Abstract
The Escherichia coli protein SufI (FtsP) has recently been proposed to be a component of the cell division apparatus. The SufI protein is also in widespread experimental use as a model substrate in studies of the Tat (twin arginine translocation) protein transport system. We have used SufI-GFP (green fluorescent protein) fusions to show that SufI localizes to the septal ring in the dividing cell. We have also determined the structure of SufI by X-ray crystallography to a resolution of 1.9 Å. SufI is structurally related to the multicopper oxidase superfamily but lacks metal cofactors. The structure of SufI suggests it serves a scaffolding rather than an enzymatic role in the septal ring and reveals regions of the protein likely to be involved in the protein–protein interactions required to assemble SufI at the septal ring.
Abbreviations used: Tat, twin arginine translocation; GFP, green fluorescent protein; T1, mononuclear type I; LB0N, LB without 10g NaCl per liter; NCS, non-crystallographic symmetry
Keywords: SufI, FtsP, Tat, cupredoxin, X-ray crystallography
Introduction
Currently, around 20 cell division genes have been identified in the bacterium Escherichia coli (reviewed in Refs. 1–6). Most of these genes have the designation fts, which stands for ‘filamentation temperature sensitive’ and reflects the fact that the first E. coli division genes were discovered in screens for mutants that divided normally at 30 °C but became filamentous and ultimately died at 42 °C. The fts genes share two critical properties. Firstly, loss-of-function mutations cause division defects. Secondly, the proteins encoded by these genes all localize to the midcell where they form a structure called the septal ring (also termed the divisome or septalsome). Many investigators regard the septal ring as an organelle that mediates cytokinesis. It assembles at the midcell prior to division and then constricts as division proceeds so as to remain at the leading edge of the developing septum.
Samaluru et al. have recently shown that the E. coli gene sufI has several properties of an fts gene.7 They have proposed renaming the gene ftsP to emphasize its role in division and to avoid confusion with suf genes involved in assembly of iron–sulfur clusters.7 sufI was originally identified as a multicopy suppressor of an ftsI(Ts) mutation, with the sufI designation standing for ‘suppression of ftsI(Ts)’.8 FtsI, also known as penicillin-binding protein 3, is a transpeptidase involved in synthesis of peptidoglycan cell wall during cell division.9–12 Reddy and colleagues demonstrated several additional division-related phenotypes associated with sufI, the most noteworthy being that a sufI null mutant is filamentous when grown at elevated temperature in media lacking osmotic protectants such as NaCl.7,13 Thus, sufI acts like an fts gene, but whether it is part of the septal ring that mediates cell division remains to be determined.
SufI is a water-soluble, periplasmic protein.8 The sequence of SufI shows that it is a member of the multicopper oxidase superfamily.14 However, the SufI sequence conserves only 2 of the 12 canonical copper-binding residues found in multicopper oxidases and this has led to the prediction that SufI does not bind metal cofactors.15
In addition to its involvement in cell division, SufI is of considerable interest due to its use as a model substrate in studies of the bacterial Tat (twin arginine translocation) protein transport pathway. The Tat system is used to transport folded substrate proteins from the cytoplasm to the periplasm across the cytoplasmic membrane (reviewed in Refs. 16–19). SufI has a number of favourable properties for mechanistic studies of Tat transport relative to that of other E. coli Tat substrates, including that it is water soluble, monomeric, and does not require insertion of a cofactor during folding. These properties have led to the adoption of SufI as the preferred model substrate in Tat translocation studies in E. coli.15,20–30
In this study, we examine whether SufI/FtsP resembles other Fts proteins in localizing to the division septum. We also determine the structure of SufI/FtsP with the aim of providing additional insight into the molecular function of the protein, as well as a structural basis for studying the interactions of SufI with other cell division proteins and with the Tat translocation apparatus.
Results
Fusion of SufI to green fluorescent protein
Recent genetic evidence7,13 has demonstrated a role for SufI in protecting and stabilizing the divisional assembly under conditions of stress. Specifically the evidence implies that SufI may be a septal ring protein.
To test this hypothesis, we sought to develop green fluorescent protein (GFP)-tagged versions of SufI that would allow the subcellular localization of SufI under normal growth conditions to be determined. To this end we constructed separate IPTG-inducible fusions of gfp to the 5′ or 3′ ends of sufI (Fig. 1a). We term the protein products of these genes GFP-SufI and SufI-GFP, respectively. The fusion constructs were integrated into the chromosome in a single copy at the attachment site for phage φ80 in both a wild-type and a sufI<>aph null mutant background. Isogenic strains that expressed gfp alone were constructed as controls. It is important to note that GFP does not function in the periplasm unless exported via the Tat system.31–33 Because SufI is a Tat substrate, no precautions had to be taken to direct the SufI-GFP fusion to the Tat pathway. However, for the GFP-SufI protein and for GFP alone, a Tat signal sequence from TorA was incorporated at the N-terminus of the protein during the cloning steps.34
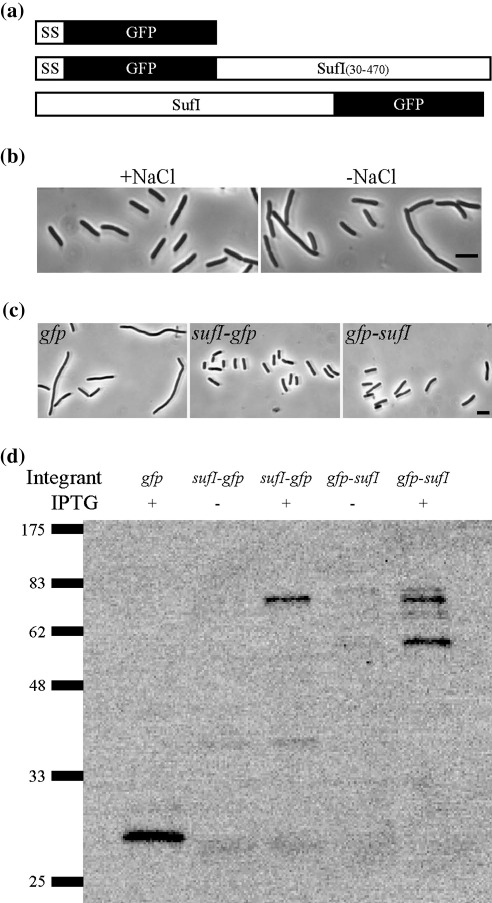
Fig. 1.
(a) Cartoon of gfp constructs. “SS” refers to a Tat signal sequence from TorA. SufI is a Tat substrate and therefore does not need an artificial signal sequence. (b) A sufI null mutant has mild division defects. EC1751 (sufI<>aph) was grown at 37 °C in LB with or without 1% NaCl, fixed, and photographed under phase contrast microscopy. The scale bar represents 5 μm. (c) GFP/SufI fusions rescue division defects. Derivatives of EC1751 (sufI<>aph) expressing the indicated gfp construct were grown in LB0N and photographed as above. The strains shown are EC1874, EC1875 and EC1876. The scale bar represents 5 μm. (d) Immunoblot of strains that express gfp, sufI-gfp or gfp-sufI under the control of an IPTG-inducible promoter. Sample concentrations were normalized by OD600, except that fourfold more of the sufI-gfp sample was loaded because this fusion gene is not highly expressed. The blot was probed with anti-GFP serum. The strains shown are gfp (EC1874), sufI-gfp (EC1876) and gfp-sufI (EC1875). Molecular mass markers are indicated at the left.
The functionality of the fusion proteins was assessed by determining whether they could complement the division defects of a sufI<>aph null mutant (obtained from the Keio collection35). When grown in standard LB containing 10 g NaCl per liter, the sufI mutant exhibited normal morphology, consistent with earlier reports.7,8,13 However, when grown in the absence of NaCl (LB0N medium), about 20% of the cells exhibited division defects that resulted in the appearance of short filaments or chains of up to three to four cells (Fig. 1b and Table 1).7 Division defects were more pronounced at 37 °C than at 30 °C and were confirmed after eviction of the Kanr cassette to create a markerless sufI<>frt deletion strain (not shown). Introduction of either the gfp-sufI or sufI-gfp fusions into the sufI<>aph strain ameliorated the division defect observed in LB0N, with the sufI-gfp fusion giving essentially complete complementation (Fig. 1c and Table 1). Thus, both GFP fusion constructs retain the functionality of SufI. Immunoblotting these strains with anti-GFP revealed major cross-reactive bands at 75 kDa consistent with the calculated mass of 75 kDa for the SufI/GFP fusions after removal of their Tat signal peptides (Fig. 1d). The immunoblot of GFP-SufI also revealed a band at about 60 kDa. Analysis of purified SufI (described below) shows that a surface loop is susceptible to clipping between residues 307–308. Cleavage at this site would produce a truncated GFP-SufI(307) with a predicted mass of 58 kDa, which correlates well with the size of the GFP-containing fragment observed in the blot. It is important to note that clipping of the surface loop does not affect the integrity of the SufI protein because the two fragments remain associated, and it is therefore reasonable to assume that the clipped protein is transport competent and functional.
