Abstract
Objectives. In September 1999, the Elizabeth Glaser Pediatric AIDS Foundation initiated a multicountry, service-based programmatic effort in the developing world to reduce perinatally acquired HIV infection. We review 6½ years of one of the world's largest programs for the prevention of mother-to-child transmission (PMTCT) of HIV.
Methods. Each PMTCT facility records patient data in antenatal clinics and labor and delivery settings about counseling, testing, HIV status, and antiretroviral prophylaxis and submits the data to foundation staff.
Results. More than 2.6 million women have accessed foundation-affiliated services through June 2006. Overall, 92.9% of women who received antenatal care or were eligible for PMTCT services in labor and delivery have been counseled, and 82.8% of those counseled accepted testing. Among women identified as HIV positive, 75.0% received antiretroviral prophylaxis (most a single dose of nevirapine), as did 45.6% of their infants.
Conclusions. The foundation's experience has demonstrated that opt-out testing, supplying mothers with medication at time of diagnosis, and providing the infant dose early have measurably improved program efficiency. PMTCT should be viewed as an achievable paradigm and an essential part of the continuum of care.
We review 6½ years of one of the world's largest programs for the prevention of mother-to-child transmission (PMTCT) of HIV in resource-limited settings. Created by the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) in September 1999 and originally titled “Call to Action,” this multinational effort provides access to services for PMTCT.
EGPAF's PMTCT program was developed on the basis of the results of clinical trial HIVNET 012, which demonstrated the efficacy of a feasible, effective, safe regimen of antiretroviral medication to reduce mother-to-child transmission of HIV.1,2 This advance presented the first real opportunity to narrow the gap between wealthier and resource-poor nations in preventing mother-to-child transmission. Prior to HIVNET 012, the regimens that had proven effective for PMTCT required antepartum administration of zidovudine (AZT) for a minimum of 4 weeks, during labor and delivery, and oral administration to the infant for at least 1 week.3–5
In Uganda, single-dose nevirapine (NVP) was reported to be more effective than were clinical trials in the Ivory Coast and Burkina Faso that used a 4-week antepartum course of AZT.4,5 Single-dose NVP could be administered orally to pregnant HIV-positive women at the onset of labor and to their infants within 72 hours after delivery at a cost of less than US $4.1 Since 2000, Boehringer Ingelheim has donated NVP to many countries for antiretroviral prophylaxis.6 The simplicity and affordability of single-dose NVP stimulated a commitment by the foundation to provide resources for the urgent expansion of PMTCT services.
METHODS
EGPAF was cofounded in 1988 by Elizabeth Glaser, Susan DeLaurentis, and Susie Zeegen.7 It has developed and administered hundreds of peer-reviewed research and implementation programs worldwide.
In September 1999, the same month that HIVNET 012 was published, EGPAF invested $1 million to initiate a multicountry, service-based programmatic effort in the developing world to reduce perinatally acquired HIV infection. Substantial contributions to the program subsequently were provided from private donors, including the Bill and Melinda Gates Foundation. In 2002, EGPAF entered into an agreement with the United States government to expand access to and scale up PMTCT services.
EGPAF solicited applications from governmental, nongovernmental, faith-based, national, and international organizations and health care facilities interested in planning, implementing, or expanding PMTCT programs. It engaged in a grant-making process that was iterative, peer reviewed, and interactive with the proposed project leaders.
EGPAF launched its first PMTCT projects in Thailand and 5 African countries (South Africa, Kenya, Uganda, Cameroon, and Rwanda) in 2000. As of June 2006, EGPAF has supported projects in 22 countries: Angola, Cameroon, China, Ivory Coast, Democratic Republic of Congo, Dominican Republic, Georgia, Honduras, India, Kenya, Lesotho, Malawi, Mozambique, Russia, Rwanda, South Africa, Swaziland, Tanzania, Thailand, Uganda, Zambia, and Zimbabwe. Published reports of EGPAF-sponsored activities in Cameroon, Zambia, and Zimbabwe are available.8–11
EGPAF-affiliated work is conducted in accordance with local ministry of health policies. EGPAF-affiliated programs integrate HIV counseling and testing and antiretroviral prophylaxis regimens into existing maternal and child health clinics. Patient flow, HIV counseling techniques and testing algorithms, drug distribution, and other aspects of program delivery differ among countries. EGPAF funds have supported clinic renovation, essential commodities, community mobilization and training of health care workers, education on infant feeding and family planning, technical assistance, evaluation and monitoring, and psychosocial support.
