Abstract
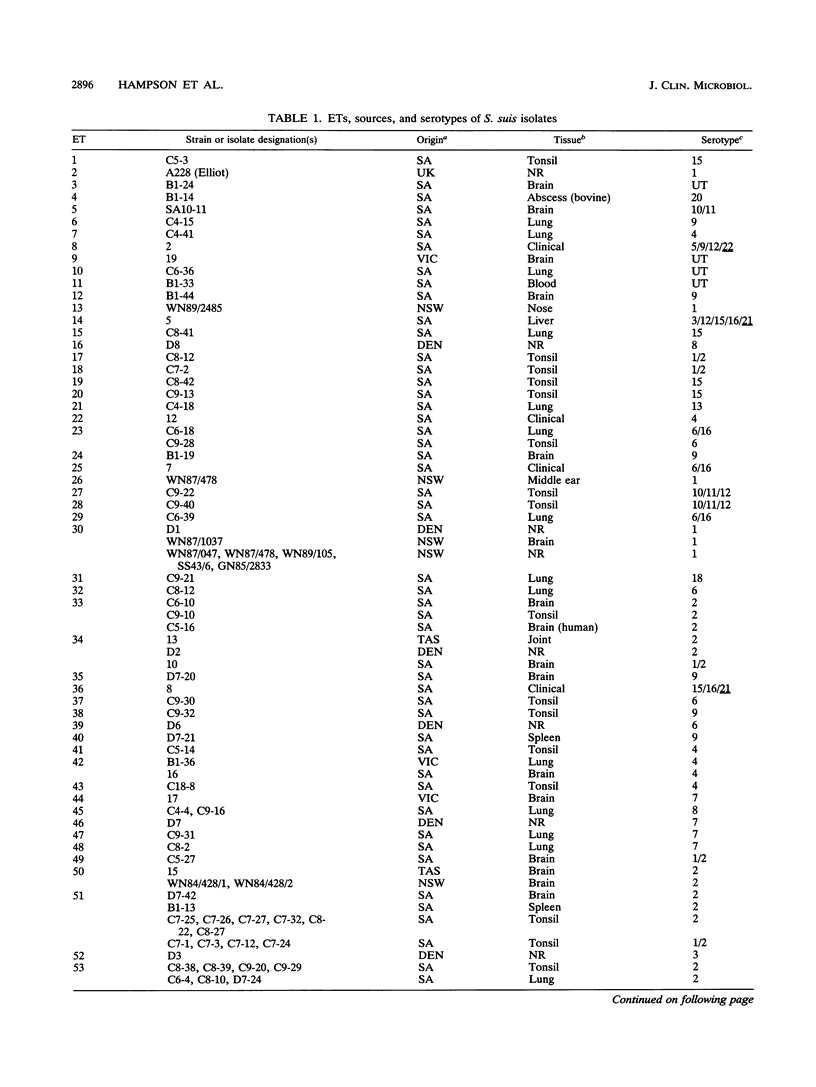
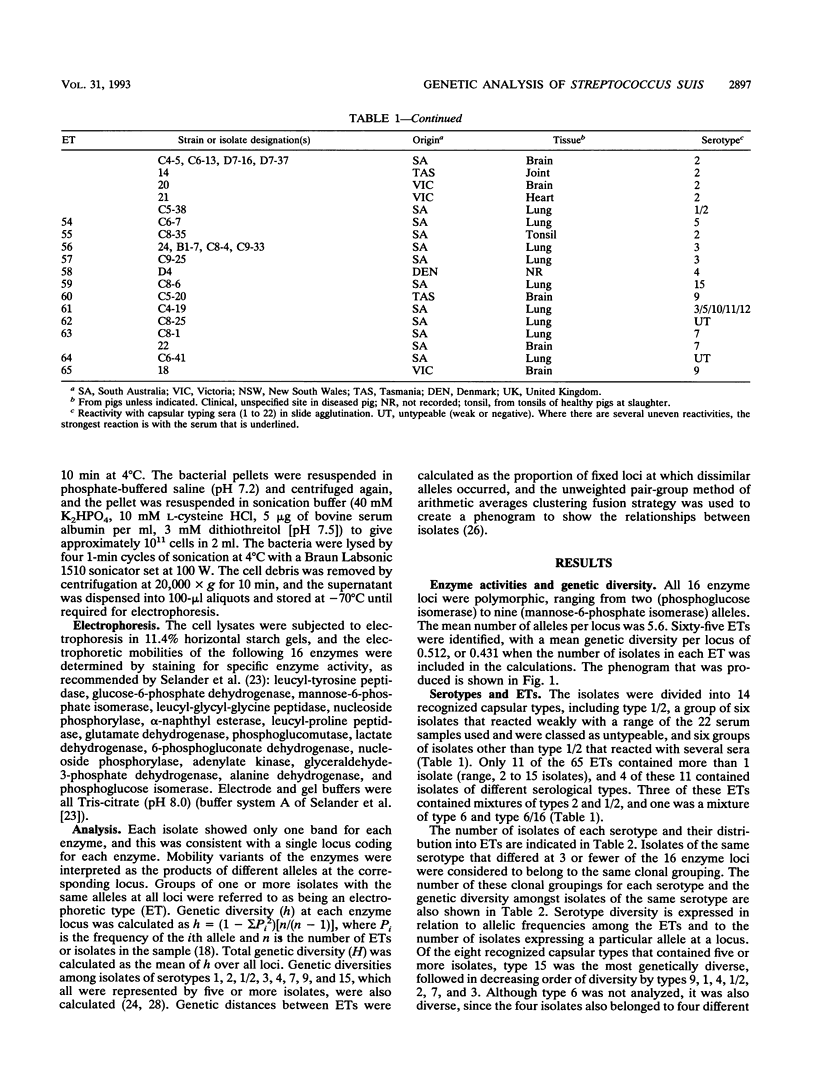
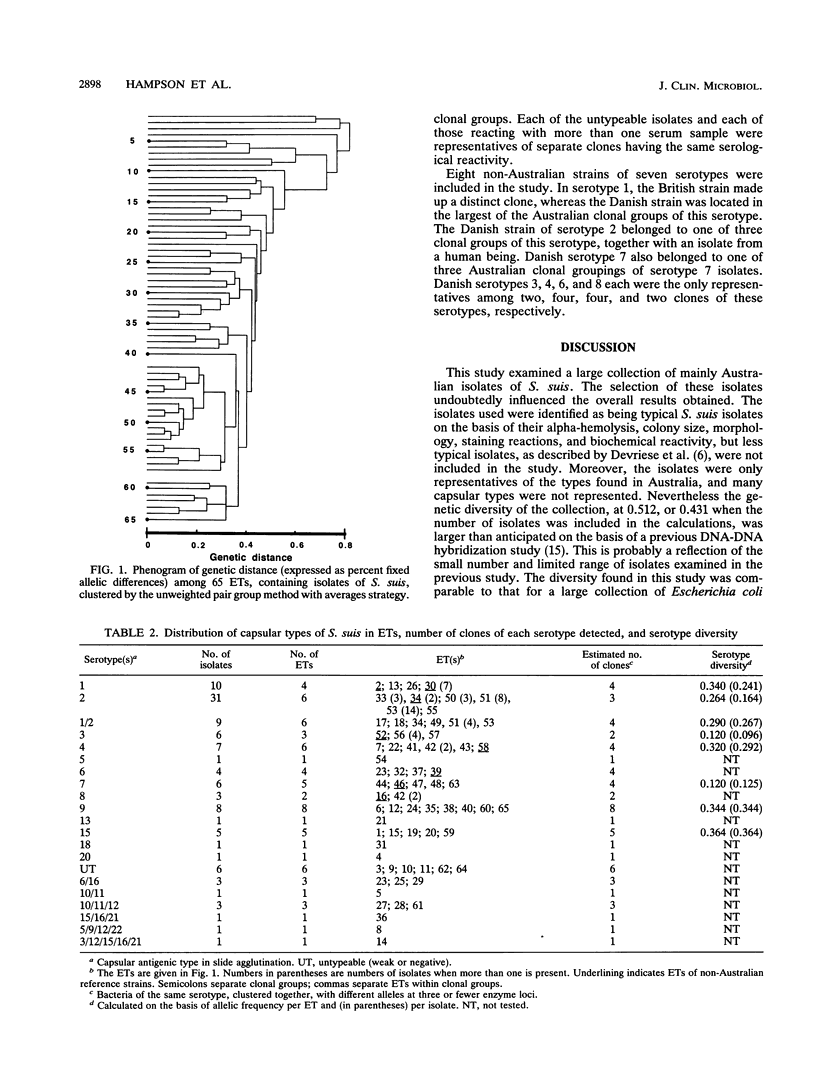
The genetic diversity of 109 isolates of Streptococcus suis, which were recovered mainly from Australian pigs, was examined by multilocus enzyme electrophoresis. The collection was genetically diverse. Sixty-five electrophoretic types (ETs) were recognized, with a mean genetic diversity per enzyme locus of 0.512, or 0.431 when the number of isolates in each ET was considered. Serotype diversity varied, being greatest for isolates of capsular serotype 15 (0.364), and then diminishing in the order of serotypes 9, 1, 4, 1/2, 2, 7, and 3 (0.120). On average, isolates from these eight serotypes represented 4.13 separate clonal groups per serotype. This diversity indicated that serotyping of S. suis for subspecific differentiation is not a reliable technique for identifying specific strains and is not a good predictor of the genetic background of a given isolate. No tendency for isolates recovered from healthy pigs to be genetically distinct from those from diseases animals was found, nor were there consistent differences between isolates recovered from animals with different disease syndromes (meningitis, pneumonia, and septicemia). Danish reference strains of serotypes 1, 2, and 7 each belonged to one of the same clonal groupings of these types found in Australia, but Danish strains of serotypes 3, 4, 6, and 8 and a strain of serotype 1 from the United Kingdom were each genetically distinct from the Australian isolates. Generally, isolates in the same ET belonged to the same serotype, but one ET contained isolates of types 6 and 6/16, and three were made up