Abstract
Traumatic spinal cord injury (SCI) in mammals causes widespread glial activation and recruitment to the CNS of innate (e.g., neutrophils, monocytes) and adaptive (e.g., T and B lymphocytes) immune cells. To date, most studies have sought to understand or manipulate the post-traumatic functions of astrocytes, microglia, neutrophils or monocytes. Significantly less is known about the consequences of SCI-induced lymphocyte activation. Yet, emerging data suggest that T and B cells are activated by SCI and play significant roles in shaping post-traumatic inflammation and downstream cascades of neurodegeneration and repair. Here, we provide neurobiologists with a timely review of the mechanisms and implications of SCI-induced lymphocyte activation, including a discussion of different experimental strategies that have been designed to manipulate lymphocyte function for therapeutic gain.
Keywords: autoimmune, lymphocyte, autoantibody, T cell, B cell, CNS injury
Introduction to adaptive immunity, lymphocyte regulation and autoimmune responses
General principles of lymphocytes and adaptive immunity
Cells of innate and adaptive immunity play fundamentally different roles during an immune response. Innate immune cells (e.g., neutrophils, monocytes, dendritic cells, etc.) provide immediate defense against infection or other inflammatory stimuli but also help activate and recruit cells of the adaptive immune system (i.e., T and B lymphocytes). This is accomplished through complex interactions involving antigen presentation and the release of various inflammatory mediators (e.g., cytokines and chemokines). Once lymphocytes recognize antigen, they proliferate, yielding large numbers of “daughter cells” or clones specific for that antigen (clonal selection or clonal expansion). Some clones persist indefinitely, providing “memory” against the inciting antigen. Childhood vaccines attempt to exploit this aspect of adaptive immunity by intentionally creating a persistent repertoire of lymphocytes with exquisite specificity for select pathogens (e.g., measles virus). More recently, therapeutic vaccines have been developed that try to exploit neuroantigen-specific T and B cell function for repairing the CNS (see below).
During an adaptive immune response, lymphocyte clones not entering the memory pool become effector cells which enter the circulation and home to sites of injury or infection in search of antigen. Therein, effector lymphocytes secrete cytokines and antibodies that orchestrate and amplify the functions of other immune cells. For example, when antibody binds to antigen, an immune complex is created that facilitates phagocytic removal of antigen. Antibodies also activate innate immune cells by cross-linking Fc receptors—specialized antibody receptors that have tyrosine-based activation motifs2,16,101. Immune complexes catalyze activation of serum and tissue complement—a system of proteins that circulate in the blood and are produced by glia and neurons in the CNS5,11,29,74,126. Activated complement proteins serve as chemotactic agents (e.g., C5a, C3b) to amplify immune cell recruitment and function and they can also directly lyse cells bearing target antigen. These antigen-specific immune responses will persist until the antigen is removed or until endogenous regulatory cascades suppress the response.
Originally, it was thought that each lymphocyte receptor was specific for a single antigen. More recently, it has become clear that a single T cell receptor (TCR) or immunoglobulin can bind epitopes found on a number of distinct antigens, i.e., they are polyspecific37,132. For example, a TCR or an antibody may be specific for a measles virus capsid protein but may also bind a protein present in CNS myelin. However, that same TCR or antibody might not bind skeletal muscle or Mycobacterium tuberculosis. Because the number of potential antigens far exceeds the number of lymphocytes found in the immune system, polyspecificity is important for optimal host-defense; however, it also introduces the potential for triggering autoimmunity.
Immunoregulation and autoimmunity
When lymphocytes recognize and become activated by self-antigens (e.g., non-pathogenic peptides, proteins, lipids or nucleic acids found in the host), autoimmune disease can develop. Due to the processes of receptor editing and positive and negative selection, most self-reactive lymphocytes are deleted or inactivated (anergized) during development. Why then do we maintain the ability to respond to self antigens throughout adulthood? Although the answer to this question is not entirely clear, there is compelling data to suggest that self-antigens are important for modulating the sensitivity of naïve lymphocytes and reducing the overall number of ligands needed to initiate an adaptive immune response68,121. In this way, autoimmune recognition plays a physiological role in adjusting the strength of the immune response. It is believed that autoimmune pathology occurs only after an ambiguous threshold of activation is surpassed in autoreactive cells. This likely requires an optimal but poorly understood interaction between antigen, antigen presenting cell and lymphocytes, with concomitant dysregulation of assorted immunoregulatory networks that maintain immunological tolerance (reviewed in24). For example, naturally occurring regulatory T cells (Tregs) suppress immune responses23,65,90,115,118. This suppression is antigen-specific, can be enhanced experimentally and is mediated by diverse mechanisms including the release of immune suppressive cytokines (e.g., TGFβ, see 1,13 for review). The fact that depletion of naturally occurring Tregs causes autoimmune disease in otherwise normal animals is evidence of the profound role for Tregs in maintaining immune tolerance106,107.
