Abstract
Systemic fungal infections pose a significant risk to patients following allogeneic hematopoietic cell transplantation (alloHCT). Voriconazole (Vfend®, Pfizer) is an oral second-generation triazole antifungal agent that offers broad spectrum of coverage against fungal species and is frequently utilized in the post-HCT setting. Herein, we describe five patients who were initially believed to be experiencing a flare of cutaneous chronic graft-versus-host disease (cGvHD), but who were actually exhibiting phototoxicity caused by voriconazole. A high index of suspicion for this adverse reaction in the post-alloHCT setting will prevent misdiagnosis and avoid inappropriate therapy for cGvHD.
Introduction
Systemic fungal infections pose a significant risk to patients following allogeneic hematopoietic cell transplantation (alloHCT). In the early post-transplant period, neutropenia is the primary risk factor and frequently fluconazole is used as effective prophylaxis against Candida infections. Late after engraftment, however, the most important fungal infection is invasive aspergillosis, particularly during periods of active graft versus host disease and increased corticosteroid use [1,2]. Voriconazole (Vfend®, Pfizer), a second-generation oral triazole antifungal agent, is the treatment of choice for invasive aspergillosis [3], and its ease of administration and favorable safety profile have resulted in increased utilization as antifungal prophylaxis, especially when there is high risk for aspergillosis [4]. Like the other azole antifungals, voriconazole inhibits cytochrome P450-dependent 14-α-sterol demethylase, which is required for ergosterol biosynthesis, resulting in destruction of the fungal plasma membrane. It is effective against aspergillus sp, molds, and yeasts [5].
Following FDA approval in 2001, expanded utilization of voriconazole has led to increased recognition of the drug’s potential side effects, which include vision changes (20%), hallucinations (15%) hepatic enzyme abnormalities (12–20%), and skin reactions (17%) [5]. In preliminary clinical studies, a photosensitive rash was reported in only 2% of patients (41/2090) [5]. Herein, we describe five patients who were believed to be suffering from recalcitrant skin cGvHD, but who, were actually experiencing cutaneous phototoxicity caused by voriconazole. A high index of suspicion for this adverse reaction in post-alloHCT patients will facilitate the proper diagnosis and avoid inappropriate therapy for cGvHD.
Materials and Methods
In this retrospective analysis, five patients with cGvHD who developed voriconazole-associated phototoxicity were identified at the National Institutes of Health (NIH) dermatology service between May 2003 and August 2007. Medical records, clinical photography, and histological specimens were reviewed. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Patient | Age/Sex | Primary disease | Hx acute skin GvHD | Hx chronic skin GvHD | cGvHD of other organ systems | Voriconazole dose | Latency between voriconazole initiation and phototoxicity | Concurrent potentially photosensitizing drugs | cGVHD therapy at time of phototoxicity diagnosis | Resolution of phototoxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15/M | Alveolar rhabdomyosarcoma | Yes | Yes | Oral | 200mg BID | 2 weeks | None | Prednisone 8mg QD | 3 weeks |
| 2 | 6/F | Pre-B cell ALL | Yes | Yes | None | 175mg BID | 6 months | HCTZ | Prednisone 25mg BID Tacrolimus 0.5mg QAM, 0.25 QPM MMF 750mg q12hr Hydrocortisone 2.5% BID (face) Fluocinolone 0.1% BID (body) |
2 weeks |
| 3 | 40/F | Large B-cell lymphoma | Yes | Yes | Ocular Oral Hepatic |
200mg BID | 42 months | Amitriptyline | Extracorporeal photopheresis Prednisone 40mg QD Fluocinolone solution (scalp) Clobetasol ointment 0.05% (body) Tacrolimus ointment (face) |
Improved in3 days, continued cGvHD flare |
| 4 | 47/M | CML | Yes | Yes | Ocular Hepatic Pulmonary |
200mg BID | 1 month | None | Prednisone 50mg QOD MMF 1gm q12hr |
4 months |
| 5 | 59/M | Myeloid metaplasia/myelofibrosis | No | No | Ocular Hepatic Pulmonary |
200mg BID | 3 months | Lisinopril Furosemide |
Prednisone 20mg QOD | 3 months |
Abbreviations: ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; HCTZ, hydrochlorothiazide; MMF, mycophenolate mofetil
Results
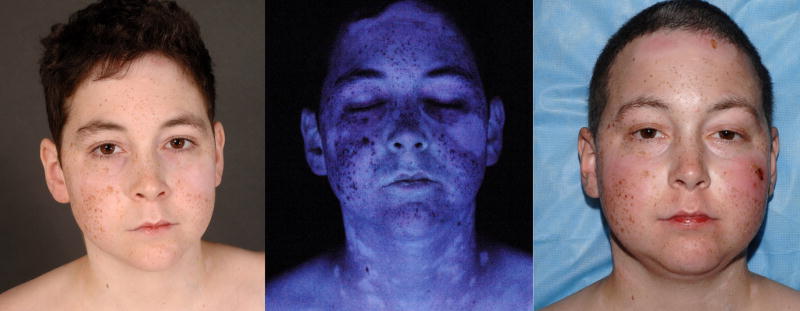
Patient 1 was a 15 year-old male diagnosed at age six with metastatic alveolar rhabdomyosarcoma. He underwent a matched nonmyeloablative allogeneic peripheral blood hematopoietic cell transplantation (alloPBHCT) following failure of multiple therapies. Acute cutaneous GvHD was confirmed by skin biopsy on day +8 post-transplant. Recurrent lichen planus-like cGvHD was noted at day +100, followed by vitiligo 20 months post-transplant (Figure 1A, B).
Figure 1.