of isolates of types 2 and 1/2. One isolate of serotype 2, which was recovered from a human with meningitis, belonged to the same ET as two isolates of serotype 2 that were recovered from pigs. The human infection was therefore likely to have been zoonotic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J. P., Zanen H. C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988 Jan-Feb;10(1):131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Beaudoin M., Harel J., Higgins R., Gottschalk M., Frenette M., MacInnes J. I. Molecular analysis of isolates of Streptococcus suis capsular type 2 by restriction-endonuclease-digested DNA separated on SDS-PAGE and by hybridization with an rDNA probe. J Gen Microbiol. 1992 Dec;138(12):2639–2645. doi: 10.1099/00221287-138-12-2639. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley F. A., Alexander T. J. The carrier site and carrier rate of Streptococcus suis type II in pigs. Vet Rec. 1980 Jul 12;107(2):40–41. doi: 10.1136/vr.107.2.40. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley F. A., Alexander T. J., Upton I., Duffus W. P. Further studies on the subclinical carrier state of Streptococcus suis type 2 in pigs. Vet Rec. 1984 May 26;114(21):513–518. doi: 10.1136/vr.114.21.513. [DOI] [PubMed] [Google Scholar]
- Devriese L. A., Desmidt M., Roels S., Hoorens J., Haesebrouck F. Streptococcus suis infection in fallow deer. Vet Rec. 1993 Mar 13;132(11):283–283. doi: 10.1136/vr.132.11.283. [DOI] [PubMed] [Google Scholar]
- Devriese L. A., Sustronck B., Maenhout T., Haesebrouck F. Streptococcus suis meningitis in a horse. Vet Rec. 1990 Jul 21;127(3):68–68. [PubMed] [Google Scholar]