Table 1.
sufI fusions to gfp rescue division
| Genetic background | Fusion | Cell length, ave ± SD (μm) | % of cells > 10 μm |
|---|---|---|---|
| WT | gfp | 4.7 ± 1.3 | 0.4 |
| ΔsufI | gfp | 8.8 ± 7.0 | 20.2 |
| ΔsufI | gfp-sufI | 6.1 ± 1.9 | 2.6 |
| ΔsufI | sufI-gfp | 4.5 ± 1.3 | 0.2 |
The strains used were EC1871, EC1874, EC1875 and EC1876. All were grown in LB0N at 37 °C, fixed and photographed under phase contrast microscopy. Length data are based on measurements of 500 cells for each strain.
SufI is localized to the septal ring
The fusion proteins were tested for septal localization by fluorescence microscopy of live cells on an agarose pad. SufI-GFP and GFP-SufI clearly accumulated at the division site in about half of the cells (Fig. 2 and Table 2). This was true regardless of whether the cells were grown in LB containing or lacking NaCl (Table 2). Almost all of the cells exhibiting septal localization of SufI had readily apparent constrictions. Septal localization was not observed when cells were fixed with paraformaldehyde/glutaraldehyde (not shown). In fixed cells, only uniform periplasmic fluorescence was observed. Sensitivity to fixation has been observed previously with other periplasmic division proteins such as AmiC.34
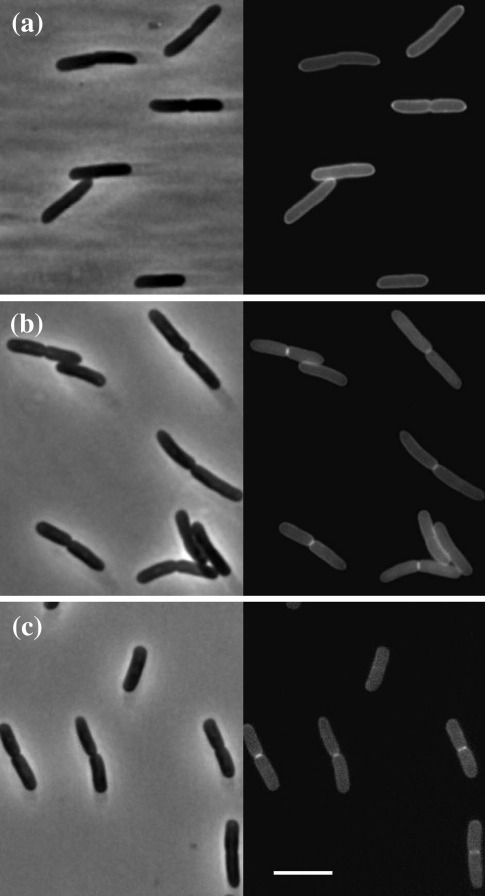
Fig. 2.
Septal localization of SufI. Phase (left) and fluorescence (right) micrographs of an sufI<>aph null mutant expressing (a) gfp, (b) gfp-sufI and (c) sufI-gfp. The scale bar represents 5 μm. The strains shown are EC1874, EC1875 and EC1876. They were grown in LB with 1% NaCl and 1 mM IPTG at 30 °C.
Table 2.
Localization of SufI
| Genetic background | NaCl | Fusion | No. of cells scored | Cell length, ave ± SD (μm) | % of cells with rings |
|---|---|---|---|---|---|
| WT | + | gfp-sufI | 122 | 5.5 ± 1.6 | 44 |
| WT | + | sufI-gfp | 184 | 3.9 ± 1.0 | 40 |
| ΔsufI | + | gfp-sufI | 302 | 5.6 ± 1.5 | 41 |
| ΔsufI | + | sufI-gfp | 216 | 5.0 ± 1.2 | 56 |
| ΔsufI | − | gfp-sufI | 201 | 6.2 ± 1.8 | 42 |
| ΔsufI | − | sufI-gfp | 208 | 4.8 ± 1.3 | 41 |
The strains used were EC1872, EC1873, EC1875 and EC1876.
Division mutants sometimes have an enlarged periplasmic space. This can lead to the enrichment of periplasmic GFP signal at division sites even when the GFP fusion protein in question does not localize to the septal ring.36 To address this concern, we also localized periplasmic GFP that was not fused to another protein. As expected, isolated GFP was more uniformly distributed throughout the periplasm than GFP fused to SufI (Fig. 2). This finding argues that septal localization of the GFP/SufI fusions is not an artifact and that any division defect in the sufI null mutant is too subtle to result in an enlarged periplasmic space (at least under these growth conditions and with this assay). It should also be noted that GFP-SufI and SufI-GFP also clearly localized to the midcell in a wild-type background, which would not be expected to have an enlarged periplasmic space (Table 2).
The periplasmic location of Tat-targeted GFP in Fig. 2 is evident from the fluorescent halo around the cells. Because neither GFP-SufI nor SufI-GFP showed a similar halo, it was not immediately obvious that those proteins were also periplasmic, although the complementation and septal localization data implied that both fusion proteins had to have reached the periplasm. We suspected the lack of a fluorescent halo in cells producing GFP-SufI or SufI-GFP was due to the low abundance of these proteins as evident from Western blotting (Fig. 1d). Indeed, when GFP-SufI and SufI-GFP were produced from multicopy plasmids, the expected halos were readily apparent (Supplemental Fig. 1). Moreover, in a Tat mutant, both GFP-SufI fluorescence and SufI-GFP fluorescence were exclusively cytoplasmic and septal localization was not observed (Supplemental Fig. 2). Taken together, these observations confirm that GFP-SufI and SufI-GFP were exported to the periplasm by the Tat system. Because we were concerned that the chaining phenotype of Tat mutants37 might obscure septal localization, we also examined GFP fusions to the well-characterized division proteins FtsZ, FtsI and FtsN. All three localized to potential division sites (Supplemental Fig. 2). Interestingly, production of GFP-FtsI in the Tat mutant inhibited division, while production of GFP-FtsN appeared to promote more frequent division.
Recruitment of SufI to the septal ring depends on FtsN
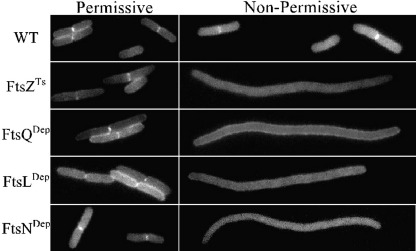
The septal ring contains 10 proteins that are essential for cell division. Studies of how these essential proteins depend on one another for recruitment to the septal ring have established a set of relationships that suggests the ring assembles by sequential addition of proteins, starting with FtsZ and ending with FtsN (reviewed in Refs. 3, 5 and 6). To determine where SufI fits into this hierarchy, we examined localization of SufI-GFP in filamentous cells that could not divide because FtsZ, FtsQ, FtsL or FtsN had been either inactivated or removed by depletion. Notably, loss of any one of these division proteins prevented SufI-GFP from accumulating at potential division sites (Fig. 3 and Supplemental Table 1). These results indicate that SufI is a late recruit to the septal ring.
Fig. 3.
Recruitment of SufI to septal rings requires other Fts proteins. The top two panel sets show a wild-type (WT) strain or ftsZ(Ts) mutant grown at 30 °C in LB (permissive) or 37 °C in LB0N (non-permissive). The strains shown are EC1873 and EC2065, both of which carry a chromosomal sufI-gfp fusion. Traditionally, 42 °C has been used as the non-permissive temperature for ftsZ84(Ts), but we found that SufI-GFP was not fluorescent at 42 °C. The bottom three panel sets show FtsQ, FtsL or FtsN depletion strains. The strains shown are JM265, JOE170 and EC1908 and carry the sufI-gfp plasmid pDSW932. These strains produce the indicated Fts protein from an arabinose-dependent promoter and were grown in the presence of arabinose (permissive) or glucose (non-permissive). The cells shown are representative. For quantitative data, see Supplemental Table 1.
Crystal structure of SufI
In an attempt to gain further functional insights about SufI, the structure of overproduced, hexahistidine-tagged SufI was determined by X-ray crystallography. SufI crystallized in two crystal forms (Table 3) and was solved by molecular replacement using the structure of the homologous E. coli CueO protein [Protein Data Bank (PDB) ID 1PF3]38 as the search model. Both the orthorhombic and monoclinic crystal forms contain two molecules in the asymmetric unit, with a common dimer interface surface of 921 Å calculated by the MSD-PISA server.39 SufI elutes from a size-exclusion chromatography column at the position expected for a monomer. In addition, analysis of the dimer interface with the MSD-PISA server gave a complexation significance score of zero. This implies that the interface is not significant and most likely arises from crystal packing.