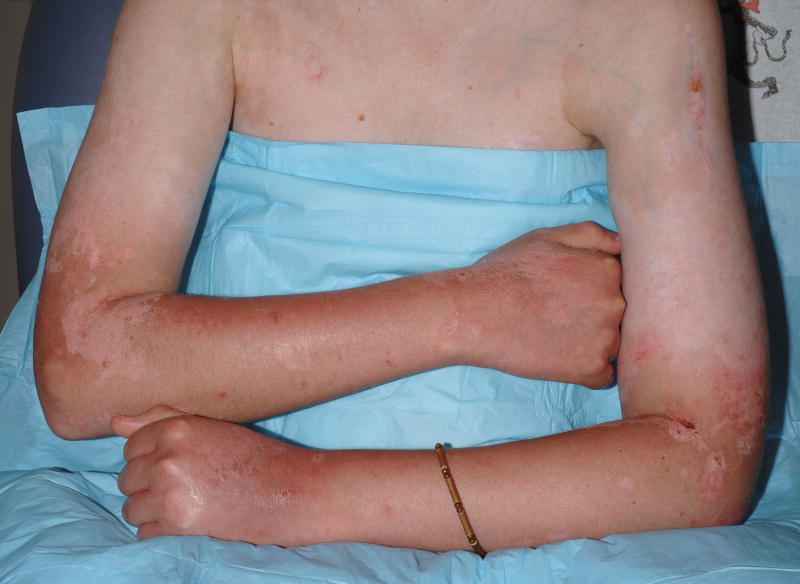
Patient 1 prior to voriconazole administration (A,B) and after treatment with eight weeks of voriconazole 200mg BID (C,D). Multiple depigmented patches are present on the head and neck area (A) and accentuate upon visualization with Wood’s lamp (B), consistent with vitiligo; (C) erythema and denuded bullae are present on the forehead, bilateral cheeks, nose, and chin; skin changes are most pronounced at sites of previous depigmentation; (D) prominent erythema on the photoexposed surfaces of the forearms and distal arms with superficial sunburn-like desquamation.
Thirty-two months post-transplant, voriconazole was initiated for a presumed pulmonary aspergillosis. Two months later, the patient developed bright erythema of the forehead, malar cheeks, hands, forearms, and arms, which his treating physicians attributed to a flare of skin cGvHD. At the time of his NIH evaluation, several erosions and intact bullae were also noted on the head, neck, arms, anterior legs, and dorsal aspects of both feet (Figure 1C, D).
A diagnosis of phototoxicity and drug-induced pseudoporphyria cutanea tarda (pseudo-PCT) secondary to voriconazole was made. Voriconazole was replaced by posaconazole and strict photoprotection was instituted. The bullae resolved in 3 weeks and the erythema gradually dissipated.
Patient 2 was a 6 year-old female who underwent a 5/6 mismatched unrelated myeloablative umbilical cord blood transplantation for pre-B cell acute lymphoblastic leukemia (ALL) 11 months before presenting to the NIH. Two months post-transplant, the patient developed biopsy-confirmed acute cutaneous GvHD that was treated with prednisone 30 mg/day and which she continued to require for recurrent skin flares. Voriconazole was initiated empirically three months post-transplant for pulmonary nodules identified on computed tomography (CT) scan. Three months later, the voriconazole dosage was increased from 125 mg twice daily to 175 mg twice daily after new pulmonary nodules were identified.
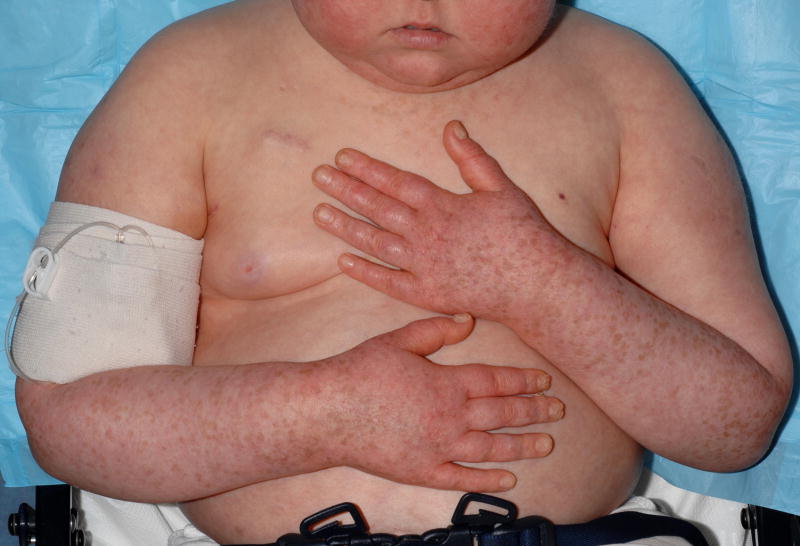
At the time of her NIH evaluation, patchy macular erythema and extensive macular pigmentation (solar lentigines) were noted on photoexposed areas of the body (Figure 2). These findings were consistent with chronic photodamage. Two weeks following replacement of voriconazole with posaconazole and institution of strict photoprotection, the erythema on photoexposed skin surfaces had faded to a light pink.
Figure 2.