HIV Counseling
Commonly, women seeking prenatal care at participating clinics receive general health education, including information about HIV and primary prevention messages. Individual or group pretest HIV counseling is provided to pregnant women, a practice that is evolving to an “opt-out” approach to testing. With the opt-out approach, women are informed that HIV testing is provided as part of an essential package of standard antenatal care; they can decline the test if they choose. The standard antenatal care package typically includes testing for syphilis, determination of hemoglobin levels, treatment for malaria, administration of vitamins and iron, tetanus immunization, and urinalysis. Women have the right to refuse HIV testing without affecting their access to other services.
National policies determine who may provide pre- and posttest counseling. Nurse midwives, nurses, physicians, medical officers, trained nonmedical personnel, and trained birth attendants are among those allowed to provide services in some PMTCT locations.
All women receive individual posttest counseling. Posttest counseling is more time-consuming than was pretest counseling, given the need to effectively tailor information for women according to serostatus. During posttest counseling, providers introduce the concept of longitudinal care during pregnancy and after delivery for both mother and infant. Addressing infant feeding options for mothers is important, because one third to one half of all mother-to-child transmissions occur postnatally through breastfeeding.12
HIV Testing
EGPAF-affiliated PMTCT programs perform HIV testing in accordance with national policies for antenatal patients. Most facilities employ rapid HIV antibody testing using Determine HIV-1/2 (Abbott Laboratories, Abbott Park, IL), which has been donated to many resource-limited countries, and most provide same-day results, improving the percentage of women receiving test results.13 In a minority of facilities, women return for a separate visit to learn their HIV serostatus. Most countries currently employ serial testing.14 National policies also determine whether nurses or other personnel in the antenatal care or labor and delivery areas can be trained and certified to perform the HIV test.
Antiretroviral Prophylaxis With Single-Dose Nevirapine
Most of EGPAF's sites encourage HIV-infected women to deliver in a facility with a skilled birth attendant where they may be given single-dose NVP under direct observation. However, many programs give single-dose NVP to women during antenatal care, advising them to take it at the onset of active labor. Some programs have also started offering women the infant dose of NVP before delivery, so that it is available to be given by the woman at home. The maternal single-dose NVP regimen is 200 mg, to be ingested at the onset of labor. When infant weight cannot be obtained, the infant dose is 0.6 mL (6 mg) of NVP syrup, to be ingested within 72 hours after delivery; otherwise, infant dose is 2 mg/kg.
Traditional birth attendants (trained and untrained) are encouraged to train and participate in PMTCT activities in areas in which they provide pre- and postnatal care. Birth attendants provide these services in Cameroon10,15 and Tanzania16 and are planned for Zambia.
An increasing number of our affiliated sites counsel and test women who are in labor and whose HIV status is unknown, either because the women have not received antenatal care or have received antenatal care without PMTCT services. Those who test positive for HIV during labor are offered NVP prophylaxis and additional postpartum counseling.
Data Collection
EGPAF requires quantitative progress reports either quarterly or semiannually, depending on the funding source. Each facility records patient data in both antenatal clinics and labor and delivery settings, with data on counseling, testing, HIV status, and antiretroviral prophylaxis, among other indicators (for a list of indicators, see the appendix available as a supplement to the online version of this article at http://www.ajph.org). The facility or program then submits a standardized form to the in-country technical adviser or the foundation program officer. EGPAF's technical assistance visits include assessing the clinic service registers used to collect these data, sampling data to ensure accuracy, and providing technical assistance to address findings or concerns. Although every effort is made to promote standardized definitions of key program indicators related to PMTCT services, such as counseling, testing, and distribution of maternal and infant doses of antiretroviral medication, there are sometimes subtle differences in these indicators according to national polices and guidelines. These data, which are collected at the facility level, represent program results among the subset of women seeking antenatal and labor and delivery services.
