adults. We hypothesized that frailty represents a dynamic process and that persons can become both more and less frail over time.10 We also hypothesized, based on previous findings,6 that age, number of comorbidities, ability to perform activities of daily living, and measures of lower-extremity physical performance would be associated with increased incidence of frailty.
METHODS
We obtained our sample from the Hispanic Established Populations for Epidemiologic Studies of the Elderly (EPESE), a longitudinal investigation of Mexican Americans 65 years and older residing in Texas, New Mexico, Colorado, Arizona, and California. Participants were identified through multistage, stratified probability sampling of selected counties, blocks, and households within defined census tracts.11 The sampling plan ensured that the results could be generalized to the approximately 500 000 older Mexican Americans living in the Southwest.11 The response rate at the beginning of the study (1993–1994) was 83% (2873 in-person interviews and 177 interviews by proxy). The participants were interviewed and examined in their homes by raters who received 20 hours of training in interviewing methods and performance-based assessments of physical functioning, including balance, gait, and functional daily living skills. The interviews were conducted in Spanish or English, according to the respondent's preference.
Follow-up interviews were conducted at 2- to 3-year intervals. We used data from wave 2, conducted in 1995 to 1996, and wave 5, conducted in 2006. Data from these 2 waves were used because they included all the measures necessary to compute the frailty index. In-person interviews at wave 2 were conducted with 2438 of the original participants (80%). We did not include proxy interviews in our analyses because of the physical nature of measurements required for the frailty index. The final sample included 2049 participants in 1995 to 1996 (baseline for our study) and 777 participants with complete data in 2006. The Hispanic EPESE methods and data are described elsewhere.12
Measurements
Frailty.
We assessed frailty according to criteria developed by Fried and Walston5 and included weight loss, exhaustion, walking speed, grip strength, and physical activity. Participants with unintentional weight loss of more than 10 pounds from the previous interview were categorized as positive for the weight loss criterion (score = 1). We assessed exhaustion with 2 items from the Center for Epidemiologic Studies Depression (CES-D) Scale: “I felt that everything I did was an effort” and “I could not get going.” Respondents were asked, “How often in the last week did you feel this way?” and answers were scored 0 for rarely or none of the time (< 1 day), 1 for some or a little of the time (1–2 days), 2 for a moderate amount of the time (3–4 days), or 3 for most of the time (5–7 days).13 Participants scoring 2 or 3 on either of these 2 items were categorized as positive for the exhaustion criterion (score = 1).
Walking speed was assessed over a 16-foot timed walk. Participants were asked to walk as fast as felt safe. Participants whose speed fell into the lowest 20%, adjusted for height and gender, were scored as positive for this criterion. Those unable to perform the test were also categorized as positive (score = 1). Grip strength was assessed with a hand-held dynamometer (Jaymar Dynamometer, J. A. Preston Corp, Jackson, MI), with different criteria for men and women. Participants unable to perform the grip strength test and those in the lowest 20%, adjusted for body mass index (BMI; weight in kilograms divided by height in meters squared) and stratified by gender, were categorized as positive for the weakness criterion (score = 1). The original frailty index5 used the short version of the Minnesota Leisure Time Activity Questionnaire to assess physical activity; we used the Physical Activity Scale for the Elderly (PASE).14 Participants who scored in the lowest 20% of the PASE, adjusted by gender, were categorized as positive for the low physical activity criterion (score = 1).
We followed the 20% guideline for creating the cutpoints for selected components of the frailty index, with the protocol established by Fried et al.6 This will allow comparison of our findings with results of other investigations that use the frailty index.
The summary frailty score ranged from 0 to 5, with a higher score indicating increased frailty. For some comparisons, participants who scored 0 on the summary frailty index were categorized as not frail. Participants scoring 1 or 2 were considered prefrail, and those scoring 3 or higher were categorized as frail. The original frailty scale has shown good predictive validity for reduced mobility, activities of daily living (ADL) dysfunction, hospitalization, and mortality among White and African American men and women 65 years and older.5,6
Covariates.