Mechanisms of trauma-induced autoimmune disease: Lessons from Multiple Sclerosis
Despite the presence of multiple immune regulatory checkpoints, autoimmune disease does occur. Multiple sclerosis (MS) is the most common and best-understood CNS autoimmune disease. Like its primary animal model, experimental autoimmune encephalomyelitis (EAE), MS results when lymphocytes bind antigens on healthy myelin and axons causing demyelination and axon injury with the subsequent onset of neurological dysfunction77. Controversy surrounds the precise mechanism(s) responsible for triggering MS. The prevailing theory is that a viral infection activates polyspecific lymphocyte clones that also recognize CNS autoantigens85,133. In this scenario, lymphocytes that become activated by viral antigens bypass the blood-brain barrier where they become reactivated by myelin and/or axonal proteins that share sequence homology with the viral protein. Repeated exposure to the virus may trigger disease relapse in established MS4.
Autoreactive lymphocytes also can infiltrate the CNS subsequent to BBB dysfunction caused by high levels of circulating cytokines released during infection or following idiopathic microvascular trauma35,55,75,92,97,119,137. Recent data also indicate dysregulation of Tregs in MS patients102,128. Once autoreactive lymphocytes bypass mechanisms of immune tolerance and gain access to the brain or spinal cord, they cause inflammation and cell death culminating in demyelination, axonal degeneration and neurological deterioration56,127. The autoimmune response is self-propagating and is characterized by recurrent BBB dysfunction with continued upregulation of molecules needed for T cell activation, i.e., MHC class II and costimulatory molecules on resident glia or infiltrating innate immune cells45,46,50,58,63,78,100. As a result of ongoing pathology, new CNS antigens are released that have the potential to ligate and activate other autoimmune lymphocytes 25,26,71,79,84. This phenomenon is known as epitope spreading and can perpetuate neuroinflammation and pathological progression.
Obviously, the etiology of SCI and MS is different. However, there are some surprising commonalities (discussed below; also see Popovich et al.94) and in both cases, it appears that mechanisms of immunological tolerance are suppressed, resulting in the onset and maintenance of a chronic autoimmune response.
Autoimmunity induced by Traumatic SCI
Autoimmune reactions are triggered by traumatic SCI in animals and humans. In rats, SCI activates MBP-reactive T cells capable of causing neuroinflammation and transient paralysis94. In SCI humans, the frequency of MBP-reactive T cells increases, reaching levels that approximate those seen in MS patients60. Also, >50% of SCI patients have increased levels of serum and CSF antibodies specific for galactocerebroside, MBP and GM-1 gangliosides43,86,123. We have recently shown that CNS autoantibodies are significantly elevated in the circulation of >90% of SCI mice6. A preliminary analysis of potential autoantigen targets in SCI mice suggests that the breadth of autoimmune responses elicited by SCI extends beyond the predicted repertoire of neuroantigens (e.g., MBP) (Figure 1). Indeed, because SCI-induced autoimmune responses are so prevalent, it is logical to question how they are initiated and their pathophysiological significance.
Figure 1.
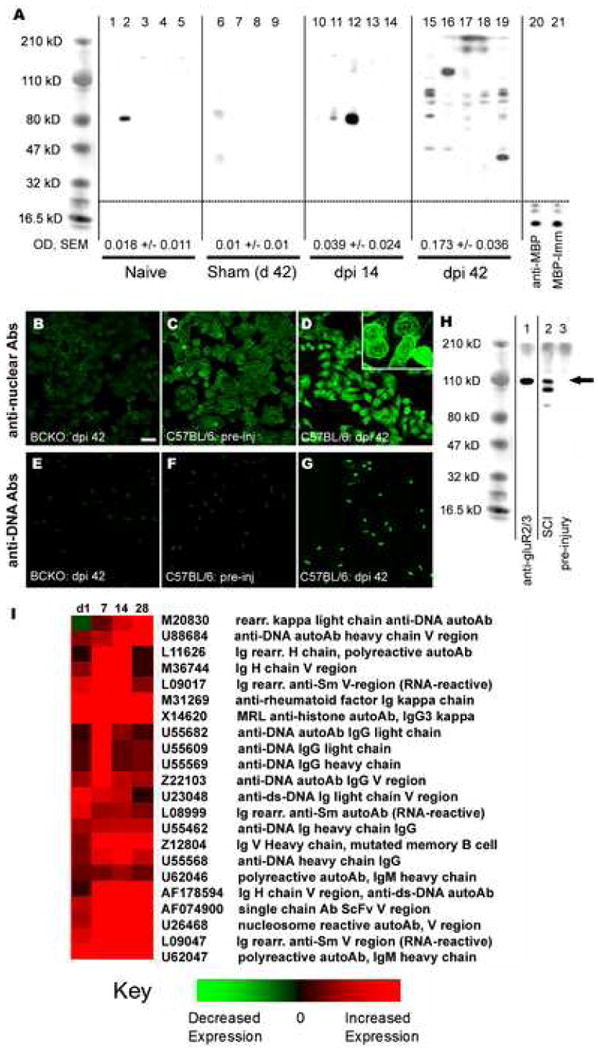
SCI triggers production of antibodies specific for systemic and CNS antigens. (A) Sera from naïve (n=5; lanes 1-5), sham (n=4; lanes 6-9) or SCI mice at 14 (n=5; lanes 10-14) or 42 days post-injury (n=5; lanes 15-19) were used to probe homogenized CNS proteins. Lane 20 was probed with a commercial anti-MBP antibody (1:40,000) and lane 21 with serum (1:200) from a mouse immunized 35d previously with 200 μg guinea-pig MBP in adjuvant. Quantitation of mean band intensity in each lane within a group is shown and reveals marked induction of anti-CNS antibodies at 42 dpi vs. naïve, sham and 14 dpi groups (OD ± SEM at the bottom of each column; *** p< 0.001 vs naive, **p < 0.01 vs sham, *p < 0.05 vs dpi 14, ANOVA with Tukey's post test; mean binding in samples from sham or uninjured mice were not different from zero; t-test). (B-G) Sera were used to probe HEp-2 (B-D) and Crithidia luciliae (E-G) substrate slides (for anti-nuclear and anti-DNA antibodies, respectively). Sera from SCI B cell knockout (BCKO, B&E) and uninjured (C&F) mice fail to show