The technical adviser or program officer reviews and submits program data to EGPAF's monitoring and evaluation staff. EGPAF's scientific director reviews reports for inconsistencies, trends over time, and variability between facilities, and provides feedback on challenges and improvements. Queries are sent to in-country technical staff or facility personnel for appropriate corrections.
EGPAF staff enter the quantitative data from the progress reports, including corrections, in Filemaker Pro 8 (FileMaker Inc, Santa Clara, CA). Program staff in the United States collaboratively analyze the data and create summary presentations to internal and external stakeholders.
Technical Assistance
EGPAF provides on-site monitoring to programs at least once every 6 months. Technical assistance was initially provided through a contract with Family Health International, but it is presently the responsibility of EGPAF itself. Recommendations from consultants or EGPAF staff are incorporated into the program and, when appropriate, shared across facilities. Facilities also share technical expertise and lessons learned by visiting other facilities and participating in a biannual program implementers' meeting.
RESULTS
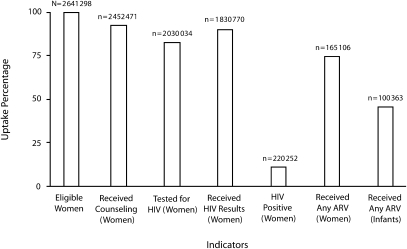
Over 6½ years, EGPAF's multinational PMTCT program increased significantly in scope. Cumulatively, more than 2.6 million women accessed antenatal services or were eligible for PMTCT services in labor and delivery settings through June 2006 (Figure 1). Overall, 92.9% of these women were counseled and 82.8% of women counseled accepted testing. Among women identified as HIV positive, 75.0% received antiretroviral prophylaxis, as did 45.6% of their infants.
FIGURE 1.
Selected indicators showing cumulative results of the Elizabeth Glaser Pediatric AIDS Foundation's program for the prevention of mother-to-child transmission (PMTCT) of HIV, through June 30, 2006.
Note. Data shown (left to right) are as follows: women eligible for PMTCT services in antenatal care (ANC) and in labor and delivery ward; women receiving counseling; women tested for HIV; women receiving their test results; women who were HIV positive; women receiving antiretroviral (ARV) prophylaxis; infants receiving ARV prophylaxis.
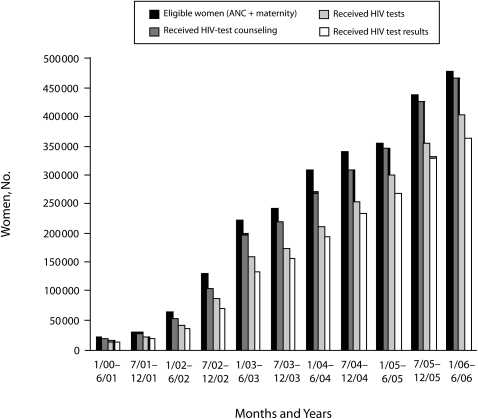
In 2000, 11 753 pregnant women were seen in antenatal clinics at 9 facilities in which the EGPAF supported PMTCT. Most of these women (93.7%) received counseling, and more than half (68.3%) were tested for HIV, of whom 86.9% received their results. The following year, there were 47 facilities with EGPAF-supported PMTCT services and a 370% increase in the number of women accessing prenatal care. Of these women, 89.9% received counseling and 81.1% were tested for HIV, of whom 90.6% received their results. In 2002, there was another 450% increase in the number of women provided access to PMTCT services at EGPAF-affiliated facilities, which now numbered 122; 82.3% of these women were counseled, and 80.7% were tested for HIV, of whom 83.8% received their test results. Program growth continued in 2003, when 2.4 times as many women as in the previous year were seen in antenatal clinics at 357 facilities; in 2004, when the number of women accessing services at 613 facilities grew 140% and in 2005, which saw 120% growth in the number of women accessing PMTCT services at EGPAF-affiliated facilities, which now numbered 958 (Figures 2 and 3).