We examined several variables potentially related to frailty and classified as sociodemographic factors, medical conditions, and behavior, physical, or cognitive performance measures. Sociodemographic factors included age (continuous), gender, marital status, financial status, and years of schooling completed (continuous). Marital status was recorded as married, single (never married), separated, divorced, or widowed and was coded into 2 categories: currently married and unmarried. A proxy measure of socioeconomic status was based on financial strain and assessed the difficulty respondents had in meeting monthly bill payments: 1 indicated a great deal, 2 indicated some, 3 indicated a little, and 4 indicated none.
Medical conditions and comorbidities were self-reported. Participants were asked if they ever had a physician diagnosis of heart attack, stroke, arthritis, cancer, hip fracture, or diabetes. We assigned a score for the total number of a respondent's medical conditions, with a potential range of 0 to 6. Smoking was recorded as current smoker versus never-smoker or ex-smoker. Height was measured with a tape placed against the wall and weight with a Metro 9800 scale (Metro Corp, Las Cruces, NM).
Behavior, physical, and cognitive performance measures included assessment of ADL and instrumental activities of daily living (IADL). Respondents were asked if they needed help doing 7 ADL tasks, including walking across a small room, bathing, grooming, dressing, eating, transferring from bed to chair, and toileting.15 Respondents who indicated that they needed help or were unable to do a task were scored as having an ADL disability. For the IADL items, respondents were asked if they were able to do 10 activities, including using a telephone, driving, shopping, preparing meals, performing light housework, taking medications, handling money, doing heavy housework, walking up and down stairs, and walking half a mile.16 Respondents who were unable to complete 1 or more of the tasks were coded as having an IADL disability.
We also examined lower-extremity function with the Short Physical Performance Battery,17 which includes assessment of standing balance, a short walk, and repeated chair stands (i.e., the time required to stand from a chair and return to sitting 5 times). We did not include the short walk from this scale in the total score because walking is a component of the frailty index. A total score, derived from a combination of the balance and chair-stand measures, had a possible range of 0 to 8, with higher scores indicating better functioning.18
Other measures included scores from the Mini-Mental State Exam (MMSE), both English and Spanish versions.19 The Spanish MMSE has been successfully used in community surveys of Mexican Americans.20 Depressive symptoms were measured with the CES-D scale.13 Two questions from the Somatic and Retarded Activities subscale of the CES-D are included in the frailty index. We used the remaining subscales of the CES-D (Positive Affect, Negative Affect, and Interpersonal) as continuous variables in selected analyses.
Data Analysis
Our analyses used descriptive and univariate statistics for continuous variables and contingency tables for categorical variables, with significance determined by the χ2 test. We used multivariable linear regression to examine the association of variables collected in 1995 to 1996 with frailty status in 2006. We computed 3 models with the frailty index rating in 2006 as the dependent variable in each model. In the first model, sociodemographic variables were included as predictors (age, gender, marital status, years of education, and financial strain). In the second model, we added medical conditions and health status variables, including arthritis, diabetes, cancer, previous heart attack or stroke, smoking status, and BMI. In the final model, we added physical performance measures (IADL and ADL ratings, balance and chair-stand scores from the Short Physical Performance Battery, and the positive and negative affect scales from the CES-D). We included a cumulative comorbidity index along with MMSE scores. All models included the baseline (1995–1996) frailty index as a covariate.
In selecting covariates, we considered clinical importance and previous disability and frailty research with Hispanic and non-Hispanic populations.10,11 We computed regression diagnostics including a covariance matrix with all continuous independent variables, goodness-of-fit statistics (by the χ2 test), regression coefficients, standard errors, and R2 values. All statistics were 2 sided, with P < .05. We conducted analyses with SPSS version 15.0 (SPSS Inc, Chicago, IL).