anti-nuclear or anti-DNA binding. In contrast, sera from SCI C57BL/6 mice (D&G) reveal strong binding to nuclear antigens and DNA. High power (inset in D) shows labeling consistent with binding to nuclear membranes, nucleosomes and/or centromeres. (H) Sera from SCI mice indicate possible binding to neuronal glutamate receptors [∼110 kD; compare anti-GluR2/3 positive-control labeling (lane 1) with SCI (dpi 42) sera labeling in lane 2]. Pre-injury sera failed to bind neuronal antigens (lane 3; arrow). Scale = 25 μm (B-G). (I) Gene chip data shows upregulation of selected genes encoding autoreactive immunoglobulins within the mouse SCI contusion site. Data are expressed relative to genes found in the spinal cord of mice given laminectomy (sham) surgery but no SCI. (A-H reprinted with permission from The Journal of Neurochemistry, November 2006, Volume 99(4), pg 1073-1087.)
Mechanisms of lymphocyte activation after SCI
Presumably, the trauma and vascular injury caused by SCI overcome mechanisms of peripheral tolerance and initiate the earliest phases of lymphocyte activation. This may occur subsequent to neuroantigens being released into the blood and lymphatics with drainage into spleen and lymph nodes41,64,73. Also, cells present in the injury site may sequester debris and carry CNS antigens to secondary lymphoid organs (spleen and lymph nodes) via these same humoral routes54. There, neuroantigens would be processed and presented by antigen presenting cells (e.g., dendritic cells) to lymphocytes, triggering lymphocyte activation. Support for peripheral priming of autoimmunity after SCI comes from our studies in mice showing that the number of activated T and B cells increases in the spleen and bone marrow within 24 hours of SCI6,53. By three days, T cells isolated from secondary lymphoid tissues of SCI rats are capable of causing transient hind limb paralysis and spinal cord inflammation when they are injected intravenously into naïve recipients94. The pathogenic potential of SCI-activated B cells still remains to be directly tested, but early indications suggest that B cells also are pathological6. Data from other models also confirm a direct link between primary CNS pathology and peripheral lymphocyte activation36,41,64,91.
Once lymphocytes gain access to the injury site, they persist indefinitely6,59,110,120. Indeed, T and B cell numbers increase in the mouse SCI lesion through at least 9 weeks post-injury. This occurs despite complete restoration of BBB integrity17,93,129, suggesting that intraspinal cytokine/chemokine gradients exist chronically and are able to upregulate integrin expression on endothelia and nearby cells9,12,70,80,109. These chemokine gradients and adhesion molecules represent molecular targets for manipulating the effects of intraspinal lymphocytes after SCI10,15,34,39,40. The persistence or progressive increase in lymphocyte numbers may also be explained by lymphocyte reactivation and proliferation within the injured spinal cord. Indeed, intraspinal lymphocytes co-localize with parenchymal microglia, perivascular macrophages, infiltrating monocytes and B cells. All are cells that express the MHC class II antigens and costimulatory molecules (e.g., CD80, CD86) necessary for lymphocyte activation6,59,95,96,108,120. The presence of large T and B cell clusters in the injured spinal cord that are morphologically identical to germinal centers found in lymph node and spleen (sites of active lymphocyte proliferation and differentiation) further supports the hypothesis that cells are reactivated locally6. Similar “ectopic” lymphoid follicles have been described at sites of chronic autoimmune inflammation (e.g., synovium in rheumatoid arthritis, meninges in MS)61,112,114. Additional support for local activation comes from data showing intraspinal expression of genes encoding autoantibodies specific for systemic autoantigens (Fig. 1). Preliminary data suggest that potent lymphocyte survival/activation factors (e.g., APRIL or BAFF81,135) are expressed in the injured spinal cord (data not shown). The chronic expression of these factors could support autoimmune lymphocyte survival and function. As such, therapies designed to block these factors may prove beneficial by reducing the effects of post-injury autoimmunity. But regardless of why lymphocytes persist indefinitely at the lesion site, there is no doubt that these cells are uniquely positioned to influence post-injury degenerative and regenerative processes.