FIGURE 2.
Number of women in the Elizabeth Glaser Pediatric AIDS Foundation's program for the prevention of mother-to-child transmission (PMTCT) of HIV who were eligible for PMTCT services in antenatal care or labor and delivery ward, received counseling, received HIV testing, and received HIV test results, by time interval.
Note. ANC = antenatal care.
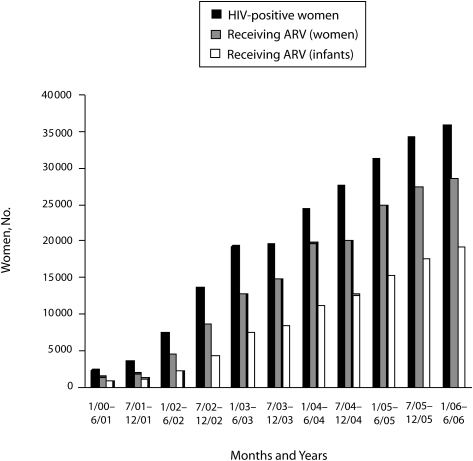
FIGURE 3.
Number of women in the Elizabeth Glaser Pediatric AIDS Foundation's program for the prevention of mother-to-child transmission who tested positive for HIV and the number of women and infants who received antiretroviral (ARV) prophylaxis, by time interval.
The most notable changes over time were observed in maternal provision of HIV testing and receipt of mother and infant doses of antiretroviral prophylaxis (Figure 4). A total of 84.9% of counseled women in antenatal care and labor and delivery service received HIV testing from July 1, 2005, through June 30, 2006, compared with 68.3% of the women in 2000.
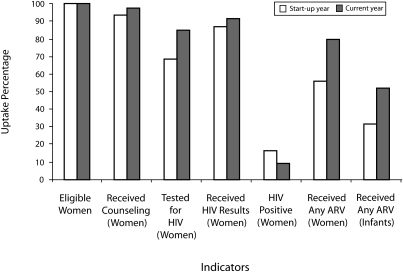
FIGURE 4.
Selected indicators showing results during the start-up year (2000) and most recent year for which data are available (July 1, 2005–June 30, 2006): the Elizabeth Glaser Pediatric AIDS Foundation's program for the prevention of mother-to-child transmission (PMTCT) of HIV.
Note. Data shown (left to right) are as follows: women eligible for PMTCT services in antenatal care (ANC) and in labor and delivery ward; women receiving counseling; women tested for HIV; women receiving their test results; women who were HIV positive; women receiving antiretroviral (ARV) prophylaxis; infants receiving ARV prophylaxis.
The greatest increases over time in uptake of key PMTCT services were in documented receipt of antiretroviral prophylaxis by mothers and infants. The percentage of women receiving antiretroviral prophylaxis was measured at 79.6% for the most recent year for which data are available and 55.7% for the first year of the program. A total of 56 104 women were reported to have received antiretroviral prophylaxis in the most recent year; if provision had remained at 55.7%, an estimated 39 275 women would have received this critical service during the same time period. Provision of infant antiretroviral drug prophylaxis was reported to be 52.3% in the last year and 31.2% in the first year. A total of 36 859 doses of infant prophylaxis were dispensed in the last year. If provision in the last year had remained at 31.2%, only 22 000 infants would have received prophylaxis.