- Gogolewski R. P., Cook R. W., O'Connell C. J. Streptococcus suis serotypes associated with disease in weaned pigs. Aust Vet J. 1990 Jun;67(6):202–204. doi: 10.1111/j.1751-0813.1990.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Gottschalk M., Higgins R., Jacques M., Beaudoin M., Henrichsen J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J Clin Microbiol. 1991 Nov;29(11):2590–2594. doi: 10.1128/jcm.29.11.2590-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk M., Higgins R., Jacques M., Mittal K. R., Henrichsen J. Description of 14 new capsular types of Streptococcus suis. J Clin Microbiol. 1989 Dec;27(12):2633–2636. doi: 10.1128/jcm.27.12.2633-2636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Blackall P. J., Woodward J. M., Lymbery A. J. Genetic analysis of Actinobacillus pleuropneumoniae, and comparison with Haemophilus spp. Taxon "minor group" and Taxon C. Zentralbl Bakteriol. 1993 Jun;279(1):83–91. doi: 10.1016/s0934-8840(11)80494-9. [DOI] [PubMed] [Google Scholar]
- Higgins R., Gottschalk M., Beaudoin M., Rawluk S. A. Distribution of Streptococcus suis capsular types in Quebec and western Canada. Can Vet J. 1992 Jan;33(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- Hommez J., Wullepit J., Cassimon P., Castryck F., Ceyssens K., Devriese L. A. Streptococcus suis and other streptococcal species as a cause of extramammary infection in ruminants. Vet Rec. 1988 Dec 10;123(24):626–627. [PubMed] [Google Scholar]
- Lee J. I., Hampson D. J., Combs B. G., Lymbery A. J. Genetic relationships between isolates of Serpulina (Treponema) hyodysenteriae, and comparison of methods for their subspecific differentiation. Vet Microbiol. 1993 Jan;34(1):35–46. doi: 10.1016/0378-1135(93)90005-r. [DOI] [PubMed] [Google Scholar]