Table 3.
SufI X-ray data collection, processing and refinement
| Data set | Orthorhombic | Monoclinic |
|---|---|---|
| A. Data collection and processing | ||
| X-ray source | ESRF ID29-1 | ESRF ID23-1 |
| Detector | ADSC scanner | MAR scanner |
| Space group (Z) | P212121 (8) | P1211 (4) |
| Unit cell parameters | ||
| a (Å) | 54.47 | 64.12 |
| b (Å) | 89.55 | 48.88 |
| c (Å) | 153.63 | 131.96 |
| α (°) | 90 | 90 |
| β (°) | 90 | 95.9 |
| γ (°) | 90 | 90 |
| Wavelength | 0.97370 | 0.87300 |
| Resolution limits (Å) | 77.4–1.9 (2.0–1.9) | 64.0–2.6 (2.74–2.6) |
| Completeness (%) | 98.4 (98) | 99.5 (96.8) |
| Measured reflections | 201,631 | 160,469 |
| Unique reflections | 58949 | 24830 |
| Multiplicity | 3.4 (2.9) | 6.5 (5.7) |
| Rmerge | 0.083 (0.397) | 0.154 (0.425) |
| I/σ(I) | 5.2 (1.9) | 3.4 (1.6) |
| B. Refinement | ||
| Crystal | Orthorhombic, P212121 | Monoclinic, P1211 |
| Resolution range | 39.8–1.9 (2.0–1.9) | 59.8–2.6 (2.74–2.6) |
| Rall (%) | 19.3 (20.9) | 21.6 (26.2) |
| Rwork (%) | 19.1 (20.8) | 21.4 (26.1) |
| Rfree (%) | 22.9 (24.1) | 24.6 (28.0) |
| rmsd bond lengths (Å) | 0.005 | 0.005 |
| rmsd bond angles (°) | 0.94 | 1.2 |
| Residues modelled (range) | Chain A: 422 (30–295, 312–330, 335–471) | Chain A: 422 (30–295, 314–469) |
| Chain B: 420 (31–43, 46–295, 313–470) | Chain B: 422 (29–295, 315–469) | |
| Water molecules modelled | Chain A: 219 | Chain A: 34 |
| Chain B: 193 | Chain B: 28 | |
| Average B (protein) (Å2) | Chain A: 28.9 | Chain A: 32.7 |
| Chain B: 31.5 | Chain B: 32.3 | |
| Average B (water molecules) (Å2) | Chain A: 35.9 | Chain A: 22.3 |
| Chain B: 37.6 | Chain B: 24.0 | |
| Residues in favoured regions of Ramachandran plot (%) | 95.3 | 88.9 |
| Residues in forbidden regions of Ramachandran plot (%) | 0.2 | 1.9 |
| PDB identifier | 2UXT | 2UXV |
Values for the highest-resolution shell are given in parentheses.
The two molecules of each asymmetric unit are essentially the same within experimental error (rmsds over 408 and 406 Cα atoms of 1.1 and 0.9 Å for the orthorhombic and monoclinic crystals, respectively). Of the two data sets, the orthorhombic crystals diffracted to higher resolution (1.9 Å compared to 2.6 Å). The overall structure of SufI (Fig. 4a) is very similar to that of CueO and contains three cupredoxin-like domains. These domains consist of a beta sandwich made up of seven strands in two beta sheets, although some variations may have one or two additional strands. The beta sheets are found in variations of the Greek key beta barrel, which have some of the beta strands adjacent in space despite not being adjacent in sequence. As with CueO, SufI has a long linker peptide between domains two and three that runs around the surface of the molecule.
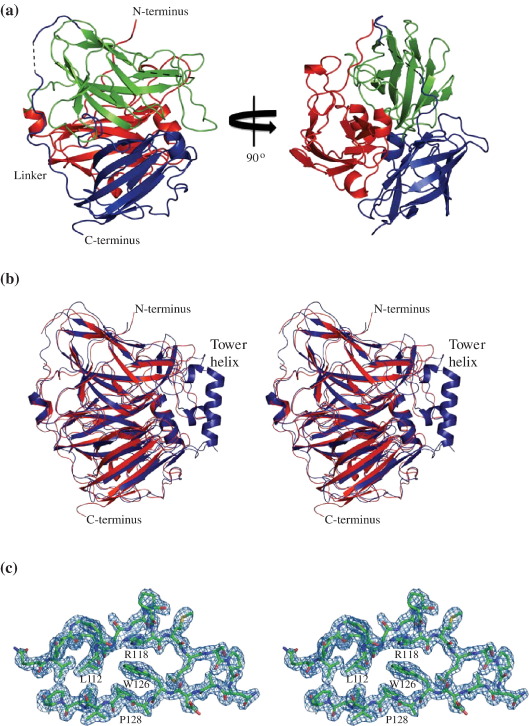
Fig. 4.
Structure of SufI. (a) Cartoon representation of SufI. The structure shown is for chain A of the orthorhombic space group with domain 1 shown in red, domain 2 in green and domain 3 in blue. Regions of missing density are shown as black dotted lines. The arrow in the region of missing density indicates where the protein is subject to proteolytic cleavage. (b) Stereo view showing CueO (blue) overlaid on the orthorhombic SufI (red) structure. PDB ID 1KV7 was overlaid onto the orthorhombic chain A of SufI with the program CCP4-Lsqkab. The positions of the N- and C-termini of the proteins are shown and the tower region of CueO is labelled for reference. The orientation is as in (a). (c) Stereo view showing representative electron density (2Fo − Fc) of orthorhombic chain A, residues 107–131 contoured at 1σ. The positions of the invariant residues leucine 112, arginine 118, tryptophan 126 and proline 128 are labelled. The images were generated with Pymol.40
Due to proteolysis, the SufI preparation used for crystallization is missing the first 17 amino acids of the 27-amino-acid N-terminal Tat signal peptide. The remaining 10 amino acids of the signal peptide are not ordered in any of the crystal forms. Previous circular dichroism studies of the E. coli SufI signal peptide have shown that it lacks a defined secondary structure in aqueous solution,41 which suggests that even if the full-length signal peptide were present it would be disordered. Other residues missing from the final model are listed in Table 3. In all the models, there is missing density for residues 296–311, which include the region where SufI is susceptible to proteolytic cleavage (above). No electron density was observed for the hexahistidine affinity tag, with the exception of the arginine residue of the affinity tag linker in chain A of the orthorhombic structure. Figure 4c shows electron density representative of the quality of the data and phases.
The main areas of difference between the two crystal forms lie between residues 42–47, 55–66 and 326–334. Residues 42–47 are found in a loop that leads into the first domain, while residues 326–334 compose the long linker between domains 2 and 3. The electron density for both of these regions was poor and often missing entirely for the side chains. This suggests that these differences between the two crystal forms arise from flexibility of the molecule in these regions. Residues 55–66 are found in an extended loop in the first domain: a short alpha-helical segment is present in this loop in the orthorhombic form, which is unravelled in the monoclinic form.
SufI belongs to the multicopper oxidase family of proteins but does not bind copper
The crystal structure coordinates of a SufI monomer were submitted to the DALI server.42 The closest structural relative identified by the DALI server is E. coli CueO, with a Z-score of 53. An overlay of the two structures (Fig. 4b) has an rmsd of 1.46 Å over 406 Cα atoms.
CueO contains four copper atoms, which are found in a mononuclear type I (T1) copper centre and a trinuclear copper centre. SufI lacks all but two of the copper-binding residues found in CueO and has been assumed not to bind copper.15 Consistent with this prediction, no electron density corresponding to copper atoms was observed in the SufI structure. The absence of bound copper or other metals in the SufI structure was confirmed by calculating an anomalous difference density Fourier map using CCP4-FFT, which failed to identify any significant peaks.
The ability of SufI to bind metals could not be ruled out due to the use of metal chelate affinity chromatography during the purification protocol, which might remove bound metal ions from the protein. However, soaking of SufI crystals with a cocktail of metal ions did not result in the identification of any metal-binding sites, and using the CHED server43 to detect/predict metal binding sites also failed to identify any putative sites.
