Abstract
Gastric cancer is a common malignancy worldwide with a high mortality rate. Multiple single agents have produced response rates ranging from 5% to 45% in patients with metastatic gastric cancer. Combination chemotherapy regimens with two or three agents have more than doubled survivorship compared with best supportive care. Recent clinical trials include the evaluation of docetaxel, cisplatin, and 5-fluorouracil (5-FU), showing a survival advantage compared with cisplatin and 5-FU, although with high rates of toxicity, particularly neutropenia and neutropenic fever. The REAL-2 trial evaluated epirubicin and cisplatin with either infusional 5-FU (ECF) or capecitabine (ECX), and epirubicin and oxaliplatin with either infusional 5-FU (EOF) or capecitabine (EOX); results favored the EOX regimen. Oxaliplatin-containing triplets appear to have a favorable safety profile compared with cisplatin-containing triplets. Additional randomized data are available for the oral fluoropyrimidine, S-1. In a randomized trial comparing 5-FU vs. irinotecan plus cisplatin vs. S-1, conducted by the Japan Clinical Oncology Group, S-1 was associated with a favorable safety profile, response rate, and time-to-treatment failure with longer survival compared with 5-FU. The Japanese SPIRITS trial was a phase III comparison of S-1 vs. S-1 plus cisplatin and showed favorable 1- and 2-year survivals and a progression-free survival advantage for S-1 and cisplatin. Cooperative group strategies are evaluating the role of chemotherapy combinations, including cisplatin and docetaxel with biologic agents. In addition, there is interest in developing oxaliplatin-based combinations. The expanding list of potential molecular markers for gastric cancer may provide the opportunity to better understand the biology of the disease and to develop new treatment strategies.
Although of relatively low incidence in the United States, gastric cancer is a common disease worldwide with a high mortality rate because of the late stage at diagnosis in many cases and high relapse rates. Gastric cancer is clearly a complex disease, with many clinical, pathologic, and molecular/genetic features. The expanding list of potential molecular markers for gastric cancer provides the opportunity to better understand the biology of the disease and to develop new treatment strategies.
PROGNOSTIC/PREDICTIVE FACTORS
Examples of important clinical and pathologic features that can affect prognosis, for example, include age, sex, primary site (distal one-third, middle one-third, gastroesophageal junction, and proximal onethird), Lauren histotype (diffuse, intestinal, mixed), number of lymph nodes resected, number of negative lymph nodes resected, and depth of invasion (Table 1).1
Table 1.
Gastric Cancer Prognostic Factors
| Clinical | Pathologic | Molecular/Genetic |
|---|---|---|
| Sex | Stage (many systems)
AJCC/pTNM Lauren/Ming MI Borrman |
VEGF/VEGF-C expression |
| Primary Site | DNA-repair error | |
| Antrum or pyloric | K-ras mutation | |
| Body or middle 1/3 | c-met amplification | |
| GE junction | K-sam | |
| Depth | Histological type
Diffuse Intestinal Mixed Not available |
C-erb B-2 amplification |
| Tumor localization | EGFR | |
| Lymph nodes | EGF | |
| Venous invasion | TGF alpha | |
| Neovascularization | VEGF | |
| Size | Differentiation (grade) | p53 LOH |
| Presence of bone marrow micro-metastases | p53 (mutation) | |
| Presence of Helicobacter pylori | APC LOH | |
| R classification | uPA system | |
| L/V classification | E-cadherin | |
| Disseminated epithelial cells |
Abbreviations: AJCC = American Joint Committee on Cancer; APC = adenomatous polyposis coli gene; EGF = epidermal growth factor; EGFR = EGF receptor; GE = gastroesophageal; LOH = loss of heterozygosity; L/V = lymphatic/vascular; MI = Maruyama Index; pTNM = pathologic tumor-node-metastasis stage; R = resection; TGF = transforming growth factor; uPA = urokinase-type plasminogen activator; VEGF = vascular endothelial growth factor.