- Mogollon J. D., Pijoan C., Murtaugh M. P., Kaplan E. L., Collins J. E., Cleary P. P. Characterization of prototype and clinically defined strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1990 Nov;28(11):2462–2466. doi: 10.1128/jcm.28.11.2462-2466.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowicz C. J., Pointon A. M., Davies P. R. Streptococcus suis isolated from pigs in South Australia. Aust Vet J. 1989 Nov;66(11):377–378. doi: 10.1111/j.1751-0813.1989.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Perch B., Pedersen K. B., Henrichsen J. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J Clin Microbiol. 1983 Jun;17(6):993–996. doi: 10.1128/jcm.17.6.993-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. D., Blackmore D. K. Prevalence of Streptococcus suis types 1 and 2 in domestic pigs in Australia and New Zealand. Vet Rec. 1989 Apr 15;124(15):391–394. doi: 10.1136/vr.124.15.391. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Sihvonen L., Kurl D. N., Henrichsen J. Streptococcus suis isolated from pigs in Finland. Acta Vet Scand. 1988;29(1):9–13. doi: 10.1186/BF03548386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecht U., Wisselink H. J., van Dijk J. E., Smith H. E. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect Immun. 1992 Feb;60(2):550–556. doi: 10.1128/iai.60.2.550-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. M., Connaughton I. D., Fahy V. A., Lymbery A. J., Hampson D. J. Clonal analysis of Escherichia coli of serogroups O9, O20, and O101 isolated from Australian pigs with neonatal diarrhea. J Clin Microbiol. 1993 May;31(5):1185–1188. doi: 10.1128/jcm.31.5.1185-1188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