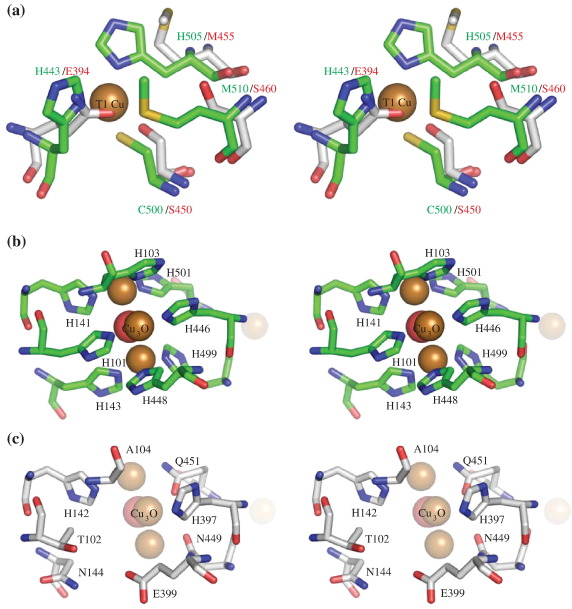
In CueO, the T1 centre is slightly buried within domain 3.38 Figure 5a shows the T1 copper centre in CueO overlaid onto the corresponding region in SufI. This illustrates how the N and S donor ligands favoured by copper have been almost totally replaced by O donor ligands in SufI. The trinuclear copper centre of CueO is located between domains 1 and 3 (Ref. 44) and a water-filled cavity is found at the equivalent position in SufI. In CueO, the trinuclear centre is coordinated by eight histidine residues (Fig. 5b). All but two of these histidine residues have been replaced in SufI with amino acids that would not normally be expected to coordinate copper (Fig. 5c). The two histidine residues retained in SufI are residues that in CueO ligate two different atoms of the trinuclear copper centre. In the SufI crystal structures these two histidines are bridged by a water molecule.
Fig. 5.
Comparison of the copper-binding sites of CueO and the equivalent regions of SufI. (a) Stereo view of the copper-binding residues of the T1 copper-binding site of CueO (green sticks) overlaid on the corresponding region of SufI (white sticks). The structure of CueO (PDB ID 1KV7) was overlaid on the orthorhombic chain A of SufI with the program CCP4-Lsqkab. The residues are labelled in green for CueO and red for SufI. The T1 copper of CueO is shown as a space-filling sphere. (b) Stereo view representation of the trinuclear copper-binding site of CueO showing the copper-binding residues as stick models. The trinuclear centre is shown as space-filling spheres. (c) The corresponding residues of SufI shown as sticks with the corresponding position of the trinuclear centre of CueO shown as transparent space-filling spheres. The structure of CueO (PDB ID 1KV7) was overlaid on the orthorhombic chain A of SufI with the program CCP4-Lsqkab. The images were generated with Pymol.40
A further difference between the CueO and SufI structures is the absence, in SufI, of the methionine-rich helix and loop, the so-called tower of the CueO structure. This region sits over the T1 copper site in CueO38,45 and constitutes an additional copper-binding site.44 Taken together, these limited sequence differences are sufficient to destroy the ability to bind copper, leading to the unusual situation of highly homologous proteins with very different biological functions.
Identification of potential functionally important regions of SufI
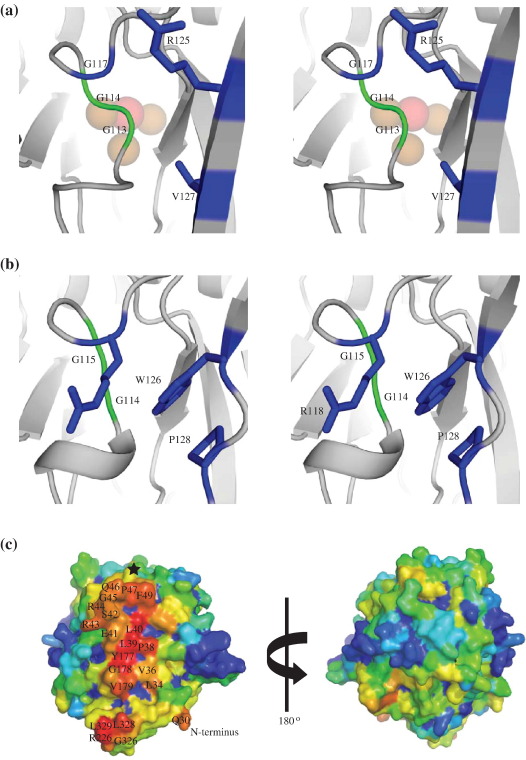
In an attempt to identify structural features that might be relevant to the specific function of SufI, we looked for residues that are conserved between SufI proteins from different organisms but are not found in CueO (Fig. 6). This approach identified two regions of particular note. The first, close to the N terminus at residues 41–47, is the motif ExRRGxP. Unfortunately, the density in this region, especially around the two arginines, is poor or missing in the models. This implies that the loop is very flexible, which could be important for any functional role it may play. The second region of interest is focussed on residues 118–128 in domain 1. Glycine residues at positions 114 and 115 of E. coli SufI are conserved in both SufI and CueO. In E. coli CueO, these glycine residues form part of a DGX motif conserved amongst all multicopper oxidases, which sits at the bottom of a surface-accessible region directly above the trinuclear copper centre (Fig. 7a) and is implicated in binding of dioxygen and, potentially, proton donation.50,51 However, in the SufI structure, the conserved residues arginine 118, tryptophan 126 and proline 128 are positioned in the space above the same glycines, occluding access from the surface of the molecule (Fig. 7b). This is additional evidence that the SufI molecule has lost the ability to accept ligands and/or substrates in the pocket that in CueO accommodates the Cu centre.
Fig. 6.
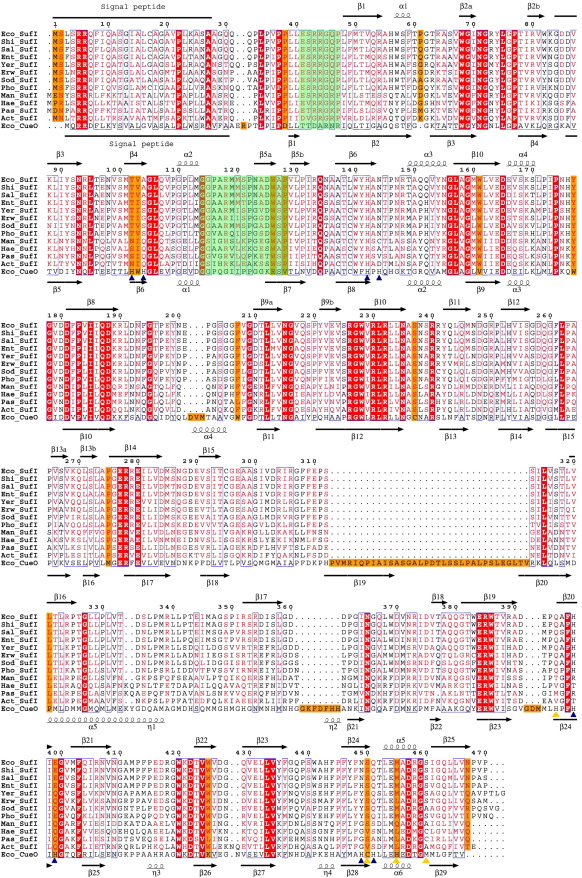
Sequence alignment of SufI proteins with CueO. Putative SufI sequences from various bacteria were identified with the computer program BLAST.46 The SufI sequences were aligned with one another and also with CueO from E. coli with ClustalW47 and coloured with WebESPript.48 Key: white character inside red box, strict identity; red character, similarity across SufI sequences; blue frame, similarity between conserved SufI sequences and CueO; orange box, differences between conserved SufI sequences and CueO. Two regions identified as having potential roles in SufI function are highlighted in green. Secondary structural elements for E. coli SufI and CueO are indicated above and below their respective sequences. Yellow triangles identify the type 1 copper centre coordinating residues in CueO, while blue triangles identify the residues in CueO that coordinate to the trinuclear copper centre. Eco, Escherichia coli; Shi, Shigella flexneri; Sal, Salmonella typhimurium; Ent, Enterobacter sp. 638; Yer, Yersinia enterocolitica; Erw, Erwinia carotovora; Sod, Sodalis glossinidius; Pho, Photorhabdus luminescens; Man, Mannheimia succiniciproducens; Hae, Haemophilus influenzae; Pas, Pasteurella multocida; Act, Actinobacillus pleuropneumonia.
Fig. 7.