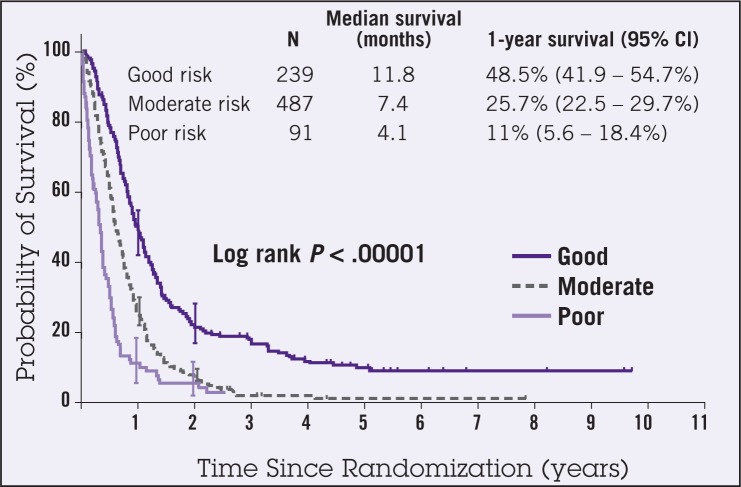
Chau et al performed a multivariate prognostic factor analysis of data obtained from 1,080 patients with advanced and metastatic esophagogastric cancer who participated in three multicenter randomized controlled trials.2 The regimens evaluated in the trials included ECF (epirubicin, cisplatin, 5-FU), FAMTX (5-FU, doxorubicin [Adriamycin], methotrexate), MCF (mitomycin, cisplatin, 5-FU), and protracted venous infusion 5-FU with or without mitomycin. The analysis identified four independent poor prognostic factors, including performance status, liver metastases, peritoneal metastases, and elevated alkaline phosphatase level. A prognostic index was created demonstrating significant differences in 1-year survival for good-, moderate-, and poor-risk groups (48.5%, 25.7%, and 11%, respectively [P < .00001]) (Figure 1). In addition, physical functioning, role functioning, and global quality of life had significant effect on overall prognosis. Patients were less likely to respond to chemotherapy if the performance status was ≥2, if peritoneal metastases were present, and if alkaline phosphatase measured ≥100 U/L (Table 2). Although an expanding list of various clinical, pathologic, and molecular/genetic prognostic and potentially predictive factors is emerging, outside of stage, optimal treatment strategies based on prognostic/predictive factors have yet to emerge.
Figure 1.
Overall survival by prognostic index (n=871). Abbreviation: CI = confidence interval. From Chau et al.2 Reprinted with permission from the American Society of Clinical Oncology.
Table 2.
Multivariate Baseline Prognostic Model
| Factors | Hazard Ratio | 99% CI | P |
|---|---|---|---|
| Performance status | |||
| 0–1 | 1 | ||
| 2–3 | 1.575 | 1.251 to 1.981 | < .0001 |
| Liver metastases | 1.409 | 1.139 to 1.743 | < .0001 |
| Peritoneal metastases | 1.329 | 1.013 to 1.743 | .007 |
| Alkaline phosphatase ≥ 100 U/L | 1.412 | 1.136 to 1.755 | < .0001 |
| Borderline significant factors | |||
| Hemoglobin ≤ 11 g/L | .011 | ||
| White blood cell count | .06 | ||
| Previous esophagectomy or gastrectomy | .054 | ||
Abbreviations: CI = confidence interval.
From Chau et al.2 Reprinted with permission from the American Society of Clinical Oncology.
Several presentations during the 2007 American Society of Clinical Oncology (ASCO) annual meeting focused on improving our understanding of esophageal and gastric adenocarcinoma biology. In the retrospective study of 184 patients with stage I through III gastric cancer, patients with the greatest risk of developing metastases had elevated carcinoembryonic antigen (CEA) levels, lymphovascular invasion, proximal localization, and extracapsular lymphatic extension.3
In another analysis, correlations between gene expression and survival time were reported in 40 patients. Gene expression profiling by microarray and real-time reverse-transcriptase polymerase chain reaction (RT-PCR) identified five genes that were related to survival.4 In an effort to define a relationship between single nucleotide polymorphisms (SNP) and efficacy of 5-FU and oxaliplatin in advanced gastric cancer, the XPD-C156A SNP showed clinical correlation both for response and toxicities.5
Four abstracts evaluated potential predictive markers for patients receiving the oral fluoropyrimidine, S-1. Park et al investigated the association between CYP2A6*9 genetic polymorphism and treatment outcomes for 50 patients who received S-1 plus docetaxel for metastatic gastric carcinoma, showing that the polymorphism is a potential predictive marker for efficacy and toxicity.6 A study of 151 patients receiving S-1 chemotherapy, including subsets of patients who also were treated with cisplatin with or without irinotecan, demonstrated that dihydropyrimidine dehydrogenase (DPD), epidermal growth factor receptor (EGFR), and excision repair cross-complementing gene 1 (ERCC1) gene expressions were correlated with survival, with the suggestion that low dihydrofolate reductase (DHFR) gene expression could be a predictor for prolonged time to progression in patients treated with S-1.7
Another exploratory study of 55 patients receiving S-1 monotherapy suggested that a 6-bp insertion/deletion in the thymidylate synthase 3′-untranslated region (TS 3′-UTR) was associated with tumor response and overall survival.8 Microarray analysis of patients on a phase I/II study of S-1 and irinotecan showed that the fibroblast growth factor (FGF) signaling pathway and nicotinate and nicotinamide metabolism pathway may predict response to the combination chemotherapy.9
A summary of other potential molecular targets for patients with adenocarcinoma of the esophagus has recently been featured in Gastrointestinal Cancer Research, including epidermal growth factor receptor (EGFR), HER-2/neu, vascular endothelial growth factor (VEGF), and cyclooxygenase 2 (COX-2), as well as genomic methylation and transcriptional profiles, chromosomal 1q21 alterations, transcription factor kappa B, and activation of embryonic sonic hedgehog signaling pathway.10
Most investigators believe that the best opportunity to advance treatment strategies for patients with adenocarcinoma of the esophagus, gastroesophageal junction, and stomach will evolve with improved understanding of tumor and host biology. The current trend in gastric cancer research is to incorporate laboratory investigations as correlates in treatment-based clinical trials, particularly in those evaluating a host of new biologic agents in an attempt to improve the benefits of combination chemotherapy.