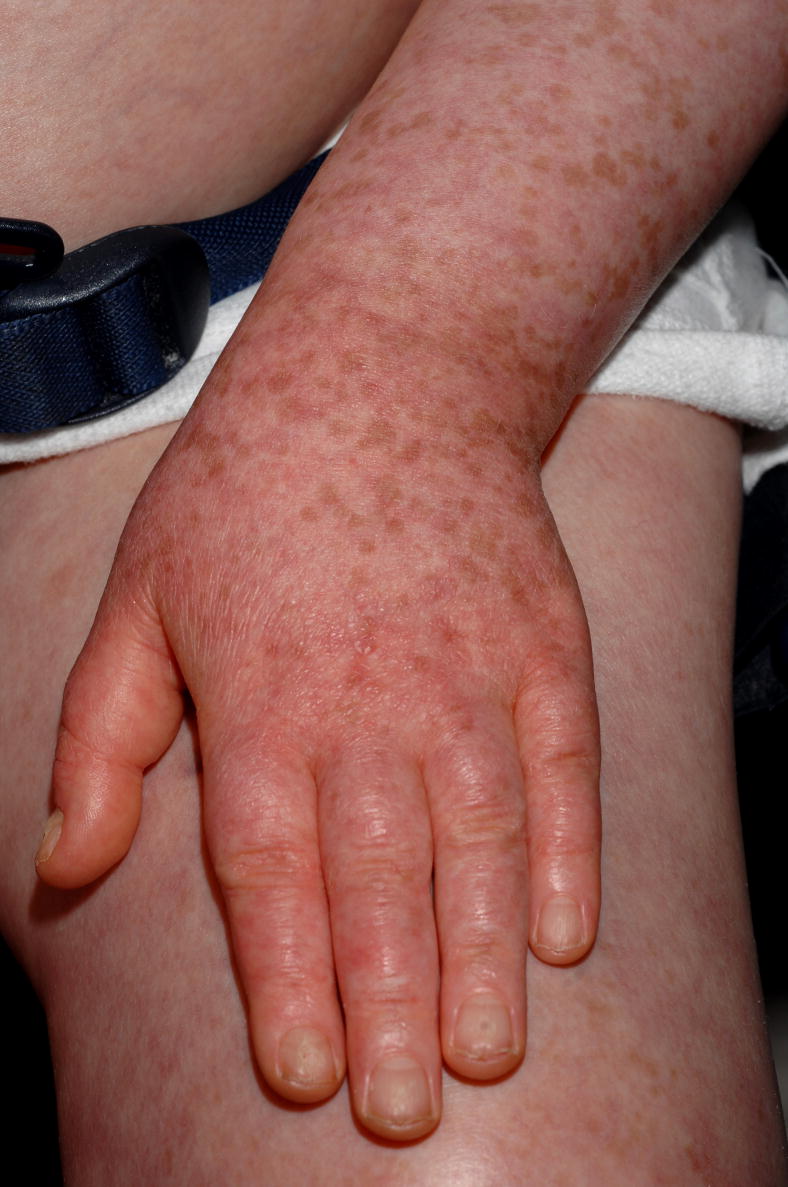
Patient 2 following eleven months treatment with voriconazole 175mg BID. Erythema and multiple round tan/brown macules on the dorsal surfaces of the hands and forearms suggestive of ongoing phototoxicity and chronic photodamage, respectively. Similar erythema and macular pigmentation is present on the upper chest.
Patient 3 was a 40 year-old female with relapsed mediastinal, large B-cell lymphoma resistant to chemotherapy and radiation, who underwent a matched non-myeloablative alloPBHCT. The patient developed acute skin GvHD on day +44 and progressive, treatment-resistant sclerotic skin manifestations of cGvHD 15 months post-transplant. Voriconazole was instituted three years prior to the patient’s initial visit following pulmonary aspergillosis.
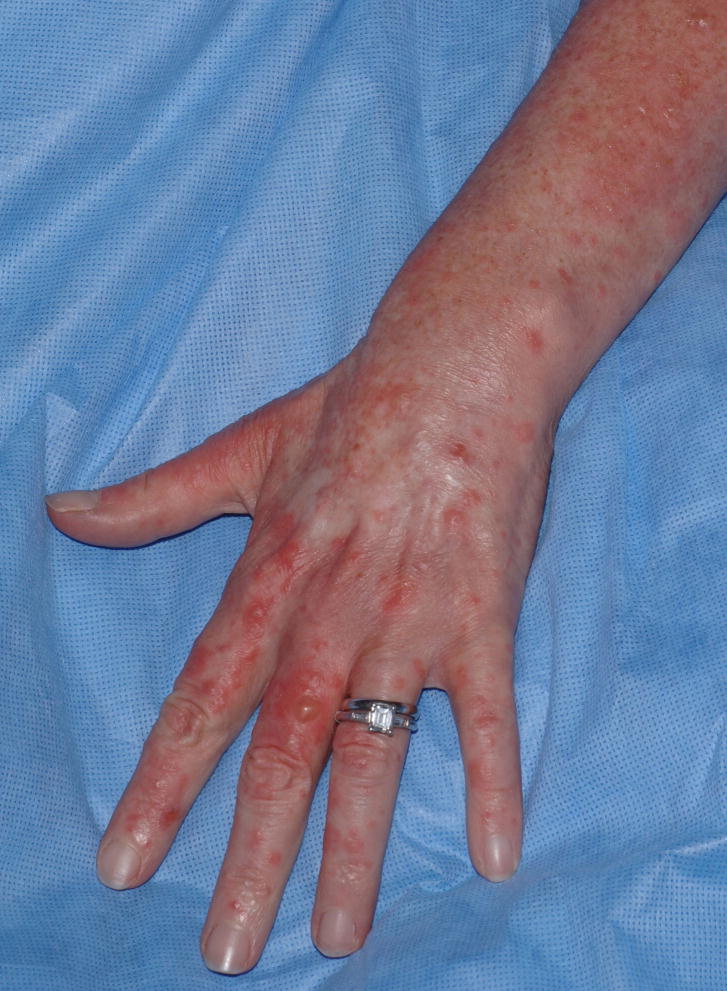
Six years, five months post-transplant, the patient presented to the NIH with new bullae on brightly erythematous and edematous sun-exposed areas of the upper and lower extremities as well as the V-area of the neck. Three days prior to presentation, she spent several hours at a picnic, reportedly primarily under the shade of a tree. Vesicles, bullae, and papules were noted on her hands and extensor forearms that evening (Figure 3A). A 3 mm punch biopsy taken from the left hand demonstrated epidermal necrosis most consistent with a phototoxic drug reaction (Figure 3B). The patient experienced marked improvement in erythema of photoexposed skin after switching from voriconazole to posaconazole and strict photoprotection, but she continued to have erythematous and erosive skin disease in the photoprotected areas, including her abdomen and back. The prednisone dosage was increased to 60 mg daily and the patient received rituximab weekly for four weeks in an attempt to control the GvHD. Three months later, she died of pseudomonal sepsis.
