Abstract
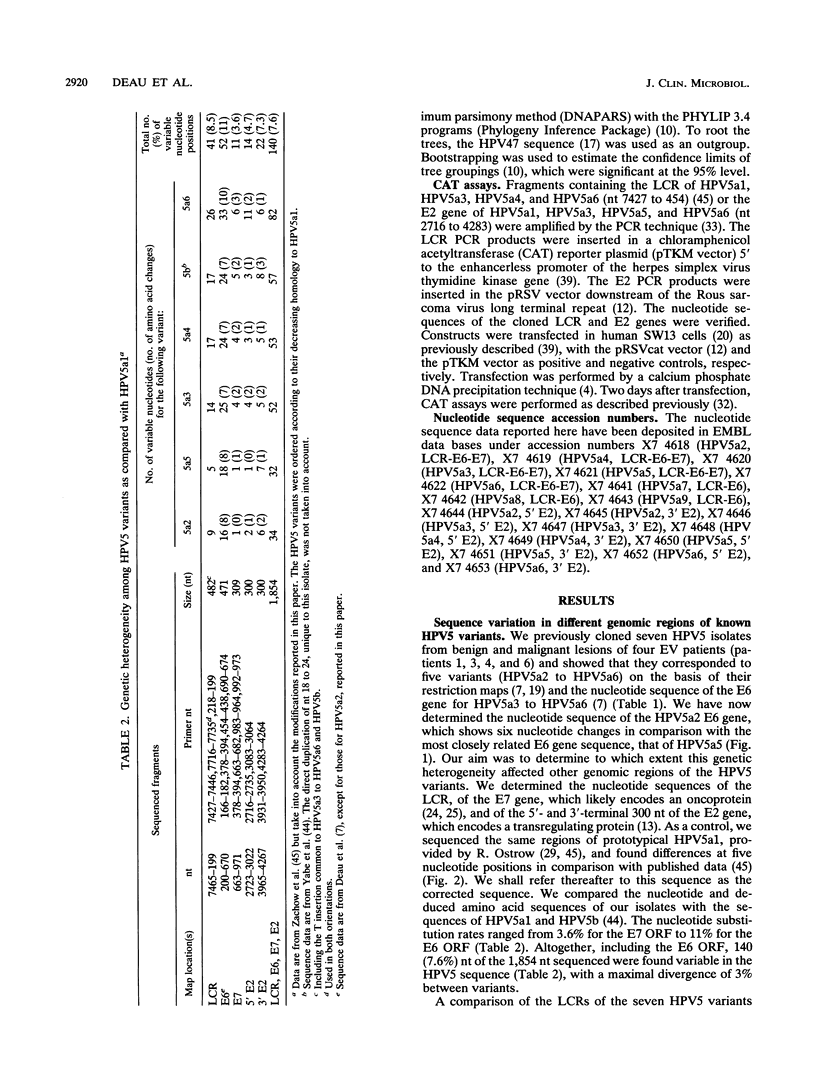
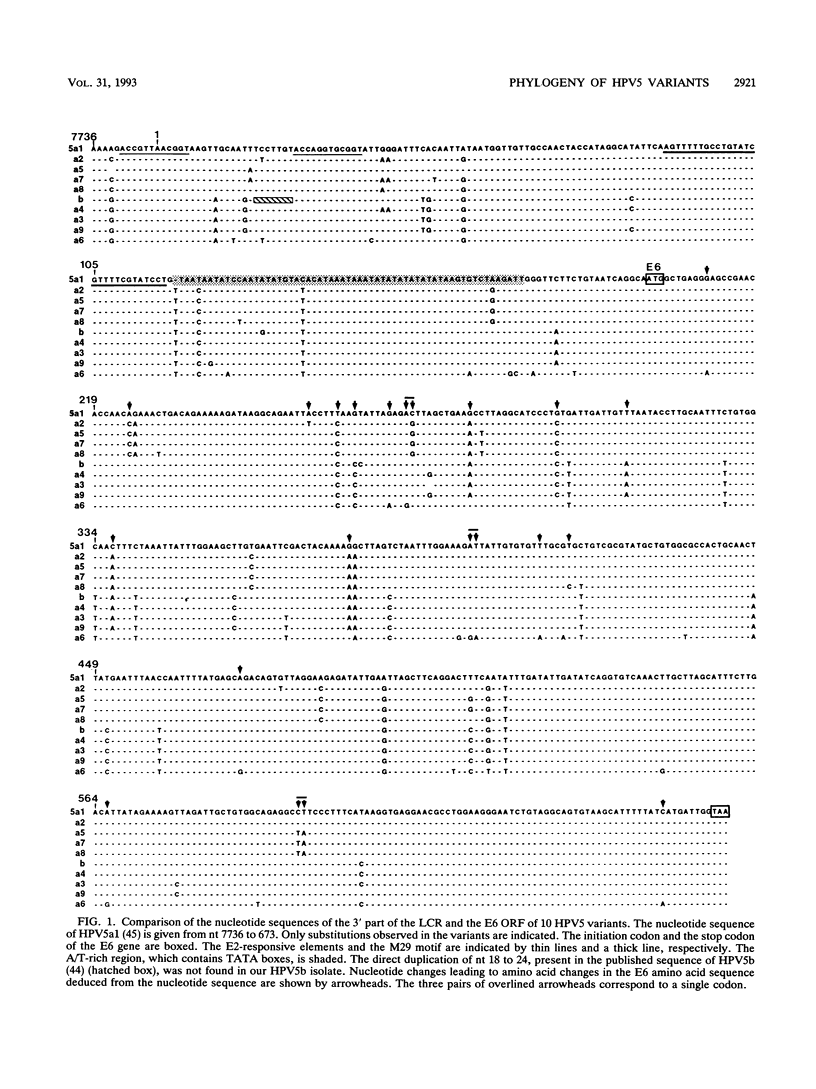
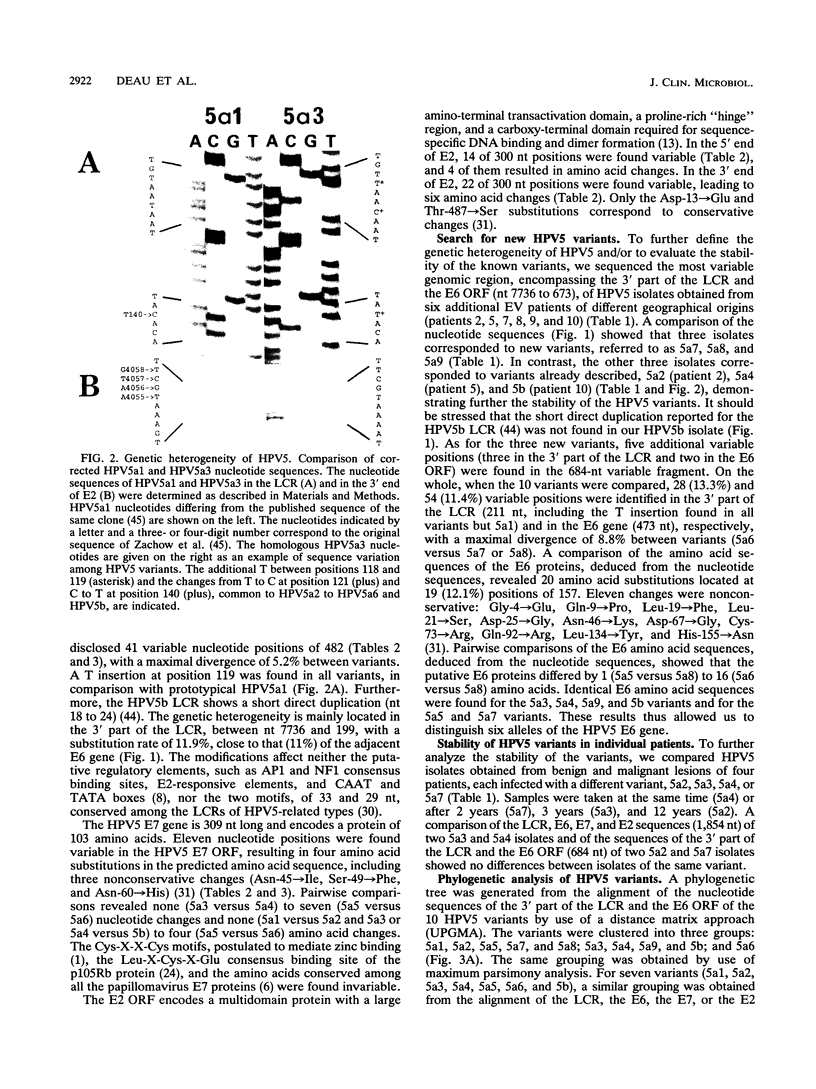
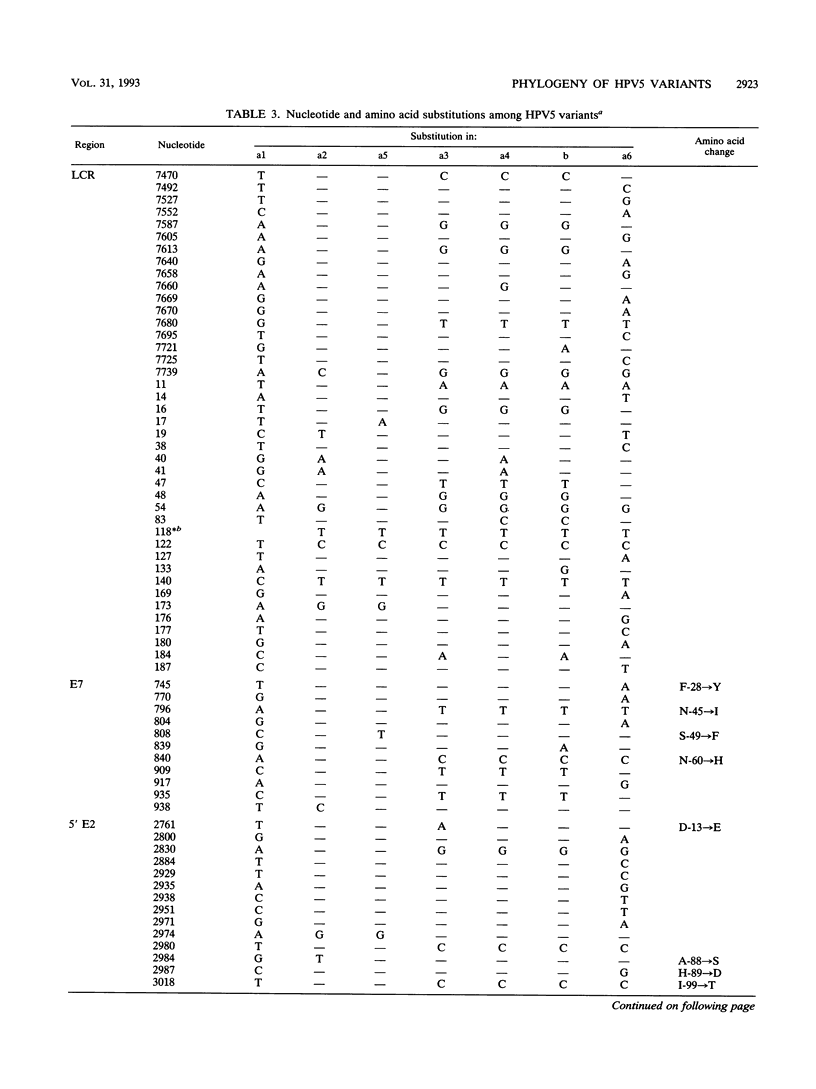
Variants of oncogenic human papillomavirus type 5 (HPV5), specifically associated with epidermodysplasia verruciformis, were recognized on the basis of the genetic heterogeneity of the E6 open reading frame (ORF). To further evaluate the genetic heterogeneity of HPV5, we sequenced the long control region (LCR), the E7 ORF, and the terminal parts of the E2 ORF of five previously characterized HPV5 variants and compared the data with the published HPV5a1 and HPV5b sequences. Alignment of the variants showed 140 (7.6%) variable nucleotides of 1,854 sequenced. Nucleotide substitution rates varied from 3.6% in the E7 ORF to 11% in the E6 ORF. By sequencing the variable region encompassing the LCR 3' part and the E6 ORF of isolates from six additional epidermodysplasia verruciformis patients, we identified three new variants and three already known variants, indicating the stability of HPV5 variants. This stability was further demonstrated by the identity of isolates obtained years later from benign and malignant lesions of three patients. Phylogenetic analysis of the 10 HPV5 variants distributed them into three groups, tentatively defining subtypes a, b, and c. The phylogenetic grouping shows no geographical dependence, a fact that may be related to the host restriction that characterizes HPV5 infections. No differences in the enhancer potential of the LCR or in the transactivating properties of the E2 protein assayed in vitro were observed among HPV5 variants. Whether HPV5 variants possess distinct biological properties in vivo remains to be determined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. S., Lowy D. R., Schiller J. T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989 Mar;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr B. B., Benton E. C., McLaren K., Bunney M. H., Smith I. W., Blessing K., Hunter J. A. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989 Jan 21;1(8630):124–129. doi: 10.1016/s0140-6736(89)91143-4. [DOI] [PubMed] [Google Scholar]