RESULTS
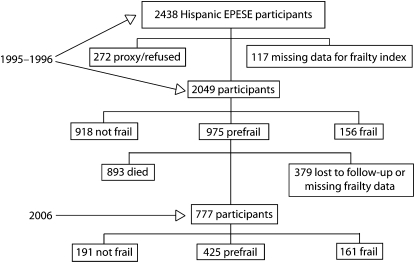
From 1995–1996, 2438 Mexican Americans were available for the Hispanic EPESE interview. Interviews for 272 participants included information collected from proxies, and another 117 participants were missing data necessary to compute the frailty index. Thus, we analyzed data from 2049 participants with a frailty index. Of these, we classified 918 as not frail, 975 as prefrail, and 156 as frail.
During the 10-year period between wave 2 of the Hispanic EPESE (1995–1996) and 2006, 893 (43.6%) of the participants died and another 379 were lost to follow-up or were missing data to compute the frailty index in 2006. In 2006, 777 participants were alive and supplied enough information to complete the frailty index. Of these, 191 were now defined as not frail, 425 as prefail, and 161 as frail. Figure 1 illustrates the frailty status of participants at baseline and follow-up. The 379 participants lost to follow-up or with missing data were older (mean age = 83.43 years [SD = 5.60] versus 82.46 years [SD = 4.48]) and had more comorbidites and ADL and IADL limitations than did the 777 participants assessed at follow-up.
FIGURE 1.
Description of the sample at baseline (1995–1996) and follow-up (2006): Hispanic Established Populations for Epidemiologic Studies of the Elderly, Southwest United States.
Table 1 includes the demographic and participant characteristics for the sample at baseline (n = 2049) and follow-up (n = 777) by frailty category. The percentage of respondents categorized as not frail decreased from 45% in 1995 to 1996 to 25% in 2006, and the percentage of persons identified as frail increased from 7% at baseline to 21% at follow-up. Statistically significant changes (P < .05) in covariates between baseline and follow-up were more frequent among persons identified as frail than among respondents who were prefrail or not frail (Table 1).
TABLE 1.
Sample Characteristics at Baseline (1995–1996) and at 10-Year Follow-Up (2006): Hispanic Established Populations for Epidemiologic Studies of the Elderly, Southwest United States
| Not Frail, No. (%) or mean (±SD) |
Prefrail, No. (%) or mean (±SD) |
Frail, No. (%) or mean (±SD) |
||||
| 1995–1996 | 2006 | 1995–1996 | 2006 | 1995–1996 | 2006 | |
| Total | 918 (45) | 191 (25) | 975 (48) | 425 (55) | 156 (7) | 161 (21) |
| Age, y | 72.89 (±4.95) | 81.28 (±3.81)* | 75.10 (±6.23) | 82.70 (±4.50)* | 79.33 (±7.25) | 83.21 (±4.91)* |
| Women | 532 (58) | 118 (62)* | 580 (60) | 263 (62) | 83 (53) | 105 (65)* |
| Married | 510 (56) | 84 (44)* | 528 (54) | 180 (42)* | 71 (45) | 57 (35)* |
| Education, y | 4.83 (±3.91) | 4.88 (±3.81) | 4.89 (±3.89) | 5.14 (±3.86)* | 5.02 (±3.91) | 4.55 (±3.29)* |