Opt-Out Testing
The provision of universal counseling with an opt-out approach to testing appears to enhance use, as reported in Cameroon11 and Malawi.17 The Malawi program, which receives funding and technical support from EGPAF and is administered by the University of North Carolina, reported a cumulative 15.1% seroprevalence among pregnant women receiving antenatal care services. On the basis of this rate, 7335 of the 48 578 pregnant women seen at the Malawi PMTCT facilities from January 2002 to December 2004 would be expected to be HIV positive. The Malawi program used opt-in testing, in which women are informed they can elect to be counseled and tested for HIV if they want (the test was not required in standard antenatal care). Using this approach, 4586 women were identified as HIV positive, or only 62.5% of the estimated HIV-positive women. Of these, 1964 women received antiretroviral prophylaxis (42.8% of those identified as HIV positive, but only 26.8% of those estimated to be HIV positive).
In early 2005, the program implemented universal group pretest counseling and opt-out testing. From January 2005 through June 2006, the Malawi PMTCT program received 31 797 pregnant women in their antenatal clinics, of whom 4801 would be predicted to be HIV positive on the basis of a seroprevalence rate of 15.1%. The program identified 4329 HIV-positive women, or 90.2% of the number expected to be HIV positive. A total of 4564 HIV-positive women received antiretroviral prophylaxis (over 100% of those identified as HIV positive were reported to receive ARV prophylaxis because this reporting window included women identified as HIV positive from the previous interval). Of the women expected to be HIV positive, 95. 1% received ARV prophylaxis. With the opt-out approach, a substantial improvement from the first 2 years of the program was achieved.17
Services to Prevent Mother-to-Child Transmission in Labor and Delivery Settings
National PMTCT programs have recognized the opportunity to provide counseling and testing in the labor and delivery setting, and some programs are introducing these services. Including these services in labor and delivery settings allows for identification of HIV-positive women who have not received counseling and testing in the antenatal setting. HIV counseling and testing is offered to women arriving in labor with unknown serostatus, including women who did not attend an antenatal clinic or attended one without PMTCT services.
In July 2005, EGPAF's PMTCT program started to document the number of eligible women in labor and delivery settings in addition to the number of women in antenatal clinic settings. From July 2005 to June 2006, a total of 919 975 women accessed the program's services, with 6.9% of them accessing services only in labor and delivery. Percentages by country of women accessing services only in labor and delivery are as follows: Kenya, 7.8% of 68 034 women; Mozambique, 16.9% of 38 772; Rwanda, 3.6% of 26 729; Swaziland, 34.1% of 15 787; and Tanzania, 16.0% of 102 980.
Provision of Maternal and Infant Nevirapine
Initially, most facilities dispensed NVP only in labor and delivery settings when women arrived for delivery. With the recognition that some women may deliver at home or arrive too late in labor to receive their NVP tablet, the policy has changed so that facilities are encouraged to dispense NVP to the mother either at a fixed point in gestation (such as at 28 weeks) or upon diagnosis of HIV.18 Significant improvement in the percentage of HIV-positive women receiving antiretroviral prophylaxis upon diagnosis of HIV has been described for Cameroon and Kenya.18
A pilot program in Tabora, Tanzania, reported similar improvement with the policy change. The program, which took place in Nzega, Igunga, and Sikonge districts, compared provision of antiretroviral prophylaxis for HIV-positive women given a tablet at 28 weeks gestation from April to June 2006 to provision of prophylaxis at HIV diagnosis from July to October 2006. The proportion of HIV-positive women receiving antiretroviral prophylaxis increased as follows: Nzega, from 42.9% (116 of 270) to 94.9% (340 of 358); Igunga, from 54.8% (96 of 175) to 102.9% (242 of 235); Sikonge, from 66.6% (80 of 120) to 102.0% (152 of 149). (Women who were identified as HIV positive elsewhere but received antiretroviral prophylaxis at EGPAF sites are included in the numerator but not the denominator, resulting in some percentages slightly higher than 100%.) Cumulatively, for Tabora, 52.6% of HIV-positive women received antiretroviral prophylaxis with the 28-week policy vs 99% with the revised policy. Given the strong temporal relationship between the policy change and improvement in receipt of antiretroviral prophylaxis, the data suggest that providing NVP upon diagnosis of HIV improves the provision of antiretroviral prophylaxis to HIV-positive women.