- Chan S. Y., Ho L., Ong C. K., Chow V., Drescher B., Dürst M., ter Meulen J., Villa L., Luande J., Mgaya H. N. Molecular variants of human papillomavirus type 16 from four continents suggest ancient pandemic spread of the virus and its coevolution with humankind. J Virol. 1992 Apr;66(4):2057–2066. doi: 10.1128/jvi.66.4.2057-2066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987 Feb 20;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Ensser A., Pfister H. Epidermodysplasia verruciformis associated human papillomaviruses present a subgenus-specific organization of the regulatory genome region. Nucleic Acids Res. 1990 Jul 11;18(13):3919–3922. doi: 10.1093/nar/18.13.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschle D., Dürst M., ter Meulen J., Luande J., Eberhardt H. C., Pawlita M., Gissmann L. Geographical dependence of sequence variation in the E7 gene of human papillomavirus type 16. J Gen Virol. 1992 Jul;73(Pt 7):1829–1832. doi: 10.1099/0022-1317-73-7-1829. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham J., Dostatni N., Gauthier J. M., Yaniv M. The papillomavirus E2 protein: a factor with many talents. Trends Biochem Sci. 1991 Nov;16(11):440–444. doi: 10.1016/0968-0004(91)90172-r. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Icenogle J. P., Sathya P., Miller D. L., Tucker R. A., Rawls W. E. Nucleotide and amino acid sequence variation in the L1 and E7 open reading frames of human papillomavirus type 6 and type 16. Virology. 1991 Sep;184(1):101–107. doi: 10.1016/0042-6822(91)90826-w. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Favre M., Obalek S., Jablonska S., Orth G. Premalignant lesions and cancers of the skin in the general population: evaluation of the role of human papillomaviruses. J Invest Dermatol. 1990 Nov;95(5):537–542. doi: 10.1111/1523-1747.ep12504887. [DOI] [PubMed] [Google Scholar]