RANDOMIZED CLINICAL TRIALS OF CHEMOTHERAPY FOR ADVANCED GASTRIC CANCER
Previous randomized clinical trial data have demonstrated that survival and quality of life are superior for advanced gastric cancer patients who receive chemotherapy compared with best supportive care.11 Over the years, a number of single-agent chemotherapy trials have confirmed that gastric cancer is a relatively “chemosensitive” disease. Based on these observations, the trend has been to investigate different combination chemotherapy regimens, both in the phase II and randomized phase III trial settings. The following summarizes some of the important phase III trials for patients with advanced gastroesophageal or gastric cancer, with an emphasis on the most recent reports.
The Earlier Era
Historically, there has been interest in anthracycline combination regimens for advanced gastric cancer. For example, the European Organisation for Research and Treatment of Cancer (EORTC) reported the comparison of FAM (5-FU, doxorubicin, and mitomycin C) vs. FAMTX (5-FU, doxorubicin, and methotrexate), which demonstrated an overall survival advantage favoring FAMTX (10.5 vs. 7.3 months).12,13 A comparison of FAMTX with EAP (etoposide, doxorubicin, cisplatin) showed no statistically significant survival advantage for either regimen.14 However, when compared with best supportive care, FAMTX resulted in a significant survival advantage (3 months vs. 10 months, respectively).15
The combination of 5-FU and cisplatin also emerged as a standard regimen for gastric cancer. A comparison of 5-FU vs. FAM vs. 5-FU and cisplatin showed a longer survival for 5-FU and cisplatin that was not significant. However, response rate and time to progression improvements significantly favored the cisplatin combination.16 Interest in ECF (epirubicin, cisplatin, 5-FU) resulted in a comparison of ECF with FAMTX, suggesting an advantage for the ECF regimen.17 This earlier era of randomized clinical trials concluded in 2000, with publication of the randomized phase III comparison of FAMTX vs. ELF (etoposide, leucovorin, 5-FU) vs. cisplatin and 5-FU. There was no advantage for any of the three regimens with respect to response and overall survival.18
Recent Chemotherapy Trials
Since 2005, combination chemotherapy trials for advanced gastric cancer have focused on the integration of other chemotherapy agents, including docetaxel, irinotecan, oxaliplatin, capecitabine, and S-1.
One of the most significant recently published trials for patients with advanced gastric cancer was reported by the V-325 Study Group, which conducted a randomized, multinational, phase III study of docetaxel (75 mg/m2, day 1) and cisplatin (75 mg/m2, day 1) plus fluorouracil (750 mg/m2 per day, days 1–5) given every 3 weeks (DCF), compared with cisplatin (100 mg/m2, day 1) and fluorouracil (1,000 mg/m2 per day, days 1–5) every 4 weeks (CF).19 The median follow-up was 13.6 months, at which time 77% of the 445 patients had progressed.