Figure 3.
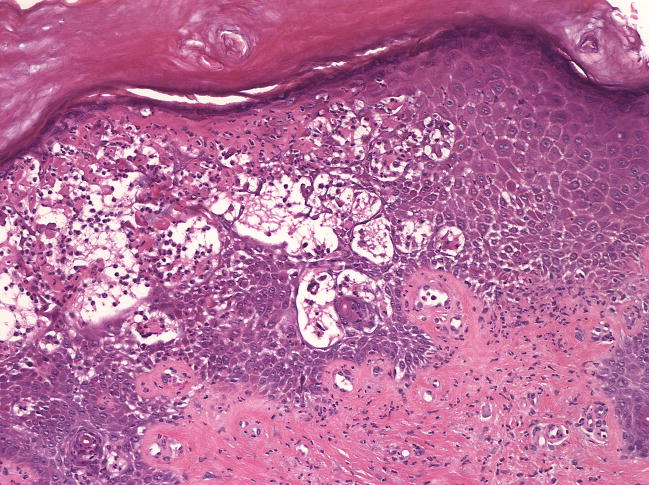
A) Patient 3 following 36 months of voriconazole 200mg BID. The patient had ongoing cGvHD of the skin with erythema and sclerosis, but developed acute exacerbation of erythema with new intact bullae formation after sun exposure; B) skin biopsy demonstrates epidermal necrosis at all levels associated with a moderately dense neutrophilic infiltrate (hematoxylin and eosin, 10x magnification).
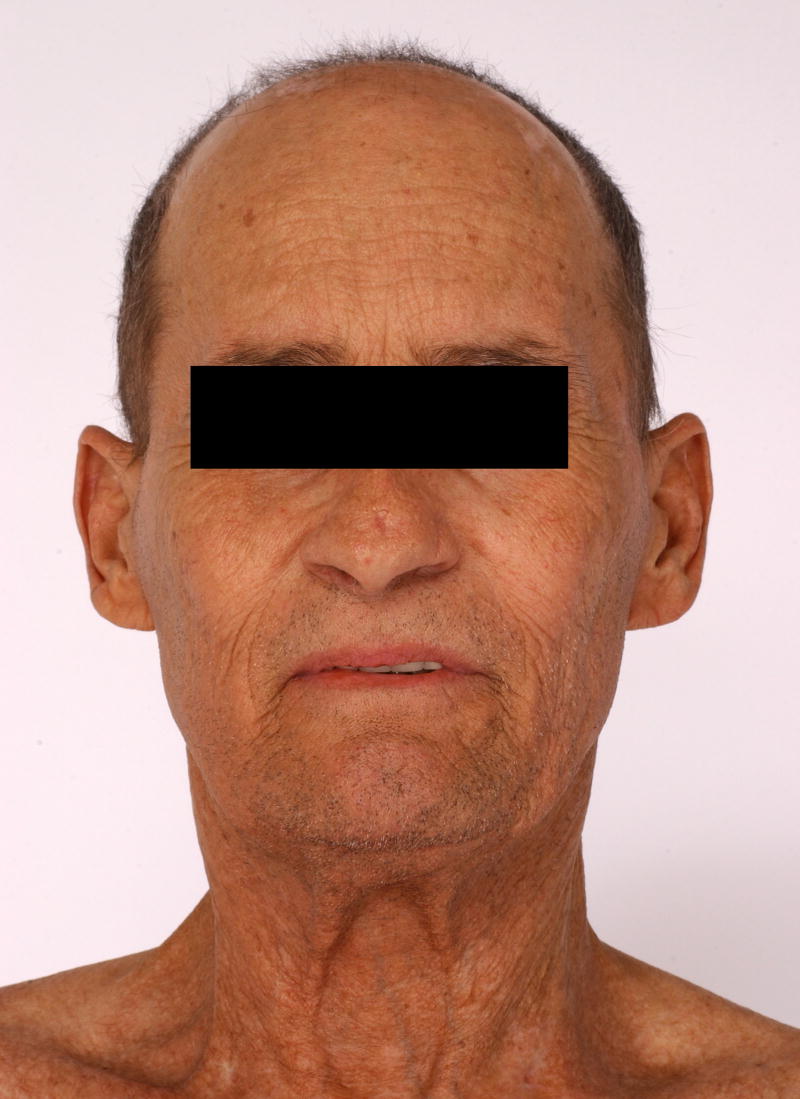
Patient 4 was a 47 year-old male who underwent a matched related nonmyeloablative alloPBHCT for chronic myelogenous leukemia (CML). One month post-transplant, the patient developed acute cutaneous GvHD. Similar eruptions recurred over his torso for the next several years. Thirty-one months post-transplant, voriconazole was initiated for a presumed pulmonary aspergillosis. Three months later the patient presented to the NIH with an 8-week history of coarse, erythematous to violaceous scaly, thickened skin on his cheeks and posterior and lateral neck (Figure 4). A 3 mm biopsy from the neck was suggestive of cutaneous lupus. The anti-nuclear antibody (ANA) titer was 5.4EU (0.0–0.9). Anti-ENA screen and anti-ds DNA titers were negative. Voriconazole-induced phototoxicity was diagnosed and the patient’s antifungal treatment was changed to itraconazole. Four months later, the skin erosions had resolved and the violaceous discoloration improved significantly.