- Kiyono T., Adachi A., Ishibashi M. Genome organization and taxonomic position of human papillomavirus type 47 inferred from its DNA sequence. Virology. 1990 Jul;177(1):401–405. doi: 10.1016/0042-6822(90)90500-q. [DOI] [PubMed] [Google Scholar]
- Kremsdorf D., Favre M., Jablonska S., Obalek S., Rueda L. A., Lutzner M. A., Blanchet-Bardon C., Van Voorst Vader P. C., Orth G. Molecular cloning and characterization of the genomes of nine newly recognized human papillomavirus types associated with epidermodysplasia verruciformis. J Virol. 1984 Dec;52(3):1013–1018. doi: 10.1128/jvi.52.3.1013-1018.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsdorf D., Jablonska S., Favre M., Orth G. Biochemical characterization of two types of human papillomaviruses associated with epidermodysplasia verruciformis. J Virol. 1982 Aug;43(2):436–447. doi: 10.1128/jvi.43.2.436-447.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitz A., McCombs W. M., 3rd, Johnston D., McCoy C. E., Stinson J. C. New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst. 1973 Aug;51(2):691–697. [PubMed] [Google Scholar]
- Lutzner M. A., Blanchet-Bardon C., Orth G. Clinical observations, virologic studies, and treatment trials in patients with epidermodysplasia verruciformis, a disease induced by specific human papillomaviruses. J Invest Dermatol. 1984 Jul;83(1 Suppl):18s–25s. doi: 10.1111/1523-1747.ep12281128. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Orth G., Dutronquay V., Ducasse M. F., Kreis H., Crosnier J. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983 Aug 20;2(8347):422–424. doi: 10.1016/s0140-6736(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Mathieu A., Avril M. F., Duvillard P., Blanchet-Bardon C., Orth G., Barranger C., Bognel C., Charpentier P., Girinsky T., Prade M. Epidermodysplasie verruciforme et papillomavirus: deux observations. Présence d'HPV 5 dans une métastase ganglionnaire. Ann Dermatol Venereol. 1985;112(9):741–742. [PubMed] [Google Scholar]