Identification and localization of conserved residues in SufI. (a) CueO: the conserved residues Gly113 and Gly114 (green sticks) sit above the trinuclear copper centre (shown as transparent spheres). Residues Gly117, Arg125 and Val127 (blue sticks) form the brim of a surface cavity that exposes the 112–114 loop (DGG in CueO, conserved as DGX across all multicopper oxidases) to the solvent. (b) SufI: in green, the glycine residues Gly114 and Gly115 corresponding to the glycines in (a); in blue, the residues Arg118, Trp126 and Pro128, which are all highly conserved across SufI sequences and prevent solvent access to the 112–114 loop in the SufI structure. (c) A surface representation of SufI, coloured red (surface residue most conserved) through orange, yellow, and green, to blue (least conserved). The N- and C-termini of the protein are shown for reference and highly conserved surface residues are labelled. A black star marks the location of the residues 118–128 that cover the pocket corresponding to the CueO catalytic centre. The right-hand panel shows a second view of the SufI surface, rotated with respect to the left-hand panel by 180° around the vertical axis. Images were generated using Pymol40 with (c) drawn using a PDB file produced by the WHISCY server.49
Using the WHISCY server,49 we examined the surface conservation of SufI residues in an attempt to identify regions of the molecular surface that may be involved in protein–protein interactions. We found a large number of conserved residues clustered to one face of SufI (Fig. 7c), including the conserved residues 41–47 discussed above (indicated by a black star in Fig. 7c). Given the role of SufI as a cell division protein and its localization to the septal ring during cell division, we speculate that this conserved face represents a binding site for interaction with other Fts proteins.
Discussion
In this study, we show that both N- and C-terminal SufI/GFP fusions accumulate at the division site in constricting cells. Such localization is characteristic of Fts proteins and supports the proposal of Reddy and colleagues that SufI is a bona fide Fts protein.7,13 Interestingly, SufI localized to the septal ring regardless of whether NaCl was present in the growth medium, implying that SufI is not recruited to the ring in response to stress. The observation that sufI mutants are sensitive to stress probably reflects redundancy rather than a specific function for SufI in protecting the divisional apparatus from stress.7
Studies of Fts protein localization in various mutant backgrounds revealed that, at least in E. coli, the septal ring assembles via a largely linear pathway (reviewed in Refs. 3, 5 and 6). Here we have shown that SufI is a late recruit to the septal ring, as its localization depends on FtsZ, FtsQ, FtsL and FtsN. In this context, it is important to note that FtsN is the last known essential division protein recruited to the septal ring. Two additional observations also argue that SufI is a late recruit. First, septal localization was observed in only about half of the cells, which were growing with a doubling time of about 40 min. This percentage is similar to the ‘late’ proteins FtsN and AmiC.34,52 In contrast, ‘early’ proteins such as FtsZ and FtsA show septal localization in 70–90% of the cells.53–55 Second, almost all of the cells exhibiting localization were already constricting, which raises the intriguing possibility that an event associated with the onset of cytokinesis (rather than the mere presence of a protein in the septal ring) is involved in SufI recruitment.
SufI is the fourth soluble periplasmic protein shown to localize to the septal ring. The other three proteins are AmiC, EnvC and TolB.34,36,56 The latter is part of the trans-envelope Tol–Pal complex implicated in facilitating constriction of the outer membrane during cytokinesis.56 AmiC and EnvC are both peptidoglycan hydrolases.36,57 Localization of TolB and AmiC to septal ring assemblies requires FtsN, as reported here for SufI.34,56 Dependency studies have not been reported for EnvC, but given that it appears to join the septal ring prior to the onset of constriction, it is likely to localize ahead of SufI, AmiC and, perhaps, TolB.56 Indeed, it is tempting to speculate that peptidoglycan hydrolysis by EnvC contributes to forming the nascent constrictions that might be important for recruitment of SufI and AmiC to the division site.
While our results firmly establish that the physiological function of SufI is in cell division, the biochemical function remains to be determined. The structure of SufI, determined here at a resolution of 1.9 Å by X-ray crystallography, confirms that the protein belongs to the multicopper oxidase family. However, it also shows that SufI does not bind copper. Indeed, access to the region of the structure that forms the active site in multicopper oxidases is blocked in SufI by highly conserved residues. Together, these observations suggest that SufI is neither a copper-binding protein nor an enzyme. At present we favor the notion that SufI's role in cell division is to serve as a scaffolding protein that helps to maintain the coherence of the septal ring during constriction.
The ability of SufI overproduction to rescue a variety of fts mutants7 (S.J.R.A, E.P. and D.S.W., unpublished data) suggests that SufI engages in protein–protein interactions that contribute to the stability of the septal ring. Sequence analysis identified several conserved residues in SufI that were all found to map to the same face of the protein. This suggests that this face may form a binding surface for interactions with other Fts proteins at the septal ring. The osmotic remedial nature of the division defect in sufI null mutants could be explained if septal rings are somewhat unstable in the absence of SufI and if unfavorable ionic/osmotic conditions exacerbate that instability. With the insights gained from the structure in combination with the phenotypic assays described here and elsewhere,7,13 it should be possible to test these ideas by targeted mutagenesis of the conserved surface residues likely to be involved in protein–protein interactions.
SufI is used extensively as the model substrate in mechanistic studies of the Tat protein translocation pathway. Since the Tat apparatus translocates substrate proteins in the folded state, knowledge of the 3D structure of the substrate is a prerequisite for detailed studies of substrate-transporter interactions in this system. The structure of SufI reported here will allow rational site-specific labelling and engineering of SufI for use in the experimental analysis of Tat mechanism.
Materials and Methods
Strains and media
Strains used are listed in Table 4. CRIM vectors were integrated into the φ80 att site using the helper plasmid pAH123 as described.61,62 EC309 was constructed by P1 transduction with DRC14 as donor and EC251 as recipient. EC1908 was constructed by P1 transduction with TB54 as donor and EC251 as recipient. Strains were grown in LB with or without 10 g NaCl per liter (LB0N). Antibiotics were used at the following concentrations: ampicillin 200 μg/ml, chloramphenicol 30 μg/ml, kanamycin 40 μg/ml and spectinomycin 50 μg/ml for selection of plasmids and 35 μg/ml for chromosomal integrants.
Table 4.
Strains and plasmids
| Strain or plasmid | Relevant features | Source or reference |
|---|---|---|
| Strain | ||
| DADE | MC4100 ΔtatABCDΔtatE | Wexler et al.58 |
| DRC14 | MC4100 ftsZ84(Ts) leu∷Tn10 | D. RayChaudhuri |
| EC251 | K12 wild-type MG1655 | Arends and Weiss59 |
| EC309 | EC251 ftsZ84(Ts) leu∷Tn10 | This study |
| EC839 | K12 wild-type BW25113 | Baba et al.35 |
| EC1751 | BW25113 sufI<>aph Kanr | Baba et al.35 |
| EC1871 | EC839 attPφ 80∷pDSW964(P204∷sstorA-gfp) | This study |
| EC1872 | EC839 attPφ 80∷pDSW979(P204∷sstorA-gfp-sufI30–470) | This study |
| EC1873 | EC839 attPφ 80∷pDSW980(P208∷sufI-gfp) | This study |
| EC1874 | EC1751 attPφ 80∷pDSW964(P204∷sstorA-gfp) | This study |
| EC1875 | EC1751 attPφ 80∷pDSW979(P204∷sstorA-gfp-sufI30–470) | This study |
| EC1876 | EC1751 attPφ 80∷pDSW980(P204∷sufI-gfp) | This study |
| EC1908 | EC251 pBAD∷ftsN (Kanr) | This study |
| EC2065 | EC309 attPφ 80∷pDSW980(P204∷sufI-gfp) | This study |
| JMG265 | KS272 ftsL∷TnphoAL81 IS 50R (Kanr)/pBAD33-LLL | J. M. Ghigo |
| JOE170 | KS272 ftsQ∷TnphoA50 (Kanr)/pJC10 | Chen et al.60 |
| TB54 | DY329 PftsN <> (aph araC PBAD) | Bernhardt and de Boer, 200136 |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIq ZΔM15 Tn10 (Tetr)] | Stratagene |
| Plasmid | ||
| pTB6 | lacIq Plac∷sstorA-gfp-T7 tag, Ampr | Bernhardt and de Boer36 |