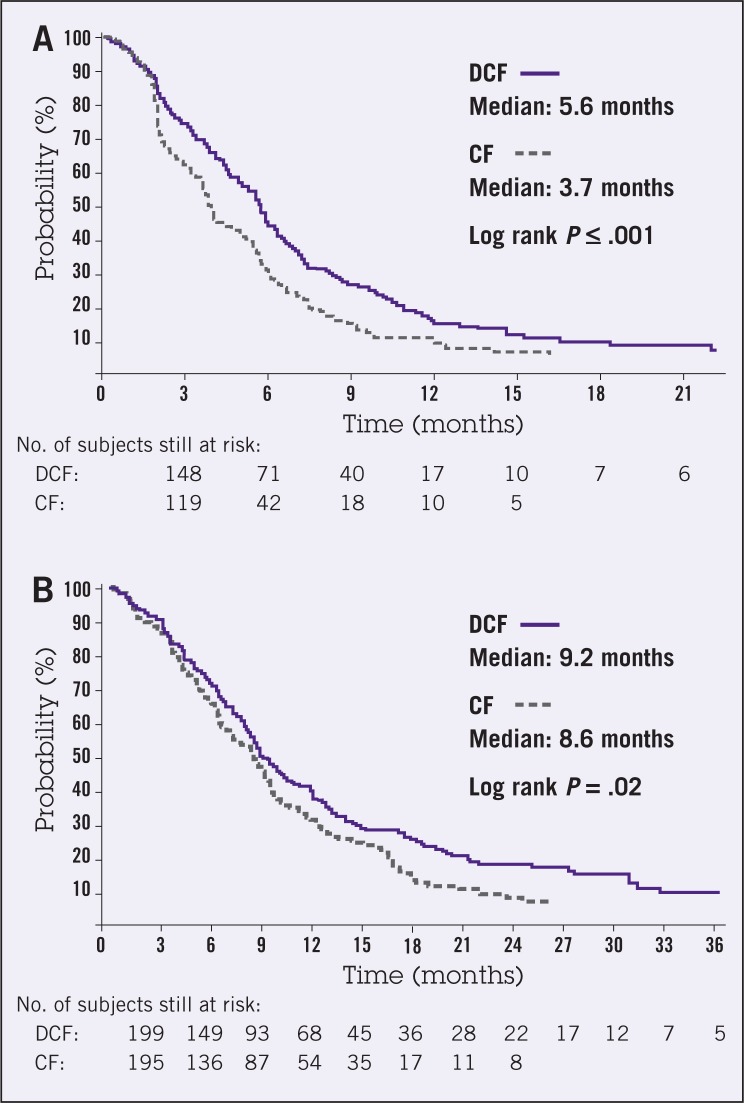
The primary end point of median time to progression (TTP) was significantly longer for DCF vs. CF (5.6 months vs. 3.7 months, respectively). Secondary end points included overall survival and response rates, both of which were superior for DCF (9.2 months vs. 8.6 months, P = .02; and 37% vs. 25%, P = .01, respectively) (Figure 2). Grade 3 or 4 treatmentrelated adverse events were more common for patients receiving DCF than CF (69% vs. 59%, respectively). In particular, grade 3/4 neutropenia was more frequent with DCF, and febrile neutropenia or neutropenic infection also was more likely to occur with DCF (29% vs. 12%, respectively). The other most frequent grade 3/4 toxicities included stomatitis, diarrhea, and lethargy.
Figure 2.
Kaplan-Meier estimates of (A) time to progression and (B) overall survival among chemotherapy-naive advanced gastric cancer patients treated with docetaxel, cisplatin, and fluorouracil (DCF) or cisplatin and fluorouracil (CF; full analysis population). From Van Cutsem et al.19 Reprinted with permission from the American Society of Clinical Oncology.
In addition, a clinical benefit analysis conducted by the V-325 Study Group showed that pain-free survival and time-to-first-cancer pain were comparable between the two groups. There was a statistically insignificant trend favoring DCF when patients were analyzed for weight loss and worsening of appetite; however, DCF significantly prolonged the time to definite worsening of Karnofsky performance status (KPS) (6.1 vs. 4.8 months, P = .009, respectively).20
Another analysis evaluated global quality of life measures, demonstrating that DCF resulted in a longer time to 5% deterioration in global quality of life compared with CF (6.5 vs. 4.2 months, respectively).21,22
The Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom recently published the REAL-2 Study, which used a 2 × 2 design to evaluate epirubicin and cisplatin plus either 5-FU (ECF) or capecitabine (ECX), or epirubicin and oxaliplatin plus either 5-FU (EOF) or capecitabine (EOX) in 1,002 patients.23 The primary end point was non-inferiority in overall survival for the regimens containing capecitabine compared with 5-FU and for those containing oxaliplatin compared with cisplatin. The prescribed doses included epirubicin (50 mg/m2, day 1), cisplatin (60 mg/m2, day 1), oxaliplatin (130 mg/m2, day 1), 5-FU (200 mg/m2 as a continuous infusion), and capecitabine (620 mg/m2 bid). Each regimen was repeated every 3 weeks.
Median overall survival durations for patients receiving ECF, ECX, EOF, and EOX were 9.9, 9.9, 9.3, and 11.2 months, respectively. Progression-free survival and response rates were similar among the regimens. A secondary analysis suggested that overall survival was longer with EOX compared with ECF (P= .02). The fluoropyrimidine regimens produced similar toxicity profiles; however, oxaliplatin compared with cisplatin demonstrated less grade 3 or 4 renal toxicity, thromboembolism, neutropenia, and alopecia, although slightly more diarrhea and neuropathy. The authors stated that EOX would provide an appropriate chemotherapy platform for incorporation of targeted agents in future advanced gastric cancer trials.