- Münger K., Scheffner M., Huibregtse J. M., Howley P. M. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- Nishikawa T., Yamashita T., Yamada T., Kobayashi H., Ohkawara A., Fujinaga K. Tumorigenic transformation of primary rat embryonal fibroblasts by human papillomavirus type 8 E7 gene in collaboration with the activated H-ras gene. Jpn J Cancer Res. 1991 Dec;82(12):1340–1343. doi: 10.1111/j.1349-7006.1991.tb01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obalek S., Favre M., Szymanczyk J., Misiewicz J., Jablonska S., Orth G. Human papillomavirus (HPV) types specific of epidermodysplasia verruciformis detected in warts induced by HPV3 or HPV3-related types in immunosuppressed patients. J Invest Dermatol. 1992 Jun;98(6):936–941. doi: 10.1111/1523-1747.ep12460892. [DOI] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Jarzabek-Chorzelska M., Obalek S., Rzesa G., Favre M., Croissant O. Characteristics of the lesions and risk of malignant conversion associated with the type of human papillomavirus involved in epidermodysplasia verruciformis. Cancer Res. 1979 Mar;39(3):1074–1082. [PubMed] [Google Scholar]
- Ostrow R. S., Bender M., Niimura M., Seki T., Kawashima M., Pass F., Faras A. J. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh H., Pfister H. Human papillomavirus type 8 contains cis-active positive and negative transcriptional control sequences. J Gen Virol. 1990 Oct;71(Pt 10):2457–2462. doi: 10.1099/0022-1317-71-10-2457. [DOI] [PubMed] [Google Scholar]
- Risler J. L., Delorme M. O., Delacroix H., Henaut A. Amino acid substitutions in structurally related proteins. A pattern recognition approach. Determination of a new and efficient scoring matrix. J Mol Biol. 1988 Dec 20;204(4):1019–1029. doi: 10.1016/0022-2836(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Rogel-Gaillard C., Breitburd F., Orth G. Human papillomavirus type 1 E4 proteins differing by their N-terminal ends have distinct cellular localizations when transiently expressed in vitro. J Virol. 