In the program in Kericho, Kenya, upon being diagnosed with HIV, pregnant women are provided with a black plastic bag containing a 200-mg tablet of NVP along with a NVP-filled oral syringe wrapped in foil for them to administer to their infants shortly after delivery.18 During the data collection period (January 1–March 31, 2005), before the intervention was implemented, 72.7% of HIV-positive mothers and 57.3% of their infants received NVP doses. In the same quarter the following year, after the intervention was put into place, 94.1% of HIV-positive mothers and 85.7% of their infants received doses of NVP. By comparison, at other foundation African facilities in the same time period, 78% of HIV-positive mothers and 48.8% of their infants received doses of NVP.
DISCUSSION
Limitations
Our analyses are based on program data reported to EGPAF. Data collection has proved to be difficult over the life of the program. When the program began, there were few functioning national systems for monitoring PMTCT; the data collection process for EGPAF's PMTCT program helped to inform and shape the new national systems that were implemented. Because data on infant feeding are limited, they are not included here although infant feeding is a critical issue. Reported data on transmission rates have also been sparse; further studies are needed to better understand transmission rates in program settings. Despite these limitations, a program of this size provides useful data that can help guide programs locally and advocate for program expansion nationally and internationally.
Conclusions
The value of PMTCT programs extends beyond prevention of infant HIV infection. These programs establish a foundation for more-widespread antiretroviral therapy by training health care providers, including those in maternal and child health, enabling laboratory diagnosis of HIV, educating and counseling women about HIV, identifying infected individuals, and educating community members about preventing, transmitting, and treating HIV. These services have empowered women to make decisions about their health care and the care of their infants. They also enhance systems for procurement and distribution and create opportunities to integrate HIV prevention and care services into the health care infrastructure.
In many countries, EGPAF's PMTCT program was the first to distribute prophylactic antiretroviral agents (primarily single-dose NVP) as part of routine services in maternal and child health clinics. EGPAF's experience demonstrates that several strategies can increase access to and use of PMTCT services; these include opt-out HIV testing, providing maternal antiretroviral prophylaxis at the time of HIV diagnosis, providing the infant with antiretroviral prophylaxis in antenatal care, and offering PMTCT services in labor and delivery settings.18
Many maternal and child health clinics now give single-dose NVP to the mother at the time of diagnosis in an antenatal clinic, advising her to take it at the onset of active labor. The percentage of women receiving antiretroviral prophylaxis at facilities supported by EGPAF has increased over time (Figure 4). This increase seems primarily attributable to the dispensing of single-dose NVP at the time of diagnosis in an antenatal clinic. Women provided NVP in an antenatal care clinic might not be directly observed swallowing the dose. There is no guarantee that all women will take their medication, but women who do not deliver in a facility will have the opportunity to take the medicine only if it is in their possession at the onset of labor.18 Two published studies have measured cord blood concentrations of NVP after the medication was dispensed to women in an antenatal clinic. The percentage of women who took the medication when it was distributed in this fashion was 68% in Zambia and 94% in Uganda.19,20
Provision of the infant dose within 3 days after delivery has been difficult when infants are not born in a health care facility. The infant NVP syrup is available in a 20-mL multiuse bottle but is not available from the manufacturer in single-dose packaging. In an attempt to improve the provision of NVP syrup by HIV-exposed infants, an increasing number of facilities dispense the infant dose in an oral syringe prefilled by the health care provider when the mother is diagnosed with HIV in an antenatal clinic. An oral syringe (Baxa Corporation, Englewood, CO) is part of Boehringer Ingelheim's NVP donation program, and NVP has a demonstrated stability of 2 months (according to the Boehringer Ingelheim package insert21) in the oral syringe.22
Although provision of PMTCT services during labor poses additional operational challenges, it may contribute substantially to the number of pregnant women identified as HIV-positive and the number of women and infants subsequently provided antiretroviral prophylaxis.18
Clinical trials in nonbreastfeeding populations have demonstrated that AZT initiated as early as 12 weeks antepartum, with single-dose NVP at onset of labor, reduces transmission to 1% to 2%,23 as does highly active antiretroviral therapy24 or AZT delivered prior to an elective cesarean delivery.25,26 Successful implementation of these regimens is more complex than prophylaxis with a single drug.