Figure 4.
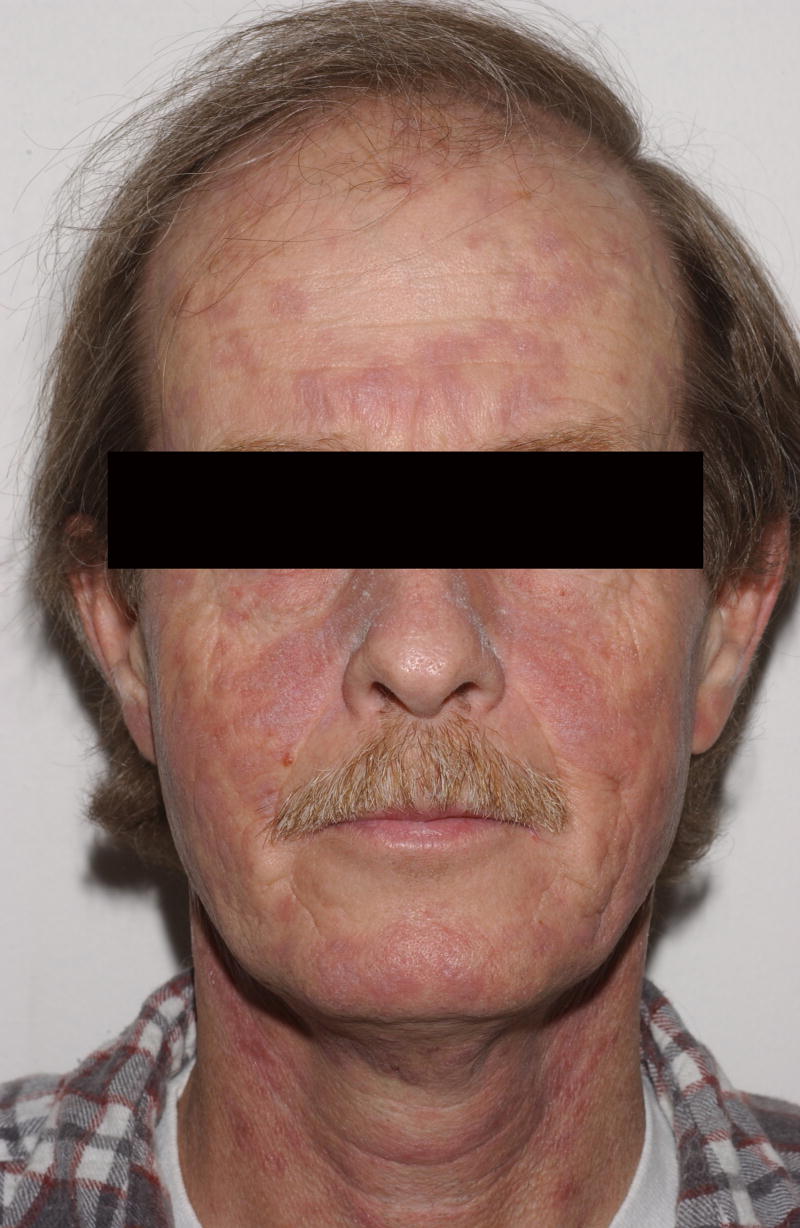
Patient 4 following 3 months of treatment with voriconazole 200mg BID. Numerous erythematous/violaceous plaques on the face.
Patient 5 was a 59 year-old male with myeloid metaplasia and myelofibrosis who underwent a matched nonmyeloablative alloPBHCT and presented to the NIH nine months post-transplantation. Additionally, four months post-transplantation the patient developed biopsy proven hepatic GvHD. The following month voriconazole was initiated for a presumed pulmonary fungal infection. At the time of his evaluation, the patient reported a 6-week history of asymptomatic scaly plaques on sun exposed areas of the arms, neck, scalp, and v-neck area of the upper chest (Figure 5A). He described a recent increase in outdoor activity following recovery from his pulmonary infection. Biopsy from the left arm showed destruction of the basal epidermal layer with necrotic keratinocytes, compatible with a diagnosis of GvHD or phototoxic drug reaction (Figure 5C). Although this patient had received voriconazole episodically in the ensuing few months, the rash continued to improve (Figure 5B) with the institution of strict photoprotection.
Figure 5.