A recently published German (Arbeitsgemeinschaft Internistische Onkologie [AIO]) randomized phase III trial for patients with metastatic gastroesophageal adenocarcinoma evaluated 5-FU and leucovorin with either oxaliplatin or cisplatin.24 The regimens included 5-FU (2,600 mg/m2 via 24-hour infusion), leucovorin (200 mg/m2), and oxaliplatin (85 mg/m2) administered every 2 weeks, or 5-FU (2,000 mg/m2 via 24-infusion), leucovorin (200 mg/m2 weekly), and cisplatin (50 mg/m2) administered every 2 weeks.
There was no statistical difference in median survival for the 220 patients who were randomized (10.7 months for the oxaliplatin regimen vs. 8.8 months for the cisplatin regimen). A subgroup analysis of patients older than age 65 years favored the oxaliplatin combination with improved response rate (41.3% vs. 16.7%), time-totreatment failure (5.4 vs. 3.2 months), progression-free survival (6 vs. 3.1 months), and overall survival (13.9 vs. 7.2 months) compared with the cisplatin regimen, respectively. The oxaliplatin combination produced less anemia, nausea, vomiting, alopecia, fatigue, renal toxicity, and thromboembolic events. However, there was less neuropathy with the cisplatin regimen.
Interest in irinotecan combination therapies resulted in the comparison of irinotecan, leucovorin, and 5-FU (IF) vs. 5-FU and cisplatin (CF) in the V-306 trial. Among 337 metastatic gastric cancer patients randomized to receive one of the two regimens, there were no significant differences in time to progression (5 months IF vs. 4.2 months CF) or overall survival (9 vs. 8.7 months, respectively).25 IF produced less grade 3/4 neutropenia, including febrile neutropenia, stomatitis, and nausea; whereas FP resulted in less diarrhea, although more FP patients withdrew from treatment because of drug-related adverse events.
The ECF regimen was further evaluated in a comparison with the combination of mitomycin (7 mg/m2 every 6 weeks), cisplatin (60 mg/m2 every 3 weeks), and protracted venous infusion 5-FU (300 mg/m2 per day) (MCF) in a randomized study of 580 patients with advanced esophagogastric cancer. There was no difference in response or survival between the two regimens, although quality of life was superior with ECF. MCF produced more thrombocytopenia and plantar-palmar erythema, whereas ECF resulted in more neutropenia and alopecia.26
During the ASCO annual meeting in 2007, Wagner et al presented results of a meta-analysis of 13 trials including 2,184 patients with advanced gastric cancer. The meta-analysis evaluated overall survival and toxicity for four different combination regimens, including 5-FU and cisplatin with vs. without anthracyclines; 5-FU/ anthracycline combinations with vs. without cisplatin; irinotecan- vs. non-irinotecan-containing regimens; and docetaxel- vs. non-docetaxelcontaining regimens. There was a significant survival advantage for the three-drug regimens including 5-FU, anthracyclines, and cisplatin; and a higher treatment-related death rate when 5-FU was administered as bolus compared with an infusional schedule. This analysis also suggested that ECF was the best-tolerated regimen and that combinations including irinotecan showed a non-significant trend toward better survival.27
Japanese investigators have a significant interest in the evaluation of oral agents, including combination regimens for patients with advanced gastric cancer. Ohtsu et al published the results of the Japan Clinical Oncology Group study, JCOG9205, which was a randomized, phase III comparison of 5-FU vs. 5-FU/cisplatin vs. uracil and tegafur (UFT) plus mitomycin (UFTM). 5-FU was administered as a 120-hour continuous infusion every 4 weeks, both as a single agent and in combination with cisplatin. UFT was administered orally at a dose of 375 mg/m2 per day bid with a weekly bolus of mitomycin (5 mg/m2).28 The study evaluated 280 patients with advanced gastric cancer. An interim analysis demonstrated that the UFTM regimen had inferior survival with higher rates of hematologic toxicity and, therefore, the UFTM arm was discontinued. In summary, the trial demonstrated a higher response rate for cisplatin and 5-FU with a longer progression-free survival but no differences in overall survival.
Most recently, the oral fluoropyrimidine S-1 (tegafur, gimestat, and otastat potassi um) has generated considerable interest and has been the subject of randomized phase III trials. Gimestat (5-chloro-2, 4- dihydroxypyridine [CDHP]) is a dihydropyrimidine dehydrogenase inhibitor, and otastat potassium (Oxo) reduces 5-FU-related gastrointestinal toxicity.
The Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group recently presented a randomized, phase III study of 5-FU vs. irinotecan and cisplatin vs. S-1 in 704 patients with advanced gastric cancer (JCOG9912).29 The dose of continuous-infusion 5-FU was 800 mg/m2 per day, days 1–5, every 4 weeks. The irinotecan/cisplatin regimen included irinotecan 70 mg/m2, days 1 and 15, and cisplatin 80 mg/m2, day 1, every 4 weeks. S-1 was administered at 40 mg/m2 orally twice daily, days 1–28, every 6 weeks. The results demonstrated significantly more toxicity with irinotecan/cisplatin compared with the other two regimens, including neutropenia and febrile neutropenia, treatment-related deaths, fatigue, anorexia, and nausea. Significantly more patients in the irinotecan/cisplatin group refused continuation of therapy secondary to toxicity. Response rates were higher with irinotecan/cisplatin than with infusional 5-FU and S-1 (38% vs. 9% vs. 28%, respectively). S-1 also demonstrated a significant non-inferiority to infusional 5-FU in survival. Irinotecan and cisplatin did not show superiority to infusional 5-FU in survival, with substantial toxicities causing treatment cessation. Investigators concluded that S-1 is an appropriate standard chemotherapy regimen for advanced gastric cancer.
The SPIRITS trial was presented by the Japanese TS-1 Advanced Gastric Cancer Clinical Trial Group.30 This randomized, phase III study evaluated S-1 vs. S-1 and cisplatin in patients with advanced gastric cancer. The dose of S-1 as a single agent was 40–60 mg bid for 28 days every 6 weeks. The S-1 and cisplatin combination included S-1 40–60 mg orally bid for 21 days every 5 weeks with cisplatin 60 mg/m2 intravenously on day 8. Evaluation of 305 randomized patients showed significant advantages favoring S-1/cisplatin over S-1 alone, respectively, in terms of survival (median survival, 13 vs. 11 months; P = .0366), progression-free survival (6 vs. 4 months; P < .0001), and overall response rate (54% vs. 31%; P = .0018). Overall, the regimens were well tolerated, with the combination producing more grade 3/4 neutropenia, anorexia, and nausea.
A third Japanese, randomized, phase III trial presented during the 2007 ASCO annual meeting and the 2008 ASCO Gastrointestinal Cancers Symposium was the IRIS study (GC0301/TOP-002), which compared S-1 with S-1 and irinotecan.31,32 S-1 as a single agent was administered at 80 mg/m2/day from days 1 through 28 every 6 weeks. The S-1 and irinotecan regimen included S-1 80 mg/m2/day, days 1 through 21, and irinotecan 80 mg/m2 on days 1 and 15, followed by a 2-week break. A total of 326 patients were randomized. Results demonstrated a significant response advantage for the combination arm (41.5% vs. 26.9%, P = .035). Neutropenia, diarrhea, and anorexia, in particular, were greater in the combination arm. There was no statistically significant overall survival difference between S-1 and S-1/irinotecan.
OTHER RECENT TRIALS IN ADVANCED GASTRIC CANCER
A host of additional phase II trials of combination chemotherapy regimens have been reported. For example, there is significant interest in improving the toxicity profile of the DCF regimen, as reported in the V-325 study. During ASCO 2007, a phase I/II trial of the combination of docetaxel, oxaliplatin, and 5-FU was reported, demonstrating an excellent safety profile.33 Further work is ongoing with this combination.
There is also interest in studying treatment strategies for gastric cancer patients who have progressed after first-line therapy, due to the lack of a clearly established second-line regimen. One recently published Korean phase II study evaluated docetaxel and epirubicin for patients with advanced gastric cancer.34 Among 34 patients enrolled, 21.8% had a partial response and 37.5% achieved stable disease. The median time to progression was 4.1 months, with an overall survival duration of 13.4 months. Neutropenia and febrile neutropenia were the principal grade 3/4 adverse events; gastrointestinal toxicity incidence was low.
Another ASCO 2007 abstract evaluated determinants of the best candidates for second-line chemotherapy for advanced gastric cancer.35 Data from 169 patients who received second-line chemotherapy were used for the analysis. Four factors that were independently associated with improved overall survival included Eastern Cooperative Oncology Group (ECOG) performance status 0–1, CEA level < 50 U/mL, one or two metastatic sites of disease, and a time to progression after first-line chemotherapy of > 4 months.
TARGETED THERAPIES
Although combination chemotherapy regimens have produced improved response rates and survival for patients with metastatic gastric cancer, overall survival remains approximately 10 months in most series. Therefore, improved strategies for the treatment of advanced gastric cancer are needed. The availability of targeted therapies has led to investigations of these agents combined with chemotherapy in patients with gastric cancer.