| BMI, kg/m2 | 28.45 (±5.05) | 26.96 (±4.52)* | 27.76 (±5.19) | 27.87 (±5.10) | 27.08 (±6.33) | 27.19 (±5.41) |
| SPPB scorea | 6.32 (±2.24) | 4.69 (±2.29)* | 4.53 (±2.13) | 2.33 (±1.43)* | 2.85 (±1.22) | 1.21 (±0.77)* |
| MMSE score | 24.94 (±3.97) | 21.71 (±3.88)* | 23.68 (±4.12) | 21.47 (±4.41)* | 21.40 (±4.88) | 19.14 (±5.63)* |
| PASE score | 114.47 (58.19) | 115.87 (61.17) | 81.95 (59.81) | 78.97 (56.85) | 22.35 (35.19) | 28.01 (36.71)* |
| CES-D scale score | 4.17 (±5.39) | 4.38 (±5.22) | 7.84 (±8.51) | 8.14 (±7.09) | 13.18 (±10.64) | 15.16 (±10.67)* |
| Daily activities | ||||||
| Total ADL problems | 0.01 (±0.20) | 0.42 (±1.02)* | 0.26 (±0.94) | 1.01 (±1.75)* | 0.93 (±1.70) | 2.13 (±2.32)* |
| Any ADL problem | 6 (1) | 43 (22)* | 94 (10) | 157 (37)* | 55 (35) | 98 (61)* |
| Total IADL problems | 0.77 (±1.67) | 1.74 (±2.24)* | 1.81 (±2.49) | 3.32 (±2.89)* | 4.77 (±2.93) | 6.09 (±2.94)* |
| Any IADL problem | 293 (32) | 105 (55)* | 511 (52) | 342 (81)* | 138 (89) | 153 (95)* |
| Interviewed in Spanish | 720 (78) | 163 (85)* | 816 (84) | 366 (86) | 130 (83) | 133 (83) |
| Medical conditionsb | ||||||
| Heart attack | 54 (6) | 20 (11)* | 110 (11) | 65 (15)* | 19 (12) | 36 (22)* |
| Stroke | 42 (5) | 14 (7) | 77 (8) | 58 (14)* | 26 (17) | 29 (18) |
| Hip fracture | 6 (1) | 3 (2) | 14 (1) | 28 (7)* | 7 (5) | 1 (7) |
| Cancer | 44 (5) | 11 (6) | 72 (7) | 35 (8) | 14 (9) | 12 (8) |
| Diabetes | 218 (24) | 54 (28) | 300 (31) | 146 (34) | 50 (32) | 77 (48)* |
| Arthritis | 373 (41) | 101 (52)* | 462 (47) | 263 (62)* | 86 (55) | 122 (76)* |
Note. BMI = body mass index; SPPB = Short Physical Performance Battery; MMSE = Mini-Mental State Exam; PASE = Physical Activity Scale for the Elderly; CES-D = Center for Epidemiologic Studies Depression; ADL = activities of daily living; IADL = instrumental ADL. Not frail was defined as a score of 0 on the frailty index, prefrail as a score of 1 or 2, and frail as a score of 3 or higher. Total sample at baseline was N = 2049 participants; at 10-year follow-up, n = 777.
Included balance and chair stand items from SPPB scale.17
Self-reported lifetime occurrence of physician-diagnosed condition.
*P < .05, for difference between baseline and 2006 for each frailty category (by analysis of variance for continuous variables and the χ2 test for categorical variables).
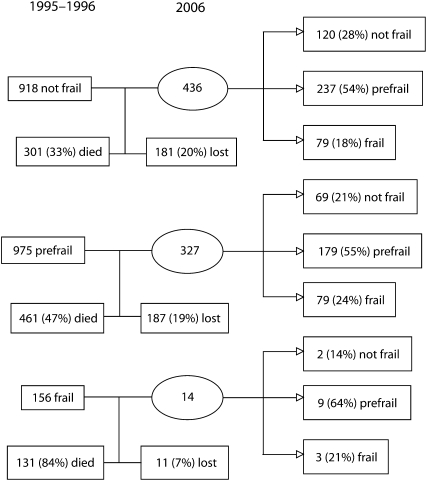
As expected, mortality was highest between baseline and 10-year follow-up among persons in the frail category. Of participants identified as frail in 1995 to 1996, 84% died by follow-up in 2006. By contrast, the mortality rates for persons identified as prefrail and not frail in 1995 to 1996 were 47% and 33%, respectively. The mortality rate across all frailty categories was 8% higher for men than for women over the 10 years.