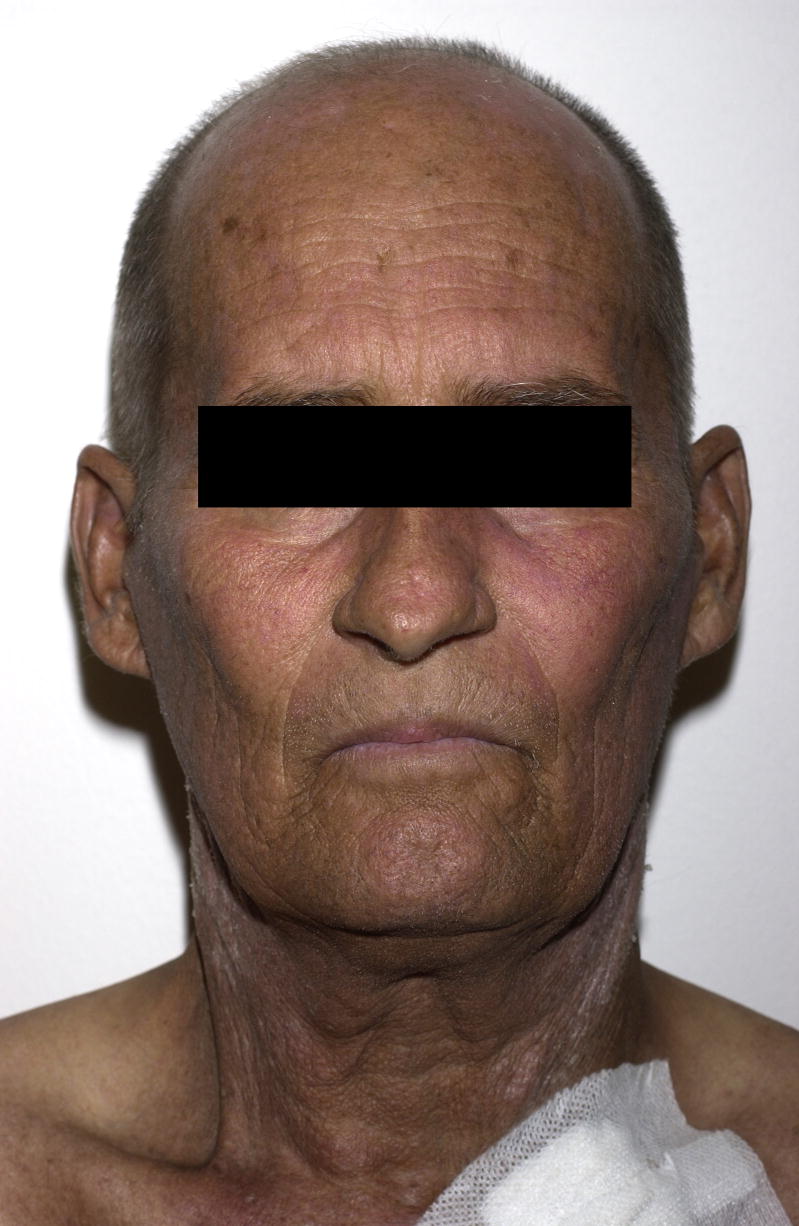
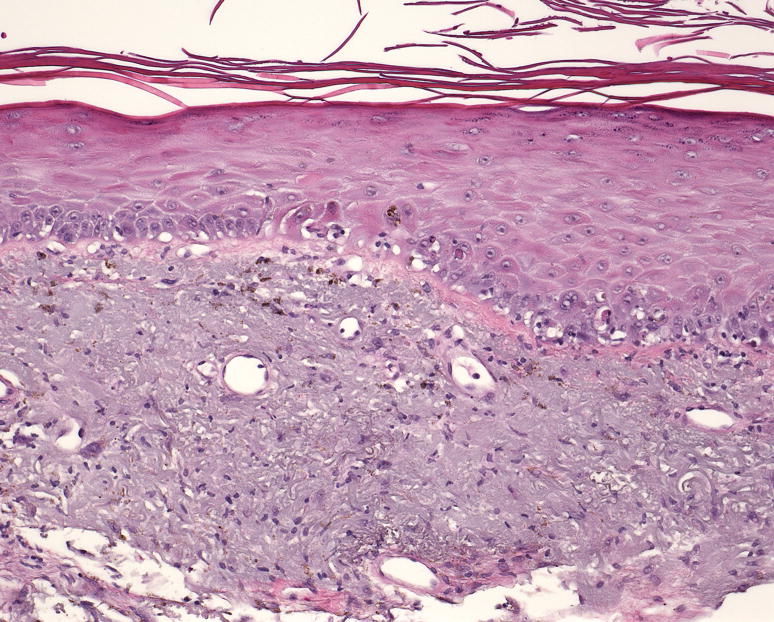
A) Patient 5 following four months treatment with voriconazole 200mg BID. Marked hyperpigmentation on the photoexposed surfaces of the face; B) pigmentation is significantly improved one year later; C) pathology demonstrated hyperkeratosis, acanthosis, destruction of the basal epidermal layer with necrotic keratinocytes, and a sparse lymphocytic infiltrate with frequent pigmented macrophages (hematoxylin and eosin, magnification 10x).
Discussion
Drug-induced photosensitivity may be photoallergic or phototoxic. Phototoxic reactions do not require prior exposure and are dependent both on the dose of drug and ultraviolet light (UVL) exposure. Thus, a high dose of a photosensitizing medication combined with prolonged and or intense UVL exposure may result in erythema and edema resembling acute sunburn [6]. Repeated UVL exposure in patients on a photosensitizing drug can simulate the effect of chronic actinic damage with development of hyperpigmentation, dry skin, and solar lentigines. Phototoxicity occurs when the appropriate wavelength of light penetrates the skin where the photosensitizing drug or its metabolite is located. The photosensitizer absorbs the UVL energy, inducing oxygen radical formation, followed by cellular damage and an inflammatory response [7]. Pseudo-PCT represents a severe form of phototoxicity with bullae formation resembling PCT without associated elevated blood porphyrin levels (Patient 1). Voriconazole is a known phototoxic agent [8–17] and it has also been reported to induce pseudo-PCT [18–21].