Functional implications of endogenous autoimmune responses triggered by SCI
Currently, the implications of post-traumatic lymphocyte activation and intraspinal accumulation remain ill-defined and controversial; what is known will be reviewed below. However, before considering if T and B cells exacerbate tissue injury or promote CNS repair, let us first consider which antigens are driving SCI-induced autoimmunity. By doing so, we hope to broaden the context in which the effects of T and B cells are considered after SCI.
In clinical and experimental SCI, only a few autoantigen targets have been documented (i.e., MBP, GM-1 ganglioside, galactocerebroside, glutamate receptor 2/3, RNA and DNA)6,43,86,123 (also see Fig. 1). More recently, we used serum antibodies from individual SCI mice to probe homogenized spinal cord proteins separated by 2D-gel electrophoresis. A preliminary proteomics analysis of the 2D gels indicates that >50 different self-proteins are being targeted by SCI autoantibodies (data not shown). Because some of these autoantigens are found throughout the body (e.g., actin, RNA/DNA), it may be appropriate to consider SCI as a trigger for CNS and systemic autoimmune disease. For example, an increase in autoantibodies that bind nuclear antigens (e.g., RNA/DNA) and glutamate receptors6 could cause or exacerbate renal insufficiency and may explain the idiopathic cognitive declines that occur in a subset of individuals with SCI30,32. Renal failure and reproductive sterility are considered to be secondary consequences of impaired neural function in para- or quadriplegics. However, it is intriguing to consider that autoimmune responses may contribute to this pathology6. Indeed, in patients with neuropsychiatric lupus, the polyspecific antibodies that bind DNA and NMDA receptors also cause kidney pathology and cortical neurodegeneration31,67,125,130. Antibodies with similar specificities are found after SCI in mice6.
It is also possible that the autoimmune responses that we interpret as being CNS-specific are in fact aberrant byproducts of pre-existing host-defense reactions. For example, if an immune response against a bacterium or virus was occurring prior to SCI, activated polyspecific T and B cells could become reactivated in the inflammatory milieu of the injury site. In the same way that environmental pathogens trigger MS onset or disease relapses, pre- or post-traumatic exposure to pathogens or unrelated systemic trauma could initiate SCI-induced autoimmune reactions via polyspecificity.
Given that an alarming number of lymphocytes specific for pathogens or systemic antigens (e.g., nucleic acids) can also react with CNS antigens3,18,31,37,49,51,72,124,130, it seems certain that SCI or any perturbation that can trigger an immune response will activate autoimmunity. However, there is little evidence that autoimmune processes cause delayed pathology or neurological decline after SCI or other forms of neural trauma (e.g., traumatic brain injury, stroke). Perhaps this is because the threshold for detecting these changes is extremely high after severe CNS injury. Indeed, in an individual who is already paralyzed, it would be difficult to discriminate a neurological deficit resulting from focal activation of T or B cells in discrete regions of the spinal cord. Alternatively, because researchers and clinicians typically focus on measuring changes in spinal-mediated motor/sensory function (both after SCI and in MS), it is possible that pathology caused by autoimmunity in systemic organs (e.g., kidney) or disturbances in cognitive function would be missed or simply attributed to neural deficits caused by SCI. An equally plausible explanation is that severe trauma activates immunoregulatory pathways that limit the extent of autoimmune pathology. For example, studies by Lafaille and colleagues showed that SCI simultaneously activates Tregs and pathogenic autoreactive T cells. In this model, Tregs were shown to control and arrest pathogenic T cells thereby limiting inflammation and injury-induced gliosis14,19,90,136. Interestingly, in transgenic mice deficient in Tregs, SCI induces pathological autoimmunity53,69. Injury-induced suppression of pathological autoimmunity may also be a consequence of dysregulated function in the sympathetic nervous system and neuroendocrine axis. We and others have shown that circulating levels of glucocorticoids are increased and that norepinephrine-mediated killing of lymphocytes is enhanced after SCI and stroke27,28,66,76,99. The resulting lymphocyte death could simultaneously limit the potential for developing autoimmunity while simultaneously increasing susceptibility to opportunistic infection.