| pJC69 | oriRR6Kγ, attPφ80, Spcr, CRIM based plasmid | Chen and Beckwith61 |
| pAH123 | λcI857, rep101ts origin, Pr-intφ80, CRIM helper plasmid Ampr | Haldimann and Wanner62 |
| pDSW204 | pTrc99A based, promoter down mutation in − 35 region (P204 promoter), Ampr, ColE1 ori | Weiss et al.12 |
| pDSW207 | gfp fusion vector derived from pDSW204, Ampr | Weiss et al.12 |
| pDSW208 | gfp-fusion vector derived from pDSW204, Ampr | Weiss et al.12 |
| pDSW210 | gfp-fusion vector with promoter down mutations in both the − 35 and − 10 regions (P206 promoter), ColE1 ori, Ampr | Weiss et al.12 |
| pDSW231 | pDSW210-ftsZ (P206∷ftsZ-gfp, Ampr) | Laboratory collection |
| pDSW234 | pDSW207-ftsI (P204∷gfp-ftsI, Ampr) | Weiss et al.12 |
| pDSW238 | pDSW207-ftsN (P204∷gfp-ftsN, Ampr) | Laboratory collection |
| pDSW932 | pDSW208-sufI (P204∷sufI-gfp, Ampr) | This study |
| pDSW962 | pDSW204-sstorA-gfp (P204∷ sstorA-gfp, Ampr) | This study |
| pDSW964 | pJC69-derivative, lacIq, P204∷sstorA-gfp, Spcr | This study |
| pDSW978 | pDSW962-sufI (P204∷ sstorA-gfp-sufI30–470, Ampr) | This study |
| pDSW979 | pJC69-derivative, lacIq, P204∷sstorA-gfp-sufI30–470, Spcr | This study |
| pDSW980 | pJC69-derivative, lacIq, P204∷sufI-gfp, Spcr | This study |
| pQE60-SufI | pQE60 sufIHis, Ampr | This study |
Plasmids
Plasmid pDSW932 (P204∷sufI-gfp, Ampr) was constructed by PCR amplification of sufI using chromosomal DNA as template and primers P985 (5′-caggaattctcactcagtcggcgtcag-3′) and P986 (5′-cctctgcaggttgttgttcggtaccggattgaccaacag-3′). The 1434-bp product was cut with EcoRI and PstI (sites underlined) and ligated into the same sites of pDSW208.12 Plasmid pDSW980 was created by digesting pDSW932 with SphI and ScaI to generate a 4454-bp fragment containing lacIq P204∷sufI-gfp. This fragment was ligated to a 2668-bp fragment of the CRIM vector pJC6961 cut with SphI and HincII. A plasmid that expresses a gfp-sufI fusion was constructed in several steps. An 870-bp fragment carrying the Tat signal sequence coding region from torA and gfp was amplified by PCR using plasmid pTB636 as template and primers P1074 (5′-cgggaattcaacaataacgatctctttcag-3′) and P1075 (5′-cgaagcttaggatccattgtatagttcatccatgcc-3′). This product was cut with EcoRI and HindIII (sites underlined) and ligated into the same sites of pDSW20412 to create pDSW962 (P204∷sstorA-gfp). A derivative of sufI lacking its Tat signal sequence was then amplified from plasmid pDSW932 with primers P1112 (5′-cggggatcccaacagcaaccgctacccgtt-3′) and P1113 (5′-ccgggatccttacggtaccggattgaccaa-3′). The 1344-bp product was cut with BamHI (sites underlined) and ligated into the same site of pDSW962. An isolate with the BamHI fragment in the desired orientation was designated pDSW978. The lacIq P204∷sstorA-gfp-sufI was moved into a CRIM vector by ligating the 3170-bp SphI–ScaI fragment from pDSW962 into SpaI–HincII-digested pJC69 to create pDWS964. pDSW979 was created by ligating the 4502-bp SphI–ScaI fragment that carries lacIq P204∷sstorA-gfp-sufI from pDSW978 into pJC69 cut with SphI and HincII. Plasmid pQE60-SufI directs the production of the E. coli SufI precursor protein with a C-terminal hexahistidine tag. The sufI gene was amplified from chromosomal DNA with primers SUFI1 (5′-gcgcgaattcgttttacatggagcaaatatg-3′) and SUFI2 (5′-gcgcagatctcggtaccggattgaccaacag-3′), digested with EcoRI and BglII (sites underlined) and cloned into the same sites in pQE60 (Qiagen) to give pQE60-SufI.
Localization of fusions between GFP and SufI
Cultures were grown at 30 °C in LB with 1 mM IPTG to an OD600 of ∼ 0.5. Slides for visualizing live cells were prepared by pipetting 40 μl of molten 1% agarose (in water) onto a pre-warmed glass slide with two spacers made of laboratory tape. A second glass slide was placed on top to create a thin agarose pad. Once cooled, the two slides were separated and 5 μl of live culture in growth medium was applied to the agarose pad and covered with a #1 coverslip. Cells were immediately photographed at 100× magnification essentially as previously described.63 Typical exposure times for GFP were about 2 s. Digital images were imported to Adobe Photoshop for cropping and making minor adjustments to brightness and contrast. Final figures were assembled in Canvas.
For assessing the dependency of SufI-GFP on FtsZ, strains were grown in LB with 1 mM IPTG at 30 °C to mid log phase. Then 1 ml of culture was pelleted in a microcentrifuge and the cells were resuspended in 1 ml of LB0N. The washed cells were used at a dilution of 1:10 to inoculate 30 °C LB and 37 °C LB0N (both contained 1 mM IPTG). Cells were allowed to grow for 1.5 h, by which time the ftsZ(Ts) mutant in LB0N had become filamentous. Aliquots of the cultures were transferred to an agarose pad and photographed as described above.
For assessing dependency on FtsL, FtsQ and FtsN, the respective depletion strains carrying pDSW932 were grown in LB containing antibiotics, 0.2% l-arabinose and 10 μM IPTG. (The arabinose induces expression of the respective fts gene, while the IPTG induces expression of the sufI-gfp fusion.) Once the cultures reached mid log phase, 1 ml was pelleted in a microcentrifuge and the cells were resuspended in 1 ml of LB (for FtsL- and FtsQ-depletion strains) or 1 ml of LB0N (for the FtsN-depletion strain). The washed cells were used at a dilution of 1:50 to inoculate LB or LB0N containing antibiotics, 10 μm IPTG and either 0.2% arabinose or 0.2% glucose. Cultures were grown at 30 or 37 °C for 3–4 h, by which time the cells growing in the presence of glucose had become filamentous. Aliquots of the cultures were transferred to an agarose pad and photographed as described above.
To verify the periplasmic localization of SufI/GFP fusion proteins (Supplemental Fig. 1), transformants were grown at 30 °C in LB containing Amp and 10 μM IPTG to mid log phase. Aliquots of the cultures were transferred to an agarose pad and photographed as described above.
To examine localization of SufI and various division proteins in a tat null mutant (Supplemental Fig. 2), strain DADE was transformed with a series of gfp fusion plasmids. Transformants were grown at 30 °C in LB containing Amp to log phase. Then, aliquots of the cultures were transferred to an agarose pad and photographed as described above. IPTG was included at 10 μM only in the case of the ftsZ-gfp fusion; the remaining gfp constructs were adequately expressed in the absence of IPTG. Because some of the fusion constructs were not well tolerated in the DADE background, the length of the growth period was different for different strains. GFP, GFP-SufI, SufI-GFP and FtsZ-GFP overnight cultures were diluted 1:1000 and grown for 4 h. GFP-FtsI and GFP-FtsN overnight cultures were diluted 1:20 and grown for 1 h.
Complementation of a ΔsufI mutant
Strains were grown at 37 °C in LB to mid log phase. Then an aliquot was harvested by centrifugation and the cell pellet was taken up in one-tenth volume LB0N with 1 mM IPTG. An aliquot of this suspension was used to inoculate LB0N with 1 mM IPTG and the culture was grown at 37 °C. Samples were removed periodically, fixed and photographed by phase contrast microscopy to analyze morphology. Cell lengths were measured with Image-Pro software version 4.1 (Media Cybernetics, Silver Spring, Md.).
Immunoblotting
GFP-SufI and SufI-GFP were detected by immunoblotting as described.64
Overproduction of SufI
Plasmid pQE60-SufI was used for the overproduction of C-terminally hexahistidine-tagged SufI. The plasmid was transformed into the Δtat strain DADE harbouring pREP4; cells were grown at 37 °C with shaking in LB medium to mid log phase and induced by addition of IPTG to 2 mM final concentration. After 6 h further growth, cells were harvested by centrifugation (7000g, 15 min). Cell pellets were resuspended in 20 mM Mops (pH 7.2), 200 mM NaCl and 30 mM imidazole (buffer A) and disrupted by several passes through a French pressure cell at an operating pressure of 8000 psi. Cell debris was removed by centrifugation and the cleared lysate was applied to a nickel-charged, 5-ml HiTrap HP column (GE Healthcare) equilibrated in buffer A. SufI was eluted using a linear gradient of imidazole to 500 mM in buffer A. Fractions containing SufI, as assessed by SDS-PAGE, were pooled, concentrated using a 4-ml 30-kDa molecular mass cutoff Amicon Ultra (Millipore) and loaded onto a Superdex-75 10-300 column (GE Healthcare) equilibrated in 20 mM Mops (pH 7.2), 200 mM NaCl. The elution position of SufI from the column corresponded to an apparent molecular mass of approximately 55 kDa. N-terminal sequencing of the purified SufI showed that the first 17 residues corresponding to part of the signal peptide had been cleaved (data not shown).