In the five patients described, the diagnosis of phototoxicity was confounded by a history of cutaneous cGvHD and the inability to reliably distinguish between cGvHD and phototoxicity based on routine histological methods. We based the diagnosis of voriconazole-induced phototoxicity on a number of clinical features, including distinct photodistribution of skin lesions, a history of UVL exposure contemporaneous with drug administration, the absence of signs of reactivation of GvHD in other organ systems, and improvement of skin lesions upon discontinuation of voricaonazole and/or institution of strict photoprotection. Although biopsies were performed in 3 cases, the histopathologic findings of phototoxicity, including necrotic or apoptotic keratinocytes in various layers of the epidermis, vacuolization of the cells in the basal layer, and a mild superficial perivascular lymphohistiocytic infiltrate may also be observed in exanthematous GvHD. Similarly, the presence of epidermal necrosis is reflected in the severity of both GvHD and phototoxicity and cannot definitely separate either disease process [22–24]. Even the presence of eosinophils in the dermal infiltrate, a frequent finding in cutaneous drug reactions, does not reliably distinguish between phototoxic drug reactions and GvHD [22,25]. As exacerbations of cutaneous cGvHD may be triggered by both phototoxic and non-phototoxic drug eruptions, careful monitoring for persistent skin symptoms is warranted following identification and discontinuation of the causative agent.
Although voriconazole has been available for a number of years and is frequently used for prophylaxis [4], it is not yet approved for this indication. Posaconazole, a recently approved orally bioavailable antifungal, has been shown to prevent invasive aspergillosis in patients at high risk for infection, including patients with severe acute or chronic GvHD on treatment with corticosteroids [26]. Posaconazole has proven to be effective in patients with systemic mycosis refractory to voriconazole or who develop intolerable side effects with voriconazole [27]. It has broad-spectrum activity against Candida, Aspergillus, and Zygomycetes species [28,29]. Posaconazole-associated photosensitivity has not been described in the literature and we have not observed it in our cGvHD patients.
In our experience, the incidence of phototoxicity associated with voriconazole in the post-HCT setting is significantly higher than that reported in the initial clinical trial data. Whereas the initial voriconazole clinical trials enrolled hospitalized patients, our NIH population consisted primarily of outpatients with greater opportunity for exposure to UVL.
Because UVL exposure may induce a flare of cutaneous cGvHD independent of concurrent phototoxic drug exposure [30, 31], sun avoidance and photoprotection are recommended for all patients evaluated in our cGvHD clinic. Furthermore, patients with cGvHD are often exposed to other potential photosensitizing agents in addition to voriconazole, including trimethoprim/sulfamethosazole, hydrochlorthiazide, and furosemide. Specific recommendations include sun avoidance at peak hours, use of protective clothing, and liberal application of both physical and chemical sunblocks that absorb both UV-A and UV-B radiation. Laundry rinse-cycle additives are also available in order to increase the UV protective factor of clothing [30].
Although the exact incidence of cutaneous drug reactions (CDR) is difficult to quantify, they are among the most frequent adverse drug reactions, accounting for approximately 10–20% of all reported adverse events. CDRs are most consistently associated with exposure to antimicrobial agents, including sulfonamides, flouroquinolones, penicillins, and cephalosporins [32, 33]. The most common CDR is a morbilliform rash, characterized by fine pink macules and papules on the trunk which eventually coalesce and spread to the extremities. Skin lesions typically begin 1–2 weeks following drug exposure and fade slowly following discontinuation. By contrast, urticarial hypersensitivity reactions usually develop within 1–2 days of drug initiation, and individual lesions resolve within 24 hours. The most worrisome CDRs are erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis, a spectrum of severe drug hypersensitivity with variable skin involvement ranging from localized dusky targetoid plaques to widespread skin sloughing with mucosal membrane involvement and significant associated mortality. Often symptoms of a severe CDR do not present until several weeks after initial drug exposure [34].
Attention to the details of the clinical presentation and the drug administration history are of utmost importance in establishing the correct diagnosis in patients with cGvHD, given their complex medical history and the high likelihood of polypharmacy. In this setting, when confronted with a new onset eruption that is not considered diagnostic of cGvHD based on NIH consensus criteria [35], helpful clinical criteria in defining a CDR include: 1) exclusion of other causes for the eruption, such as viral exanthem; 2) identification of a temporal relationship between drug use and onset of the rash 3) improvement following drug cessation; and reactivation of the rash should upon rechallenge (if performed) [36].
As voriconazole has become a frequent choice for antifungal prophylaxis and treatment in the setting of alloHCT, the possibility of voriconazole-induced phototoxicity should be considered in all new skin eruptions, even those that simulate a flare of cutaneous cGvHD in patients with known cutaneous cGvHD. The recognition of voriconazole phototoxicity is especially important as efficacious treatment alternatives are readily available, and accurate diagnosis of phototoxic drug reaction will prevent misdiagnosis of cGvHD and unnecessary immunosuppression.
Acknowledgments
Funding/Support: This research was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute. Ms. Patel was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 2.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 3.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 4.Wingard JR, Carter SL, Walsh TJ, et al. Results of a Randomized, Double-Blind Trial of Fluconazole (FLU) vs. Voriconazole (VORI) for the Prevention of Invasive Fungal Infections (IFI) in 600 Allogeneic Blood and Marrow Transplant (BMT) Patients. ASH Annual Meeting Abstracts. 2007;110:163. [Google Scholar]
- 5.Vfend (Voriconazole, Oral and Intravenous Formulations), NDA 21–267, Briefing Document for FDA Antiviral Drugs Advisory Committee Meeting, 4 October 2001. [Accessed 2007 October 18];Pfizer Inc. [online] Available from URL: http://www.fda.gov/ohrms/dockets/ac/01/briefing/3792b2_01_Pfizer.pdf.