Finally, we must consider the possibility that some autoimmune reactions are tolerable and may in fact be beneficial. For example, autoimmune cells including those reactive with MBP have been shown to secrete neurotrophins like BDNF upon stimulation by antigen33,47,57. This is thought to protect CNS cells from degeneration and also may promote axon growth and repair 87,116. Because autoimmune cells selectively accumulate in and nearby sites of CNS injury, neurotrophins may be delivered in a context-dependent manner. The potential benefits afforded by autoimmune reactions after CNS injury are discussed in more detail below.
Manipulating adaptive immunity as a therapy for SCI
Clearly, the adaptive immune system is capable of exacerbating tissue damage and promoting various indices of CNS repair. A focus of current research is to learn how to exploit autoimmune-mediated repair while minimizing or circumventing the pathological consequences of autoreactive lymphocytes. A few of these approaches are summarized below.
Strategies seeking to enhance adaptive immunity for repair of injured spinal cord
Numerous studies have intentionally evoked autoimmune responses after SCI using active immunization or vaccine protocols (i.e., where antigens emulsified in immune-stimulating adjuvants are injected into the injured subject). Huang et al. immunized SCI mice with whole spinal cord homogenate in an attempt to promote axon regeneration48. The goal of this study was to increase the production of autoantibodies that would bind proteins in myelin known to inhibit axon growth (e.g., MAG). Indeed, only in immunized mice were anti-myelin antibody titers increased concomitant with enhanced axon regeneration beyond the site of SCI48. A similar approach was used to successfully block the axon growth-inhibitory properties of Nogo-A83. Specifically, an intrasplenic injection of a fusion protein containing the NogoA peptide was used to promote synthesis of high-affinity anti-Nogo-A antibodies. Importantly, this immunization protocol rapidly increased anti-Nogo-A antibodies without inducing pathological autoimmunity83. Schwartz and colleagues have shown that autoantigen vaccines can also safely induce T cell-mediated neuroprotection, i.e., “protective autoimmunity” 42,62,88,113.
As an alternative to active immunization, the passive delivery of autoantibodies has been used successfully as a repair strategy in models of demyelination, Alzheimer's disease and SCI8,38,111. Rodriguez and colleagues discovered an IgM autoantibody that binds to oligodendrocyte precursor cells and stimulates their entry into the cell cycle in vitro 103,104. When injected in vivo, these antibodies promote remyelination of chemically-demyelinated spinal cord lesions105. In models of SCI, Schnell et al. showed that infusions of IN-1 antibodies, later shown to bind Nogo, stimulated long distance axon growth and functional recovery22,82,111. The repeated successes using IN-1 and later generation anti-Nogo antibodies to promote axon regeneration and functional recovery in rodents and primates has resulted in the start of a Phase I clinical trial in which humanized anti-Nogo antibodies are being tested as a treatment for human SCI20,21.
Evidence that suppressing adaptive immunity is neuroprotective after SCI
Using vaccine protocols similar to those described above, we and others have shown that autoimmune responses can exacerbate CNS pathology7,36,52,89,122,131. In fact, when used in rat and mouse models of peripheral or CNS injury, active and passive immunization protocols consistently exacerbate neuropathology and impair neurological function7,52. Surprisingly, autoimmune vaccines caused a disseminated experimental autoimmune attack (i.e., EAE) throughout the neuraxis in mice receiving a facial nerve axotomy7. Importantly, no disease developed in immunized mice not receiving a peripheral nerve injury7. These data emphasize the potential for mild nerve injury to break or overcome mechanisms of immune regulation in the periphery.
In a separate study, we performed SCI in mice possessing a T cell repertoire biased towards recognition of MBP53. We predicted that these mice would reveal the true physiological potential of MBP-reactive T cells in the context of SCI. Indeed, without using adjuvants to bias the function T cells, we could examine how neuroantigen-specific T cells influence, in a context-dependent fashion, neuropathology and recovery from SCI. If MBP-reactive T cells are neuroprotective as predicted by the concept of protective autoimmunity, then we expected that T cell activation and entry into the spinal cord would reduce demyelination and axonal injury and promote functional recovery. Conversely, if they augment the acute destructive potential of innate immunity and exacerbate antibody-mediated demyelination and axonal injury, we predicted that spinal cord pathology and associated neurological deficits would be enhanced. The data clearly support the notion that MBP-reactive T cells are neurodestructive in the context of a sterile SCI53.