The expert consultation panel of the World Health Organization (WHO) recently updated its guidelines for PMTCT to encourage the adoption of more-complex and -efficacious antiretroviral prophylaxis regimens, which may decrease the perceived risk of the development of virological resistance to NVP.27 The treatment of immunocompromised (WHO Stage 3 or 4) HIV-infected women is emphasized in the WHO guidelines. The criteria for initiation of antiretroviral therapy for pregnant women are the same as for nonpregnant women. Treating these women addresses their health, substantially decreases transmission to the infant, and minimizes the development of resistance to NVP from administration of single-dose NVP.
It is essential to expand access to PMTCT services into areas in which none currently exist and, whenever possible, to provide more-efficacious regimens. For many populations, an essential step for providing these services is access to single-dose NVP, with more-complicated regimens added incrementally. In many settings, single-dose NVP will continue to be the only feasible intervention in the immediate future.
Antiretroviral prophylaxis has proven efficacious in preventing mother-to-child transmission of HIV. Seven years have passed since the initiation PMTCT programs, but millions of new infections have occurred in infants. Only an estimated 34% of HIV-positive women in the world have access to PMTCT services.28 It is important to obtain the requisite data to substantiate different strategies while continuing to expand access to these services. A substantial decrease in infant HIV infections should be viewed as an achievable paradigm and an essential part of the continuum of care and support.
Acknowledgments
The Elizabeth Glaser Pediatric AIDS Foundation's prevention of mother-to-child transmission of HIV program appreciates the generous financial support of the US Agency for International Development, Johnson & Johnson, Boehringer Ingelheim, Jewelers for Children, the Bill and Melinda Gates Foundation, Oprah Winfrey Foundation, and Ronald McDonald House Charities. This article was made possible through the support of the Global Bureau's Center for Population, Health, and Nutrition of the US Agency for International Development under cooperative agreement GPH-A-00-02-00011-00 (Call To Action Project) with the Elizabeth Glaser Pediatric AIDS Foundation.
The Elizabeth Glaser Pediatric AIDS Foundation acknowledges the tireless efforts of its partners in the prevention of mother-to-child transmission in all 22 countries; their work made this article possible. The authors thank Lucy Alcalá, Brenda Nerima, Francois Dabis, and Thomas Welty for their contributions and helpful comments.
Note. The opinions expressed are those of the authors and do not necessarily reflect the views of the US Agency for International Development.
Human Participant Protection
No protocol approval was needed for this study.
References
- 1.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795–802 [DOI] [PubMed] [Google Scholar]
- 2.Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS 1999;13:479–486 [DOI] [PubMed] [Google Scholar]
- 3.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353:773–780 [DOI] [PubMed] [Google Scholar]
- 4.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire: a randomised trial. Lancet 1999;353:781–785 [DOI] [PubMed] [Google Scholar]
- 5.Dabis F, Msellati P, Meda N, et al. Six-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. Diminution de la Transmission Mere-Enfant Lancet 1999;353:786–792 [DOI] [PubMed] [Google Scholar]
- 6.Boerhringer Ingelheim VIRAMUNE Donation Programme for the Prevention of Mother-to-Child Transmission of HIV-1. Available at: http://www.pmtctdonations.org. Accessed December 9, 2005
- 7.The Elizabeth Glaser Pediatric AIDS Foundation. About us section of Web site. Available at: http://www.pedaids.org/AboutUs.aspx. Accessed March 14, 2008.