- 6.Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007 Jul;6(4):431–43. doi: 10.1517/14740338.6.4.431. [DOI] [PubMed] [Google Scholar]
- 7.Toback AC, Anders JE. Phototoxicity from systemic agents. Dermatol Clin. 1986 Apr;4(2):223–30. [PubMed] [Google Scholar]
- 8.Denning DW, Griffiths CE. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exp Dermatol. 2001 Nov;26(8):648–53. doi: 10.1046/j.1365-2230.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr Infect Dis J. 2002 Mar;21(3):240–8. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Cortez KJ, Walsh TJ, Bennett JE. Successful treatment of coccidioidal meningitis with voriconazole. Clin Infect Dis. 2003 Jun 15;36(12):1619–22. doi: 10.1086/375235. [DOI] [PubMed] [Google Scholar]
- 11.Vandecasteele SJ, Van Wijngaerden E, Peetermans WE. Two cases of severe phototoxic reactions related to long-term outpatient treatment with voriconazole. Eur J Clin Microbiol Infect Dis. 2004 Aug;23(8):656–7. doi: 10.1007/s10096-004-1176-7. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol. 2004 Nov–Dec;21(6):675–8. doi: 10.1111/j.0736-8046.2004.21614.x. [DOI] [PubMed] [Google Scholar]
- 13.Racette AJ, Roenigk HH, Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005 May;52(5 Suppl 1):S81–5. doi: 10.1016/j.jaad.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Hilliard T, Edwards S, Buchdahl R, et al. Voriconazole therapy in children with cystic fibrosis. J Cyst Fibros. 2005 Dec;4(4):215–20. doi: 10.1016/j.jcf.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Auffret N, Janssen F, Chevalier P, Guillemain R, Amrein C, Le Beller C. Voriconazole photosensitivity: 7 cases [in French] Ann Dermatol Venereol. 2006 Apr;133(4):330–2. doi: 10.1016/s0151-9638(06)70910-3. [DOI] [PubMed] [Google Scholar]
- 16.Serra Soler G, Delgado Sánchez O, Esteban Marcos E, Martínez-López I, Femenías Sureda M. Voriconazole-associated phototoxicity [in Spanish] Farm Hosp. 2006 Nov–Dec;30(6):386–7. doi: 10.1016/s1130-6343(06)74012-0. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007 Mar 1;44(5):e55–6. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 18.Dolan CK, Hall MA, Blazes DL, Norwood CW. Pseudoporphyria as a result of voriconazole use: a case report. Int J Dermatol. 2004 Oct;43(10):768–71. doi: 10.1111/j.1365-4632.2004.02177.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharp MT, Horn TD. Pseudoporphyria induced by Voriconazole. J Am Acad Dermatol. 2005 Aug;53(2):341–5. doi: 10.1016/j.jaad.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed. 2007 Feb;23(1):29–31. doi: 10.1111/j.1600-0781.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 21.Kwong WT, Hsu S. Pseudoporphyria associated with voriconazole. J Drugs Dermatol. 2007 Oct;6(10):1042–4. [PubMed] [Google Scholar]
- 22.Weedon D. The lichenoid reaction pattern (‘interface dermatitis’) In: Weedon D, editor. Skin Pathology. 2. New York, NY: Churchill Livingstone; 2002. p. 46. [Google Scholar]
- 23.Weedon D. Reactions to physical agents. In: Weedon D, editor. Skin Pathology. 2. New York, NY: Churchill Livingstone; 2002. p. 600. [Google Scholar]
- 24.Hood AF, Farmer ER. Interface dermatitis. In: Farmer ER, Hood AF, editors. Pathology of the skin. 2. New York, NY: McGraw-Hill; 2000. p. 204. [Google Scholar]
- 25.Nghiem P. The “Drug vs Graft-vs-Host Disease” Conundrum Gets Tougher, but There is an Answer: The Challenge to Dermatologists. Arch Dermatol. 2001 Jan;137:75–76. doi: 10.1001/archderm.137.1.75. [DOI] [PubMed] [Google Scholar]
- 26.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007 Jan 25;356(4):335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007 Jan 1;44(1):2–12. doi: 10.1086/508774. Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- 28.Boucher HW, Groll AH, Chiou CC, Walsh TJ. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs. 2004;64(18):1997–2020. doi: 10.2165/00003495-200464180-00001. [DOI] [PubMed] [Google Scholar]
- 29.Segal BH, Almyroudis NG, Battiwalla M, et al. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin Infect Dis. 2007 Feb 1;44(3):402–9. doi: 10.1086/510677. [DOI] [PubMed] [Google Scholar]
- 30.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006 Apr;12(4):375–96. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Kitajima T, Imamura S. Graft-versus-host reaction enhanced by ultraviolet radiation. Arch Dermatol Res. 1993;285(8):499–501. doi: 10.1007/BF00376823. [DOI] [PubMed] [Google Scholar]
- 32.Ibia EO, Schwartz RH, Widermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000 Jul;136(7):849–54. doi: 10.1001/archderm.136.7.849. [DOI] [PubMed] [Google Scholar]
- 33.van der Linden PD, van der Lei J, Vlug AE, Stricker BH. Skin reactions to antibacterial agents in general practice. J Clin Epidemiol. 1998 Aug;51(8):703–8. doi: 10.1016/s0895-4356(98)00041-9. [DOI] [PubMed] [Google Scholar]
- 34.Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol Rev. 2001 Sep;53(3):357–79. [PubMed] [Google Scholar]
- 35.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005 Dec;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994 Nov 10;331(19):1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]