Other data also suggest that lymphocytes are deleterious to the injured CNS. For example, SCI mice given antibodies against CXCL10, a chemokine which facilitates T cell recruitment to sites of inflammation, had reduced T cell accumulation accompanied by significant anatomical and functional preservation40. Similarly, when rats devoid of T cells (athymic nude rats) received a SCI, the lesions were significantly smaller and functional recovery was improved relative to SCI rats with T cells98. However, using a model of facial nerve axotomy, Jones and colleagues have shown that survival of axotomized motor neurons depends on the presence of an anti-inflammatory CD4+ T cells30,117. More recently, this same group expanded their analysis of T cell subsets influenced by axotomy and showed that both anti-inflammatory (Th2 or Tregs) and proinflammatory (Th1 or Th17) T cells were activated 134. This balanced activation of different T cell subsets may be important for T cells to exert a neuroprotective phenotype. Interestingly, after SCI, adaptive immunity is biased towards the Th1 proinflammatory phenotype6,53. Thus, the preferential induction of Th2 immunity may prove to be neuroprotective after SCI44. More studies are needed to prove this conclusively.
Conclusions
There is compelling evidence that SCI activates autoreactive T and B cells with the potential to exert divergent functions. On the one hand, studies have shown that endogenous autoimmunity can be enhanced to promote spinal cord repair. On the other hand, there are data that prove that autoimmune responses can exacerbate the deleterious consequences of spinal cord or peripheral nerve injury. From these conflicting data, it is becoming clear that in order to develop safe and effective therapies, we must learn how to simultaneously suppress the pathological effects of lymphocytes and boost their reparative effects without interfering with their pivotal role in host-defense. The challenge for the future is to reveal the key molecular determinants that control these divergent functions. Only then can we make treatments like this a reality.
Acknowledgments
Supported by NIH R03-NS055871, NIH NS047175 and NIH P30-NS045758
Abbreviations
- SCI
Spinal cord injury
- CNS
Central nervous system
- TCR
T cell receptor
- Treg
Regulatory T cell
- MS
Multiple Sclerosis
- EAE
Experimental autoimmune encephalomyelitis
- BBB
Blood-brain barrier
- MBP
Myelin basic protein
- CSF
Cerebrospinal fluid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Majid KB, Stefferl A, Bourquin C, Lassmann H, Linington C, Olsson T, Kleinau S, Harris RA. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;55:70–81. doi: 10.1046/j.1365-3083.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 3.Alderuccio F, Rolland JM, Toner GC, Schwarz MA, McCluskey J, Toh BH. Autoantibodies to neurons and to the cytoskeleton in small cell carcinoma with paraneoplastic sensory neuropathy. Autoimmun. 1989;5:115–123. doi: 10.3109/08916938909029149. [DOI] [PubMed] [Google Scholar]
- 4.Andersen O, Lygner PE, Bergstrom T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993;240:417–422. doi: 10.1007/BF00867354. [DOI] [PubMed] [Google Scholar]
- 5.Anderson AJ, Robert S, Huang W, Young W, Cotman CW. Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma. 2004;21:1831–1846. doi: 10.1089/neu.2004.21.1831. [DOI] [PubMed] [Google Scholar]
- 6.Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- 7.Ankeny DP, Popovich PG. Central nervous system and non-central nervous system antigen vaccines exacerbate neuropathology caused by nerve injury. Eur J Neurosci. 2007;25:2053–2064. doi: 10.1111/j.1460-9568.2007.05458.x. [DOI] [PubMed] [Google Scholar]
- 8.Asakura K, Miller DJ, Pease LR, Rodriguez M. Targeting of IgMkappa antibodies to oligodendrocytes promotes CNS remyelination. J Neurosci. 1998;18:7700–7708. doi: 10.1523/JNEUROSCI.18-19-07700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnum SR. Inhibition of complement as a therapeutic approach in inflammatory central nervous system (CNS) disease. Mol Med. 1999;5:569–582. [PMC free article] [PubMed] [Google Scholar]
- 12.Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 13.Bauman TM, Kasper LH. Novel approaches and cutting edge immunotherapies in multiple sclerosis. Front Biosci. 2004;9:2302–2322. doi: 10.2741/1398. [DOI] [PubMed] [Google Scholar]
- 14.Ben Nun A, Cohen IR. Spontaneous remission and acquired resistance to autoimmune encephalomyelitis (EAE) are associated with suppression of T cell reactivity: suppressed EAE effector T cells recovered as T cell lines. J Immunol. 1982;128:1450–1457. [PubMed] [Google Scholar]
- 15.Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 16.Beuche W, Friede RL. Myelin phagocytosis in Wallerian degeneration of peripheral nerves depends on silica-sensitive, bg/bg-negative and Fc- positive monocytes. Brain Research. 1986;378:97–106. doi: 10.1016/0006-8993(86)90289-1. [DOI] [PubMed] [Google Scholar]
- 17.Bilgen M, Dogan B, Narayana PA. In vivo assessment of blood-spinal cord barrier permeability: serial dynamic contrast enhanced MRI of spinal cord injury. Magn Reson Imaging. 2002;20:337–341. doi: 10.1016/s0730-725x(02)00504-0. [DOI] [PubMed] [Google Scholar]