Crystallization and X-ray diffraction
Crystals were grown by the vapour-diffusion method in sitting drops at 20 °C. Screens were set up with a crystallization robot (TECAN, UK). Crystallization drops were obtained by mixing 0.2 μl of protein solution in 20 mM Mops (pH 7.2), 200 mM NaCl with 0.2 μl of the crystallization screen and were equilibrated against 100 μl of mother liquor. SufI crystals were grown by mixing the protein solution at 0.75 mg/ml with EB Wizard I condition 37 (2.5 M NaCl, 100 mM imidazole, pH 8.0). Crystals were visible after 5 days with dimensions of approximately 50 μm × 100 μm × 200 μm.
Diffraction data from the SufI crystals were collected at 100 K on beam lines ID23-1 and ID29 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. The crystals were cryoprotected in 8 M sodium formate. The crystals belonged to either space group P21 or space group P212121, with both the monoclinic and orthorhombic crystals growing from the same condition, although the orthorhombic form required a longer growth period. All X-ray data integration and scaling were performed with the computer programs MOSFLM65 and Scala.66 The lattices of the two crystal forms were not found to be related. Table 3 gives the crystallographic data collection and processing statistics.
Structure determination, model building and refinement
Initial structure factor phases were obtained for the orthorhombic SufI crystals by molecular replacement. The program SCWRL 3.067 was used to model the SufI side chains onto the closely homologous blue copper oxidase CueO from E. coli (PDB ID 1PF3,38 32% sequence identity) and the resulting model was used as a search model for molecular replacement with the computer program CCP4-PHASER.66 Two copies of the search model were found in the asymmetric unit and the SufI sequence was then built into the resulting density with X-fit,68 followed by multiple rounds of iterative refinement in BUSTER-TNT69 and model building in X-fit. Non-crystallographic symmetry (NCS) restraints were applied in BUSTER-TNT, with restraints lifted for individual residues where required. Once completed, the orthorhombic SufI structure was used as a search model for molecular replacement in the monoclinic crystal form, again with CCP4-PHASER.66 This provided a good solution with two copies in the asymmetric unit. The molecules were in a conformation very similar to those in the orthorhombic form with the exception of the loop around residues 55–66, which shifted between the two crystal forms. This region was removed from the search model and the molecular replacement search was repeated. Residues 55–66 were manually rebuilt into the resultant electron density with X-fit and then multiple rounds of iterative refinement with NCS in BUSTER-TNT, followed by model building in X-fit, were performed. The NCS for individual residues was lifted locally where required. Full details of the refinement parameters for both crystal forms can be found in Table 3. The anomalous difference density was calculated with the program CCP4-FFT70 and all superpositions were performed with the program CCP4-LSQKAB.71
Protein Data Bank accession numbers
Coordinates and structure factors have been deposited in the PDB with accession numbers 2uxt and 2uxv.
Acknowledgements
We thank Kenn Rudd for the sufI<>aph mutant, Piet de Boer and Thomas Bernhardt for plasmid pTB6 and strain TB54, D. RayChaudhuri for strain DRC14, J. M. Ghigo for strain JMG265 and J. Chen for plasmid JC69 and strain JOE170. Work in D.W.'s lab was funded by the Department of Microbiology and a grant from the NIH (R01 GM59893). S.J.R.A. was supported by an NIH Training Grant in Biotechnology (T32 GM08365). The Wellcome Trust funded a studentship for M.T., and P.R. was funded by the MRC grant G0400389 to S.M.L. We thank Gérard Bricogne and the Global Phasing Consortium for access to a beta version of the refinement program Buster-TNT. F.S. is a Royal Society University Research Fellow.
Edited by M. Guss
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jmb.2008.12.043
Contributor Information
David S. Weiss, Email: david-weiss@uiowa.edu.
Susan M. Lea, Email: susan.lea@path.ox.ac.uk.
Appendix A.
Table 1 Localization of SufI-GFP in fts mutants.
References
- 1.Arends S.J.R., Williams K.B., Kustusch R.J., Weiss D.S., Erhmann M. The Periplasm. ASM Press; Washington, DC: 2007. Cell division; pp. 173–197. [Google Scholar]
- 2.Errington J., Daniel R.A., Scheffers D.J. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 2003;67:52–65. doi: 10.1128/MMBR.67.1.52-65.2003. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goehring N.W., Beckwith J. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 2005;15:R514–R526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Margolin W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell. Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicente M., Rico A.I., Martinez-Arteaga R., Mingorance J. Septum enlightenment: assembly of bacterial division proteins. J. Bacteriol. 2006;188:19–27. doi: 10.1128/JB.188.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss D.S. Bacterial cell division and the septal ring. Mol. Microbiol. 2004;54:588–597. doi: 10.1111/j.1365-2958.2004.04283.x. [DOI] [PubMed] [Google Scholar]
- 7.Samaluru H., SaiSree L., Reddy M. Role of SufI (FtsP) in cell division of Escherichia coli: evidence for its involvement in stabilization of the division assembly. J. Bacteriol. 2007;189:8044–8052. doi: 10.1128/JB.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato J., Nishimura Y., Yamada M., Suzuki H., Hirota Y. Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J. Bacteriol. 1988;170:3967–3977. doi: 10.1128/jb.170.9.3967-3977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam M., Fraipont C., Rhazi N., Nguyen-Disteche M., Lakaye B., Frere J.M. The bimodular G57–V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyzes peptide bond formation from thiolesters and does not catalyze glycan chain polymerization from the lipid II intermediate. J. Bacteriol. 1997;179:6005–6009. doi: 10.1128/jb.179.19.6005-6009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botta G.A., Park J.T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J. Bacteriol. 1981;145:333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratt B.G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl Acad. Sci. USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss D.S., Chen J.C., Ghigo J.M., Boyd D., Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy M. Role of FtsEX in cell division of Escherichia coli: viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. J. Bacteriol. 2007;189:98–108. doi: 10.1128/JB.01347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K., Go N. Function and molecular evolution of multicopper blue proteins. Cell. Mol. Life Sci. 2005;62:2050–2066. doi: 10.1007/s00018-004-5076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley N.R., Palmer T., Berks B.C. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 2000;275:11591–11596. doi: 10.1074/jbc.275.16.11591. [DOI] [PubMed] [Google Scholar]
- 16.Berks B.C., Palmer T., Sargent F. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 2005;8:174–181. doi: 10.1016/j.mib.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Cline K., Theg S.M. The Sec and Tat protein translocation pathways in chloroplasts. In: Dalbey R.E., Koehler C., Tamanoi F., editors. vol. XXV. Elsevier; San Diego, CA: 2007. pp. 455–485. (The Enzymes, Molecular Machines Involved in Protein Transport across Cellular Membranes). [Google Scholar]
- 18.Lee P.A., Tullman-Ercek D., Georgiou G. The bacterial twin-arginine translocation pathway. Annu. Rev. Microbiol. 2006;60:373–395. doi: 10.1146/annurev.micro.60.080805.142212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller M., Klosgen R.B. The Tat pathway in bacteria and chloroplasts (review) Mol. Membr. Biol. 2005;22:113–121. doi: 10.1080/09687860500041809. [DOI] [PubMed] [Google Scholar]
- 20.Alami M., Luke I., Deitermann S., Eisner G., Koch H.G., Brunner J., Muller M. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell. 2003;12:937–946. doi: 10.1016/s1097-2765(03)00398-8. [DOI] [PubMed] [Google Scholar]
- 21.Alami M., Trescher D., Wu L.F., Muller M. Separate analysis of twin-arginine translocation (Tat)-specific membrane binding and translocation in Escherichia coli. J. Biol. Chem. 2002;277:20499–20503. doi: 10.1074/jbc.M201711200. [DOI] [PubMed] [Google Scholar]
- 22.Chanal A., Santini C.L., Wu L.F. Specific inhibition of the translocation of a subset of Escherichia coli TAT substrates by the TorA signal peptide. J. Mol. Biol. 2003;327:563–570. doi: 10.1016/s0022-2836(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 23.McDevitt C.A., Buchanan G., Sargent F., Palmer T., Berks B.C. Subunit composition and in vivo substrate-binding characteristics of Escherichia coli Tat protein complexes expressed at native levels. FEBS J. 2006;273:5656–5668. doi: 10.1111/j.1742-4658.2006.05554.x. [DOI] [PubMed] [Google Scholar]