The pattern of frailty changed over time, with an increasing number of participants being classified as frail or prefrail because of their scores on the walking speed or grip strength components of the frailty index. In 1995 to 1996, the percentage of persons scoring a 1 on any of the 5 frailty items ranged from 13% for walking speed and weight loss to 7% for exhaustion and physical activity (PASE scores). In 2006, 54% of the participants scored a 1 on walking speed, 35% on grip strength, 29% on exhaustion, 22% on physical activity (PASE), and 21% on weight loss.
Results from our 3 multivariate linear regression models are shown in Table 2. The variables that were consistently significant predictors of frailty across all 3 models over the 10-year period of the study were age, smoking history, arthritis, BMI, negative affect from the CES-D scale, and number of comorbidities. We also computed cross-sectional regression models with data from both the baseline and follow-up waves. In these models, the outcome variable was the frailty index obtained concurrently with the information on the covariates. The statistically significant predictors of frailty in these models were different from the longitudinal models and included gender, heart attack, arthritis, Short Physical Performance Battery score, ADL and IADL ratings, and the Positive Affect subscale of the CES-D (data not shown).
TABLE 2.
Multiple Regression Models Predicting Frailty Index at Follow-Up (2006) From Data Collected at Baseline (1995–1996): Hispanic Established Populations for Epidemiologic Studies of the Elderly, Southwest United States
| Model 1,a b (SE) | Model 2,b b (SE) | Model 3,c b (SE) | |
| Age | 0.05* (0.01) | 0.06* (0.01) | 0.06* (0.01) |
| Gender | 0.02 (0.01) | 0.04 (0.05) | 0.08 (0.10) |
| Education | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) |
| Married | 0.10 (0.09) | 0.13 (0.08) | 0.16 (0.08) |
| Financial straina | 0.05 (0.03) | 0.05 (0.04) | 0.03 (0.04) |
| Ever smoked | 0.36* (0.15) | 0.36* (0.14) | |
| Medical conditionb | |||
| Diabetes | 0.23* (0.10) | 0.24* (0.11) | |
| Hip fracture | 0.79* (0.51) | 0.57 (0.52) | |
| Cancer | 0.15 (0.23) | 0.06 (0.24) | |
| Stroke | 0.22 (0.20) | 0.14 (0.23) | |
| Cardiac | 0.31* (0.16) | 0.26 (0.19) | |
| Arthritis | 0.17* (0.09) | 0.13* (0.08) | |
| BMI (kg/m2) | 0.03* (0.01) | 0.02* (0.01) | |
| IADL problems | 0.03 (0.02) | ||
| ADL problems | 0.12 (0.11) | ||
| SPPB scorec | 0.01 (0.02) | ||
| MMSE score | 0.03* (0.01) | ||
| Affectd | |||
| Positive affect | 0.03 (0.02) | ||
| Negative affect | 0.06* (0.01) | ||
| Comorbid conditions | 0.16* (0.05) | ||
| Frailty index at wave 2 | 0.18* (0.06) | 0.13* (0.06) | 0.09 (0.06) |
| R2 | 0.09 | 0.18* | 0.29* |
Note. BMI = body mass index; IADL = instrumental activities of daily living; SPPB = Short Physical Performance Battery; MMSE = Mini-Mental State Exam. Not frail was defined as a score of 0 on the frailty index, prefrail as a score of 1 or 2, and frail as a score of 3 or higher. All models included the baseline (1995–1996) frailty index as a covariate. Model 1 included sociodemographic variables. Model 2 added medical conditions and health status variables. Model 2 added physical performance measures and a cumulative comorbidity index.
This was the degree of difficulty paying bills (1 = a great deal, 2 = some, 3 = a little, and 4 = none).
Self-reported lifetime occurrence of physician-diagnosed condition.
This included balance and chair stand items from SPPB scale.
This was from the Center for Epidemiologic Studies Depression Scale.