- 18.Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol. 2005;12:217–224. doi: 10.1080/17402520500285247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchli AD, Rouiller E, Mueller R, Dietz V, Schwab ME. Repair of the injured spinal cord. A joint approach of basic and clinical research. Neurodegener Dis. 2007;4:51–56. doi: 10.1159/000100359. [DOI] [PubMed] [Google Scholar]
- 21.Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 24.Christen U, von Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Claudio L, Raine CS, Brosnan CF. Evidence of persistent blood-brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta Neuropathol (Berl) 1995;90:228–238. doi: 10.1007/BF00296505. [DOI] [PubMed] [Google Scholar]
- 26.Cross AH, Tuohy VK, Raine CS. Development of reactivity to new myelin antigens during chronic relapsing autoimmune demyelination. Cell Immunol. 1993;146:261–269. doi: 10.1006/cimm.1993.1025. [DOI] [PubMed] [Google Scholar]
- 27.Cruse JM, Keith JC, Bryant ML, Jr, Lewis RE., Jr Immune system-neuroendocrine dysregulation in spinal cord injury. Immunol Res. 1996;15:306–314. doi: 10.1007/BF02935314. [DOI] [PubMed] [Google Scholar]
- 28.Cruse JM, Lewis RE, Jr, Bishop GR, Kliesch WF, Gaitan E, Britt R. Decreased immune reactivity and neuroendocrine alterations related to chronic stress in spinal cord injury and stroke patients. Pathobiology. 1993;61:183–192. doi: 10.1159/000163790. [DOI] [PubMed] [Google Scholar]
- 29.Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidoff GN, Roth EJ, Richards JS. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil. 1992;73:275–284. [Review] [90 refs] [PubMed] [Google Scholar]
- 31.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 32.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3:464–472. [PubMed] [Google Scholar]
- 33.Edling AE, Nanavati T, Johnson JM, Tuohy VK. Human and murine lymphocyte neurotrophin expression is confined to B cells. J Neurosci Res. 2004;77:709–717. doi: 10.1002/jnr.20176. [DOI] [PubMed] [Google Scholar]
- 34.Eng LF, Lee YL. Response of chemokine antagonists to inflammation in injured spinal cord. Neurochem Res. 2003;28:95–100. doi: 10.1023/a:1021652229667. [DOI] [PubMed] [Google Scholar]
- 35.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fee D, Crumbaugh A, Jacques T, Herdrich B, Sewell D, Auerbach D, Piaskowski S, Hart MN, Sandor M, Fabry Z. Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J Neuroimmunol. 2003;136:54–66. doi: 10.1016/s0165-5728(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 37.Freddo L, Hays AP, Nickerson KG, Spatz L, McGinnis S, Lieberson R, Vedeler CA, Shy ME, Autilio-Gambetti L, Grauss FC. Monoclonal anti-DNA IgM kappa in neuropathy binds to myelin and to a conformational epitope formed by phosphatidic acid and gangliosides. J Immunol. 1986;137:3821–3825. [PubMed] [Google Scholar]
- 38.Frenkel D, Solomon B, Benhar I. Modulation of Alzheimer's beta-amyloid neurotoxicity by site-directed single-chain antibody. J Neuroimmunol. 2000;106:23–31. doi: 10.1016/s0165-5728(99)00232-5. [DOI] [PubMed] [Google Scholar]
- 39.Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion attenuates cellular infiltration following spinal cord contusion injury in rat. J Neurosci Res. 2000;59:63–73. [PubMed] [Google Scholar]
- 40.Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 41.Harling-Berg C, Knopf PM, Merriam J, Cserr HF. Role of cervical lymph nodes in the systemic humoral immune response to human serum albumin microinfused into rat cerebrospinal fluid. J Neuroimmunol. 1989;25:185–193. doi: 10.1016/0165-5728(89)90136-7. [DOI] [PubMed] [Google Scholar]
- 42.Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, Popovich PG. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19:753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 44.Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100–112. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. GLIA. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 46.Hickey WF, Gonatas NK, Kimura H, Wilson DB. Identification and quantitation of T-lymphocyte subsets found in the spinal cord of the Lewis rat during experimental allergic encephalomyelitis. J Immunol. 1983;131:2805–2809. [PubMed] [Google Scholar]
- 47.Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. J Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 48.Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 49.Hughes LE, Smith PA, Bonell S, Natt RS, Wilson C, Rashid T, Amor S, Thompson EJ, Croker J, Ebringer A. Cross-reactivity between related sequences found in Acinetobacter sp., Pseudomonas aeruginosa, myelin basic protein and myelin oligodendrocyte glycoprotein in multiple sclerosis. J Neuroimmunol. 2003;144:105–115. doi: 10.1016/s0165-5728(03)00274-1. [DOI] [PubMed] [Google Scholar]
- 50.Irani DN, Griffin DE. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J Immunol. 1996;156:3850–3857. [PubMed] [Google Scholar]