For example, at ASCO 2007, an AIO Upper GI Study Group phase II trial in patients with metastatic gastric cancer evaluated first-line cetuximab with weekly oxaliplatin, 5-FU, and leucovorin (FUFOX).36 The study included 52 patients, and demonstrated an overall response rate of 65.2%, time to progression of 7.6 months, and overall survival of 9.5 months.
In another phase II trial, cetuximab plus irinotecan, infusional fluorouracil, and leucovorin (FOLFIRI) was administered to 34 patients with untreated gastric or gastroesophageal junction adenocarcinoma. The response rate was 44.1%, median time to progression was 8 months, and median overall survival was 16 months.37
Two recent phase II trials of bevacizumab plus chemotherapy have also been reported. In one study, the combination of bevacizumab, cisplatin, and irinotecan was administered to 35 patients and produced a response rate of 65%, with a median time to progression of 8.3 months and a median overall survival of 10.3 months.38 A second-line trial with docetaxel and bevacizumab administered to 17 patients produced a response rate of 23.5%.39
CONCLUSIONS
Answers to some of the most important questions involving gastric cancer therapeutic interventions remain elusive. These questions include the following: (1) What are the molecular properties that characterize gastric cancer? (2) What are the early molecular alterations that eventually result in malignant transformation? (3) Can molecular alterations serve as targets for therapeutic intervention? (4) What therapeutic gain can we achieve by exploiting molecular features?
Current clinical trial strategies employ a variety of chemotherapy platforms linked to biologic therapies. For example, the ECOG recently completed a trial evaluating sorafenib vs. sorafenib, cisplatin, and 5-FU. A current randomized phase II trial, in collaboration between ECOG and the Cancer and Leukemia Group B (CALGB), includes the addition of cetuximab to three different chemotherapy regimens (ECF, irinotecan and cisplatin, and FOLFOX [5-fluorouracil, leucovorin, oxaliplatin]). Laboratory correlates are an important component of the ECOG/CALGB trial and are being incorporated in many ongoing phase II studies. The hope is that there will be a better understanding of the many complex factors, including clinical, pathologic, and molecular/genetic profiles that contribute to the clinical course of individual gastric cancer patients. Employing biologic strategies mandates a movement away from empiric clinical trial design to one that focuses on optimal collaboration between laboratory and clinical scientists.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Benson has been a consultant to and received research support from sanofi-aventis, Bristol-Myers Squibb, Pfizer, Roche, Bayer, Amgen, ImClone, and Taiho. All research support is directly negotiated by Northwestern University.
REFERENCES
- 1.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21(19):3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 2.Chau I, Norman AR, Cunningham D, et al. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22(12):2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 3.Kilickap S, Dizdar O, Harputluoglu H, et al. Predictive factors for metastasis in patients with gastric cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):641. (abstr 15056) [Google Scholar]
- 4.Yamada Y, Arao T, Nishio K, et al. Identification of prognostic biomarkers for gastric cancer by gene expression analysis. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):228s. (abstr 4623) [Google Scholar]
- 5.Keam B, Kim H, Im S, et al. Comprehensive analysis of ERCC, XPD, and XRCC polymorphisms: association with clinical outcomes in patients with advanced gastric cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):234s. (abstr 4649) [Google Scholar]
- 6.Park SR, Park MS, Park YL, et al. CYP2A6 genetic polymorphism as a predictive marker for clinical outcomes in patients with metastatic gastric carcinoma treated with S-1 plus docetaxel. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):230s. (abstr 4633) [Google Scholar]
- 7.Nishina T, Matsubara J, Toshikazu M, et al. Clinical significance of dihydropyrimidine dehydrogenase (DPD), epidermal growth factor receptor, and excision repair cross-complementing gene 1 (ERCC1) gene expression of tumor tissue in patients with advanced gastric cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):229s. (abstr 4629) [Google Scholar]
- 8.Ichikawa W, Takahashi T, Sasaki Y. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with S-1 monotherapy. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):222s. (abstr 4600) [Google Scholar]
- 9.Uetake H, Inokuchi M, Sugihara K, et al. Microarray analysis using paraffin embedded samples of gastric cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):647. (abstr 15089) [Google Scholar]
- 10.Izzo JG, Luthra R, Sims-Mourtada J, et al. Emerging molecular targets in esophageal cancers. Gastrointest Cancer Res. 2007;1(suppl 2):S3–S6. [PMC free article] [PubMed] [Google Scholar]