*P < .05
The 10-year follow-up data indicated that frailty is a dynamic process and that a person's frailty status may change over time. For example, in 1995 to 1996, 975 (48%) respondents were identified as prefrail. In 2006, 327 (33.5%) of these participants were alive and supplied sufficient information to compute a frailty index. Of these 327 participants, 69 (21%) were classified in 2006 as not frail, 179 (55%) remained prefrail, and 79 (24%) were identified as frail. Figure 2 presents the distribution of participants by frailty status in 1995 to 1996 and 2006, along with the figures for participants who died or were lost at follow-up.
FIGURE 2.
Description of transitions in frailty status from baseline (1995–1996) to follow-up (2006): Hispanic Established Populations for Epidemiologic Studies of the Elderly, Southwest United States.
DISCUSSION
We examined the trajectory of frailty in 2049 community-living Mexican Americans 65 years and older. At the end of the 10-year follow-up, 75% of our sample (mean age = 82.46 years; SD = 4.48) were classified as either prefrail or frail. Of participants identified as frail at baseline (1995–1996), 84% died by 2006. By contrast, 67% of those identified as not frail at baseline were alive at the 2006 interview. In multivariable linear regression analyses, baseline age, diabetes, arthritis, smoking status, BMI, MMSE score total, Negative Affect subscale score, and number of comorbid conditions were statistically significant predictors of frailty at the end of 10 years and accounted for 29% of the variance in the frailty index.
Our results are consistent with previous longitudinal research examining frailty among non-Hispanic White older adults,6 with a few important differences. For example, we found statistically significant relationships between diabetes, BMI, MMSE score, and negative affect and frailty ratings at follow-up.
Recent research has attempted to elucidate the relationship between obesity, disability, and mortality.21 A study of Hispanic, African American, and non-Hispanic White older adults reported hazard ratios for disability that were twice as large as the hazard ratios for mortality among individuals with a BMI over 30 kg/m2.22 Emerging evidence suggests that obesity is associated with increased risk of disability among older people.23 To the extent that frailty is a precursor to disability, our findings are consistent with previous research indicating a positive association between obesity- and disability-related morbidity among older adults.22,23
By contrast to our expectations, performance-related variables that were statistically significant predictors for frailty at follow-up included MMSE score and the Negative Affect subscale of the CES-D score, but not the measures of ADL, IADL, or balance.1,24 There has been debate in the literature regarding the role of cognition in defining frailty.25 The frailty index developed by Fried et al.6 does not include a direct measure of cognitive function. Our results suggest a relationship between cognition and frailty over time.
We also found an association between scores on the Negative Affect subscale of the CES-D at baseline and risk of frailty at 10-year follow-up. We did not include the complete CES-D score in the regression models because 2 questions from the Somatic and Retarded Activity subscale of the CES-D are used in the frailty index. The correlation between the Somatic and Retarded Activity and Negative Affect subscales was less than 0.40 in our sample. The relationship between cognitive function and negative affect at baseline and risk of frailty at follow-up suggests an association between emotion, cognition, and physical health.26 The cognitive and emotional variables (negative affect) that predicted frailty status at the 10-year follow-up were not associated with frailty ratings in cross-sectional analyses. The difference in variables associated with frailty in the cross-sectional versus longitudinal analyses may explain some of the confusion in the literature regarding risk factors and the frailty index.7
The practical importance of variables such as BMI, diabetes, smoking status, and negative affect as predictors of frailty among Mexican American older adults is that they represent modifiable characteristics or behaviors not previously identified as risk factors for frailty among non-Hispanic White or minority populations. Several authors have commented on the importance of identifying frailty or a prefrail state (subclinical disability) among older adults so that appropriate interventions can be implemented.27,28 Previous studies demonstrated the benefits of exercise, resistance training, nutritional supplementation, and hormone replacement and the importance of emotional health in reducing frailty and subsequent disability.26,29–31 These interventions may be particularly appropriate for prefrail older persons, who are at high risk for disability and adverse health outcomes and may be most responsive to intervention.32,33 Additional research is needed to examine this possibility.