1992 Feb;66(2):816–823. doi: 10.1128/jvi.66.2.816-823.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M. H. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 1992 Mar 18;84(6):394–398. doi: 10.1093/jnci/84.6.394. [DOI] [PubMed] [Google Scholar]
- Tawheed A. R., Beaudenon S., Favre M., Orth G. Characterization of human papillomavirus type 66 from an invasive carcinoma of the uterine cervix. J Clin Microbiol. 1991 Nov;29(11):2656–2660. doi: 10.1128/jcm.29.11.2656-2660.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Dostatni N., Arnos F., Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990 Aug;10(8):4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987 Nov;6(11):3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ranst M., Kaplan J. B., Burk R. D. Phylogenetic classification of human papillomaviruses: correlation with clinical manifestations. J Gen Virol. 1992 Oct;73(Pt 10):2653–2660. doi: 10.1099/0022-1317-73-10-2653. [DOI] [PubMed] [Google Scholar]
- Van der Leest R. J., Zachow K. R., Ostrow R. S., Bender M., Pass F., Faras A. J. Human papillomavirus heterogeneity in 36 renal transplant recipients. Arch Dermatol. 1987 Mar;123(3):354–357. doi: 10.1001/archderm.123.3.354. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe Y., Sakai A., Hitsumoto T., Kato H., Ogura H. A subtype of human papillomavirus 5 (HPV-5b) and its subgenomic segment amplified in a carcinoma: nucleotide sequences and genomic organizations. Virology. 1991 Aug;183(2):793–798. doi: 10.1016/0042-6822(91)91013-7. [DOI] [PubMed] [Google Scholar]
- Zachow K. R., Ostrow R. S., Faras A. J. Nucleotide sequence and genome organization of human papillomavirus type 5. Virology. 1987 May;158(1):251–254. doi: 10.1016/0042-6822(87)90263-7. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]