- 11.Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 12.Wils J, Klein DJ, Wagener TH, et al. Sequential high dose methotrexate and 5-fluorouracil combined with doxorubicin—a step ahead in the treatment of advanced gastric cancer: a trial of the EORTC Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9:827–831. doi: 10.1200/JCO.1991.9.5.827. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi W. Chemotherapy for advanced gastric cancer: review of global and Japanese status. Gastrointest Cancer Res. 2007;1(5):197–203. [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsen D, Atiq OT, Saltz L, et al. FAMTX versus etoposide, doxorubicin, and cisplatin: a random assignment trial in gastric cancer. J Clin Oncol. 1992;10:541–548. doi: 10.1200/JCO.1992.10.4.541. [DOI] [PubMed] [Google Scholar]
- 15.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Kim NK, Park YS, Heo DS, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813–3818. doi: 10.1002/1097-0142(19930615)71:12<3813::aid-cncr2820711205>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 18.Vanhoefer UV, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 20.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205– 3209. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210–3216. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 22.Ilson DH. Docetaxel, cisplatin, and fluorouracil in gastric cancer: does the punishment fit the crime? J Clin Oncol. 2007;25(22):3188–3190. doi: 10.1200/JCO.2006.10.2210. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 24.Al-Batran S-E, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26(9):1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 25.Dank M, Zaluski J, Baorone C, et al. Randomized phase 3 trial of irinotecan (CPT-11) + 5-FU/folinic acid (FA) vs CDDP + 5FU in 1stline advanced gastric cancer patients. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 23(16S):308s. (abstr 4003) [Google Scholar]
- 26.Ross P, Nicolson M, Cunningham D, et al. Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol. 2002;20:1996–2004. doi: 10.1200/JCO.2002.08.105. [DOI] [PubMed] [Google Scholar]
- 27.Wagner AD, Grothe W, Haerting J, et al. Combination chemotherapies in advanced gastric cancer: an updated systematic review and meta-analysis. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):211s. (abstr 4555) [Google Scholar]
- 28.Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus cisplatin plus tegafur plus mitomycin C in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group study (JCOG9205) J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 29.Boku N, Yamamoto S, Shirao K, et al. Gastrointestinal Oncology Study Group/Japan Clinical Oncology Group. Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin versus S-1 alone in advanced gastric cancer (JCOG9912). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):200s. (abstr LBA4513) [Google Scholar]
- 30.Narahara H, Koizumi W, Hara T, et al. TS-1 Advanced Gastric Cancer Clinical Trial Group Randomized phase III study of S-1 alone versus S-1 + cisplatin in the treatment for advanced gastric cancer (The SPIRITS trial) SPIRITS: S-1 plus cisplatin vs S-1 in RCT in the treatment for stomach cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):201s. (abstr 4514) [Google Scholar]
- 31.Chin K, Iishi H, Imamura H, et al. Irinotecan plus S-1 (IRIS) versus S-1 alone as first line treatment for advanced gastric cancer: preliminary results of a randomized phase III study (GC0301/TOP-002). 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):203s. (abstr 4525) [Google Scholar]
- 32.Imamura H, Iishi H, Tsuburaya A, et al. Randomized phase III study of irinotecan plus S-1 (IRIS) versus S-1 alone as first-line treatment for advanced gastric cancer (GC0301/TOP-002). 2008 ASCO Gastrointestinal Cancers Symposium; Orlando, FL. Jan 25–27, 2008. (abstr 5) [Google Scholar]
- 33.Ajani JA, Phan A, Ho L, et al. Phase I/II trial of docetaxel plus oxaliplatin and 5-fluorouracil (DFOX) in patients with untreated, advanced gastric or gastroesophageal cancer. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):225s. (abstr 4612) [Google Scholar]
- 34.Lim JY, Cho JY, Paik YH, et al. Salvage chemotherapy with docetaxel and epirubicin for advanced/metastatic gastric cancer. Oncology. 2007;73(1–2):2–8. doi: 10.1159/000120027. [DOI] [PubMed] [Google Scholar]
- 35.Catalano V, Graziano F, Santini D, et al. Prognostic factors in metastatic gastric cancer patients treated with second-line chemotherapy. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):638. (abstr 15169) [Google Scholar]
- 36.Lordick F, Lorenzen S, Hegewisch-Becker S, et al. Cetuximab plus weekly oxaliplatin/5FU/FA (FUFOX) in 1st line metastatic gastric cancer. Final results from a multicenter phase II study of the AIO Upper GI Study Group. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 25(18S):204s. (abstr 4526) [Google Scholar]
- 37.Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann Oncol. 2007;18:510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 38.Shah MA, Ramanathan RK, Ilson D, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 39.Enzinger PC, Fidias P, Meyerhardt J, et al. Phase II study of bevacizumab and docetaxel in metastatic esophageal and gastric cancer. 2006 ASCO Gastrointestinal Cancers Symposium; San Francisco, CA. Jan 26–28, 2006. (abstr 681) [Google Scholar]
- 40.Ichikura T, Tomimatsu S, Ohkura E, et al. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001;78(2):132–137. doi: 10.1002/jso.1133. [DOI] [PubMed] [Google Scholar]