- 24.Yahr T.L., Wickner W.T. Functional reconstitution of bacterial Tat translocation in vitro. EMBO J. 2001;20:2472–2479. doi: 10.1093/emboj/20.10.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bageshwar U.K., Musser S.M. Two electrical potential-dependent steps are required for transport by the Escherichia coli Tat machinery. J. Cell Biol. 2007;179:87–99. doi: 10.1083/jcb.200702082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLisa M.P., Lee P., Palmer T., Georgiou G. Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J. Bacteriol. 2004;186:366–373. doi: 10.1128/JB.186.2.366-373.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatzixanthis K., Palmer T., Sargent F. A subset of bacterial inner membrane proteins integrated by the twin-arginine translocase. Mol. Microbiol. 2003;49:1377–1390. doi: 10.1046/j.1365-2958.2003.03642.x. [DOI] [PubMed] [Google Scholar]
- 28.Holzapfel E., Eisner G., Alami M., Barrett C.M., Buchanan G., Luke I. The entire N-terminal half of TatC is involved in twin-arginine precursor binding. Biochemistry. 2007;46:2892–2898. doi: 10.1021/bi062205b. [DOI] [PubMed] [Google Scholar]
- 29.Moser M., Panahandeh S., Holzapfel E., Muller M. In vitro analysis of the bacterial twin-arginine-dependent protein export. Methods Mol. Biol. 2007;390:63–80. doi: 10.1007/978-1-59745-466-7_5. [DOI] [PubMed] [Google Scholar]
- 30.Sargent F., Gohlke U., De Leeuw E., Stanley N.R., Palmer T., Saibil H.R., Berks B.C. Purified components of the Escherichia coli Tat protein transport system form a double-layered ring structure. Eur. J. Biochem. 2001;268:3361–3367. doi: 10.1046/j.1432-1327.2001.02263.x. [DOI] [PubMed] [Google Scholar]
- 31.Feilmeier B.J., Iseminger G., Schroeder D., Webber H., Phillips G.J. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 2000;182:4068–4076. doi: 10.1128/jb.182.14.4068-4076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini C.L., Bernadac A., Zhang M., Chanal A., Ize B., Blanco C., Wu L.F. Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of its periplasmic localization in response to osmotic up-shock. J. Biol. Chem. 2001;276:8159–8164. doi: 10.1074/jbc.C000833200. [DOI] [PubMed] [Google Scholar]
- 33.Thomas J.D., Daniel R.A., Errington J., Robinson C. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 2001;39:47–53. doi: 10.1046/j.1365-2958.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 34.Bernhardt T.G., de Boer P.A. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 2003;48:1171–1182. doi: 10.1046/j.1365-2958.2003.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:1–11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhardt T.G., de Boer P.A. Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Mol. Microbiol. 2004;52:1255–1269. doi: 10.1111/j.1365-2958.2004.04063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley N.R., Findlay K., Berks B.C., Palmer T. Escherichia coli strains blocked in Tat-dependent protein export exhibit pleiotropic defects in the cell envelope. J. Bacteriol. 2001;183:139–144. doi: 10.1128/JB.183.1.139-144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts S.A., Weichsel A., Grass G., Thakali K., Hazzard J.T., Tollin G. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl Acad. Sci. USA. 2002;99:2766–2771. doi: 10.1073/pnas.052710499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krissinel E., Henrick K. Multiple Alignment of Protein Structures in Three Dimensions. In: Berthold M.R., editor. CompLife 2005, LNBI 3695. Springer-Verlag Berlin Heidelberg; 2005. pp. 67–78. [Google Scholar]
- 40.DeLano W. DeLano Scientific; San Carlos, CA: 2004. The PyMOL Molecular Graphics System. [Google Scholar]
- 41.San Miguel M., Marrington R., Rodger P.M., Rodger A., Robinson C. An Escherichia coli twin-arginine signal peptide switches between helical and unstructured conformations depending on the hydrophobicity of the environment. Eur J. Biochem. 2003;270:3345–3352. doi: 10.1046/j.1432-1033.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- 42.Holm L., Sander C. Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 1995;20:478–480. doi: 10.1016/s0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- 43.Babor M., Gerzon S., Raveh B., Sobolev V., Edelman M. Prediction of transition metal-binding sites from apo protein structures. Proteins. 2008;70:208–217. doi: 10.1002/prot.21587. [DOI] [PubMed] [Google Scholar]
- 44.Roberts S.A., Wildner G.F., Grass G., Weichsel A., Ambrus A., Rensing C., Montfort W.R. A labile regulatory copper ion lies near the T1 copper site in the multicopper oxidase CueO. J. Biol. Chem. 2003;278:31958–31963. doi: 10.1074/jbc.M302963200. [DOI] [PubMed] [Google Scholar]
- 45.Kataoka K., Komori H., Ueki Y., Konno Y., Kamitaka Y., Kurose S. Structure and function of the engineered multicopper oxidase CueO from Escherichia coli—deletion of the methionine-rich helical region covering the substrate-binding site. J. Mol. Biol. 2007;373:141–152. doi: 10.1016/j.jmb.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 46.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gouet P., Courcelle E., Stuart D.I., Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- 49.de Vries S.J., van Dijk A.D., Bonvin A.M. WHISCY: what information does surface conservation yield? Application to data-driven docking. Proteins. 2006;63:479–489. doi: 10.1002/prot.20842. [DOI] [PubMed] [Google Scholar]
- 50.Augustine A.J., Quintanar L., Stoj C.S., Kosman D.J., Solomon E.I. Spectroscopic and kinetic studies of perturbed trinuclear copper clusters: the role of protons in reductive cleavage of the O–O bond in the multicopper oxidase Fet3p. J. Am. Chem. Soc. 2007;129:13118–13126. doi: 10.1021/ja073905m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueki Y., Inoue M., Kurose S., Kataoka K., Sakurai T. Mutations at Asp112 adjacent to the trinuclear Cu center in CueO as the proton donor in the four-electron reduction of dioxygen. FEBS Lett. 2006;580:4069–4072. doi: 10.1016/j.febslet.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 52.Addinall S.G., Cao C., Lutkenhaus J. FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 1997;25:303–309. doi: 10.1046/j.1365-2958.1997.4641833.x. [DOI] [PubMed] [Google Scholar]
- 53.Addinall S.G., Bi E., Lutkenhaus J. FtsZ ring formation in fts mutants. J. Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Addinall S.G., Lutkenhaus J. FtsA is localized to the septum in an FtsZ-dependent manner. J. Bacteriol. 1996;178:7167–7172. doi: 10.1128/jb.178.24.7167-7172.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hale C.A., de Boer P.A. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- 56.Gerding M.A., Ogata Y., Pecora N.D., Niki H., de Boer P.A. The trans-envelope Tol–Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol. Microbiol. 2007;63:1008–1025. doi: 10.1111/j.1365-2958.2006.05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heidrich C., Templin M.F., Ursinus A., Merdanovic M., Berger J., Schwarz H. Involvement of N-acetylmuramyl-l-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 2001;41:167–178. doi: 10.1046/j.1365-2958.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- 58.Wexler M., Sargent F., Jack R.L., Stanley N.R., Bogsch E.G., Robinson C. TatD is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in sec-independent protein export. J. Biol. Chem. 2000;275:16717–16722. doi: 10.1074/jbc.M000800200. [DOI] [PubMed] [Google Scholar]
- 59.Arends S.J., Weiss D.S. Inhibiting cell division in Escherichia coli has little if any effect on gene expression. J. Bacteriol. 2004;186:880–884. doi: 10.1128/JB.186.3.880-884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J.C., Weiss D.S., Ghigo J.M., Beckwith J. Septal localization of FtsQ, an essential cell division protein in Escherichia coli. J. Bacteriol. 1999;181:521–530. doi: 10.1128/jb.181.2.521-530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J.C., Beckwith J. FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division. Mol. Microbiol. 2001;42:395–413. doi: 10.1046/j.1365-2958.2001.02640.x. [DOI] [PubMed] [Google Scholar]
- 62.Haldimann A., Wanner B.L. Conditional-replication, integration, excision, and retrieval plasmid–host systems for gene structure–function studies of bacteria. J. Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt K.L., Peterson N.D., Kustusch R.J., Wissel M.C., Graham B., Phillips G.J., Weiss D.S. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J. Bacteriol. 2004;186:785–793. doi: 10.1128/JB.186.3.785-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mercer K.L., Weiss D.S. The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J. Bacteriol. 2002;184:904–912. doi: 10.1128/jb.184.4.904-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leslie A.G.W. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsl. Protein Crystallogr. 1992;26:1–11. [Google Scholar]
- 66.Collaborative Computational Project The CCP4 suite: programs for protein crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 67.Canutescu A.A., Shelenkov A.A., Dunbrack R.L., Jr. A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci. 2003;12:2001–2014. doi: 10.1110/ps.03154503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McRee D.E. XtalView/Xfit—a versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- 69.Blanc E., Roversi P., Vonrhein C., Flensburg C., Lea S.M., Bricogne G. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004;60:2210–2221. doi: 10.1107/S0907444904016427. [DOI] [PubMed] [Google Scholar]
- 70.Read R.J., Schierbeek A.J. A phased translation function. J. Appl. Crystallogr. 1988;21:490–495. [Google Scholar]
- 71.Krissinel E., Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 Localization of SufI-GFP in fts mutants.