- 8.Kakute PN, Ngum J, Mitchell P, et al. Cultural barriers to exclusive breastfeeding by mothers in a rural area of Cameroon, Africa. J Midwifery Womens Health 2005;50:324–328 [DOI] [PubMed] [Google Scholar]
- 9.Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS 2003;17:1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welty TK, Bulterys M, Welty ER, et al. Integrating prevention of mother-to-child HIV transmission into routine antenatal care: the key to program expansion in Cameroon. J Acquir Immune Defic Syndr 2005;40:486–493 [DOI] [PubMed] [Google Scholar]
- 11.Perez F, Orne-Gliemann J, Mukotekwa T, et al. Prevention of mother to child transmission of HIV: evaluation of a pilot programme in a district hospital in rural Zimbabwe. BMJ 2004;329:1147–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler MG, Newell ML. Breast feeding and HIV-1 transmission in resource limited settings. J Acquir Immune Defic Syndr 2002;30:230–239 [DOI] [PubMed] [Google Scholar]
- 13.Malonza IM, Richardson BA, Kreiss JK, Bwayo JJ, Stewart GC. The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial. AIDS 2003;17:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsburg AS, Miller A, Wilfert CM. Diagnosis of pediatric human immunodeficiency virus infection in resource-constrained settings. Pediatr Infect Dis J 2006;25:1057–1064 [DOI] [PubMed] [Google Scholar]
- 15.Bulterys M, Fowler MG, Shaffer N, et al. Role of traditional birth attendants in preventing perinatal HIV transmission of HIV. BMJ 2002;324:222–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Msaky H, Kironde S, Shuma J, Nzima M, Mlay V, Reeler A. Scaling the frontier: traditional birth attendant involvement in PMTCT service delivery in Hai and Kilombero districts of Tanzania. ; Bangkok, Thailand: Abstract ThPeE8084 [Google Scholar]
- 17.Zimba C, Kamanga E, Chilongozi D, et al. Impact of routine counseling and testing with an opt out strategy compared to voluntary counseling and testing in the implementation of PMTCT Lilongwe, Malawi. ; Toronto, Ontario: Abstract WAE0104 [Google Scholar]
- 18.Sripipatana T, Spensley A, Miller A, et al. Site specific interventions to improve prevention of mother-to-child transmission of HIV programs in less developed settings. Am J Obstet Gynecol 2007;197(3 suppl):S107–S112 [DOI] [PubMed] [Google Scholar]
- 19.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child transmission in Lusaka Zambia. AIDS 2005;19:1309–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson JB, Parsons T, Musoke P, et al. Association of cord blood nevirapine concentration with reported timing of dose and HIV-1 transmission. AIDS 2006;20:217–222 [DOI] [PubMed] [Google Scholar]
- 21.Boerhringer Ingelheim Guidelines for the administration of Viramune 200mg tablets and 50mg/5mL oral suspension for use in the prevention of mother to child transmission (pMTCT) of HIV-1. Available at: http://www.pmtctdonations.org/ftp/Guidelines%20use%20of%20VIRAMUNE-EN.pdf. Accessed July 30, 2008
- 22.Kagaayi J, Dreyfuss ML, Kigozi G, et al. Maternal self-medication and provision of nevirapine to newborns by women in Rakai, Uganda. J Acquir Immune Defic Syndr 2005;39:121–124 [DOI] [PubMed] [Google Scholar]
- 23.Lallemant M, Jourdain G, LeCoeur S, et al. Single dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 2004;351:217–228 [DOI] [PubMed] [Google Scholar]
- 24.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA 2002;288:189–198 [DOI] [PubMed] [Google Scholar]
- 25.Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA 1998;280:55–60 [DOI] [PubMed] [Google Scholar]
- 26.The International Perinatal HIV Group The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med 1999;340(13):977–987 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infections in Infants in Resource-Limited Settings: Toward Universal Access. 2006. Available at: http://www.who.int/hiv/pub/guidelines/WHOPMTCT.pdf. Accessed September 25, 2006.
- 28.UNAIDS. 2008 Report on the Global AIDS Epidemic. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. Accessed August 11, 2008.