Strengths and Limitations
The strengths of our study included the prospective collection and analysis of data from a large sample over 10 years. We used well-established data collection procedures and measures verified in several other EPESE studies. Data collectors received extensive training and were retrained at approximately 2-year intervals. The sample included members of an understudied minority population—Mexican American older adults—who have characteristics, such as low educational level, obesity, and limited access to primary care, that may affect frailty-related morbidity and mortality. We also used a standardized definition of frailty that can be replicated in clinical and community environments. This was the first study to document transitions across levels of frailty in Mexican American older adults.
Our study also had several limitations. Health conditions and comorbidities were self-reported. Hughes et al. examined the extent and nature of bias associated with self-report versus standardized physician examination among older persons.15 Overall, these studies indicated that self-reports are valid for common medical conditions such as heart attack, stroke, and arthritis experienced by persons 65 years and older. Another limitation was that we eliminated respondents from the original sample who were not able to complete the performance tests required to compute the frailty index. Persons eliminated were older, had more comorbidities and ADL limitations, and had lower MMSE scores than the participants whose data we analyzed. Thus, persons who remained in the study represented the healthiest members of the original sample.
Our information on frailty was limited to only 2 time points, and this reduced our ability to examine changes or trends in frailty status over the 10-year period of study. This is a particular limitation in developing a better understanding of the transitions from one level of frailty to another over time. For example, we found that participants moved across different levels of frailty in both directions. Particularly interesting are persons who were classified as not frail at baseline (1995–1996) and who remained in this category 10 years later (n = 120). Also intriguing are respondents categorized as prefrail at baseline but as not frail at follow-up (n = 69). We plan to collect additional longitudinal data from the sample that will allow us to examine these transitions with more sensitive statistical models. In future research it will also be important to carefully examine social network and cultural variables that may interact with frailty and disability in complex ways.34 We found that marital status was related to frailty status (Table 2), but additional information is needed to determine how social networks, particularly social and neighborhood relationships important in acculturation, affect transitions across frailty states. This information will help us better understand health disparities in this population and address the goals of Healthy People 2010.35
Conclusions
We found an increase in the incidence of frailty at follow-up, with 84% of those identified as frail at baseline dying over 10 years. Persons identified as frail at baseline were more likely to have multiple comorbidities and to experience a significant decrease in the ability to perform basic ADL. Statistically significant predictors of frailty at 10-year follow-up included age, marital status, arthritis, diabetes, smoking status, BMI, MMSE score, Negative Affect subscale score, and number of comorbid conditions. We also found evidence of transitions across levels of frailty over 10 years, suggesting that frailty status is dynamic and that persons may move into and out of frailty states.10 The challenge of future research is to better understand this dynamic process and identify interventions and behaviors that will help older persons avoid frailty or recover optimal health after becoming prefrail or frail.
Acknowledgments
K. Ottenbacher (PI) received funding from the National Institutes of Health (grants R01 AG017638, R01 AG10939, K01 HD046682, and R03 TW007614-03).
Human Participant Protection
Informed consent was obtained from participants when they were interviewed, and the study protocol was approved by the institutional review board at the University of Texas Medical Branch.
References
- 1.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007;62:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strandberg TE, Pitkala KH. Frailty in elderly people. Lancet 2007;369:1328–1329 [DOI] [PubMed] [Google Scholar]
- 3.Dunlop DD, Song J, Manheim LM, Chang RW. Racial disparities in joint replacement use among older adults. Med Care 2003;41:288–298 [DOI] [PubMed] [Google Scholar]
- 4.Gornick ME, Eggers PW, Reilly TW, et al. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med 1996;335:791–799 [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Walston J. Frailty and failure to thrive. : Hazzard W, Blass J, Ettinger WH, Halter J, Ouslander J, eds Principles of Geriatric Medicine and Gerontology New York, NY: McGraw-Hill Professional; 1999:1387–1402 [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62:738–743 [DOI] [PubMed] [Google Scholar]
- 8.Bortz WM. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci 2002;57:M283–M288 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med 2007;120:748–753 [DOI] [PubMed] [Google Scholar]
- 10.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166(4):418–423 [DOI] [PubMed] [Google Scholar]
- 11.Markides KS, Stroup-Benham CA, Black SA, Satish S, Perkowski LC, Ostir GV. The health of Mexican American elderly: selected findings from the Hispanic EPESE. : Wykle ML, Ford AB, eds Serving Minority Elders in the Twenty-first Century New York, NY: Springer Publishing; 1999:72–90 [Google Scholar]
- 12. National Archive of Computerized Data on Aging Available at: http://www.icpsr.umich.edu/cocoon/NACDA/STUDY/02851.xml. Accessed October 6, 2008.
- 13.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–162 [DOI] [PubMed] [Google Scholar]
- 15.Hughes SL, Edelman PL, Singer RH, Chang RW. Joint impairment and self-reported disability in elderly persons. J Gerontol 1993;48:S84–S92 [DOI] [PubMed] [Google Scholar]
- 16.Lamb SE, Guralnik JM, Buchner DM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women's Health and Aging Study. Ann Rheum Dis 2000;59:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 18.Scudds RJ, Robertson JM. Empirical evidence of the association between the presence of musculoskeletal pain and physical disability in community-dwelling senior citizens. Pain 1998;75:229–235 [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci 2002;57:M181–M185 [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Alley D. Obesity, disability, and mortality: a puzzling link. Arch Intern Med 2007;167:750–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Snih S, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med 2007;167:774–780 [DOI] [PubMed] [Google Scholar]
- 23.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA 2007;298:2020–2027 [DOI] [PubMed] [Google Scholar]
- 24.Ottenbacher KJ, Ostir GV, Peek MK, Snih SA, Raji MA, Markides KS. Frailty in older Mexican Americans. J Am Geriatr Soc 2005;53:1524–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuh D, New Dynamics of Ageing (NDA) Preparatory Network A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci 2007;62:717–721 [DOI] [PubMed] [Google Scholar]
- 26.Ostir GV, Ottenbacher KJ, Markides KS. Onset of frailty in older adults and the protective role of positive affect. Psychol Aging 2004;19:402–408 [DOI] [PubMed] [Google Scholar]
- 27.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–263 [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Young Y, Rubin G, Bandeen-Roche K. WHAS II Collaborative Research Group. Self-reported preclinical disability identifies older women with early declines in performance and early disease. J Clin Epidemiol 2001;54:889–901 [DOI] [PubMed] [Google Scholar]
- 29.Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2005;60:1425–1431 [DOI] [PubMed] [Google Scholar]
- 30.Host HH, Sinacore DR, Bohnert KL, Steger-May K, Brown M, Binder EF. Training-induced strength and functional adaptations after hip fracture. Phys Ther 2007;87:292–303 [DOI] [PubMed] [Google Scholar]
- 31.Villareal DT, Steger-May K, Schechtman KB, et al. Effects of exercise training on bone mineral density in frail older women and men: a randomised controlled trial. Age Ageing 2004;33:309–312 [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Haren MT, Rolland Y, Kim MJ. Frailty. Med Clin North Am 2006;90:837–847 [DOI] [PubMed] [Google Scholar]
- 33.Torpy JM, Lynm C, Glass RM. JAMA patient page Frailty in older adults. JAMA 2006;296:2280. [DOI] [PubMed] [Google Scholar]
- 34.Hazuda HP, Espino DV. Aging, chronic disease, and physical disability in Hispanic elderly. : Markides KS, Miranda MR, eds Minorities, Aging, and Health Thousand Oaks, CA: Sage Publications; 1997:127–148 [Google Scholar]
- 35.Healthy People 2010: Understanding and Improving Health. Washington, DC: US Dept of Health and Human Services; 2000 [Google Scholar]