- 51.Jernigan M, Morcos Y, Lee SM, Dohan FC, Jr, Raine C, Levin MC. IgG in brain correlates with clinicopathological damage in HTLV-1 associated neurologic disease. Neurology. 2003;60:1320–1327. doi: 10.1212/01.wnl.0000059866.03880.ba. [DOI] [PubMed] [Google Scholar]
- 52.Jones TB, Ankeny DP, Guan Z, McGaughy V, Fisher LC, Basso DM, Popovich PG. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 55.Karpus WJ, Ransohoff RM. Chemokine regulation of experimental autoimmune encephalomyelitis: Temporal and spatial expression patterns govern disease pathogenesis. J Immunol. 1998;161:2667–2671. [PubMed] [Google Scholar]
- 56.Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WEF, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassman H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kielian T, Hickey WF. Proinflammatory cytokine, chemokine, and cellular adhesion molecule expression during the acute phase of experimental brain abscess development. Am J Pathol. 2000;157:647–658. doi: 10.1016/S0002-9440(10)64575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kil K, Zang YCQ, Yang D, Markowski J, Fuoco GS, Vendetti GC, Rivera VM, Zhang JZ. T-cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 61.Kim HJ, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162:3053–3062. [PubMed] [Google Scholar]
- 62.Kipnis J, Mizrahi T, Yoles E, Ben Nun A, Schwartz M, Ben Nur A. Myelin specific Th1 cells are necessary for post-traumatic protective autoimmunity. J Neuroimmunol. 2002;130:78–85. doi: 10.1016/s0165-5728(02)00219-9. [DOI] [PubMed] [Google Scholar]
- 63.Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- 64.Knopf PM, Harling-Berg CJ, Cserr HF, Basu D, Sirulnick EJ, Nolan SC, Park JT, Keir G, Thompson EJ, Hickey WF. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol. 1998;161:692–701. [PubMed] [Google Scholar]
- 65.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 66.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 67.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 69.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephaloymyelitis in immunodeficient anti-myelin basic protein T-cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 70.Lee YL, Shih K, Bao P, Ghirnikar RS, Eng LF. Cytokine chemokine expression in contused rat spinal cord. Neurochem Int. 2000;36:417–425. doi: 10.1016/s0197-0186(99)00133-3. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 72.Levin MC, Krichavsky M, Berk J, Foley S, Rosenfeld M, Dalmau J, Chang G, Posner JB, Jacobson S. Neuronal molecular mimicry in immune-mediated neurologic disease. Ann Neurol. 1998;44:87–98. doi: 10.1002/ana.410440115. [DOI] [PubMed] [Google Scholar]
- 73.Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/s0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- 74.Linington C, Morgan BP, Scolding NJ, Wilkins P, Piddlesden S, Compston DA. The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain. 1989;112:895–911. doi: 10.1093/brain/112.4.895. [DOI] [PubMed] [Google Scholar]
- 75.Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 76.Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 78.McGavern DB, Truong P. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J Immunol. 2004;173:4779–4790. doi: 10.4049/jimmunol.173.8.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS, Maciejewski D, Maki R, Ransohoff RM, Stokes BT. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res. 1998;53:368–376. doi: 10.1002/(SICI)1097-4547(19980801)53:3<368::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 81.Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCdelta-mediated nuclear signalling. Nature. 2004;431:456–461. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- 82.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merkler D, Oertle T, Buss A, Pinschewer DD, Schnell L, Bareyre FM, Kerschensteiner M, Buddeberg BS, Schwab ME. Rapid induction of autoantibodies against Nogo-A and MOG in the absence of an encephalitogenic T cell response: implication for immunotherapeutic approaches in neurological diseases. FASEB J. 2003 doi: 10.1096/fj.02-1203fje. [DOI] [PubMed] [Google Scholar]
- 84.Miller SD, McRae BL, Vanderlugt CL, Nikcevich KM, Pope JG, Pope L, Karpus WJ. Evolution of the T-cell repertoire during the course of experimental immune-mediated demyelinating diseases. Immunol Rev. 1995;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 85.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 86.Mizrachi Y, Ohry A, Aviel A, Rozin R, Brooks ME, Schwartz M. Systemic humoral factors participating in the course of spinal cord injury. Paraplegia. 1983;21:287–293. doi: 10.1038/sc.1983.48. [DOI] [PubMed] [Google Scholar]
- 87.Moalem G, Gdalyahu A, Leibowitz-Amit R, Yoles E, Muller-Gilor S, Shani Y, Mor F, Tooen U, Cohen IR, Schwartz M. Production of neurotrophins by activated T-cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000;15:331–345. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 88.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T-cells protect neurons from secondary degeneration after central nervous system axotomy. Nature Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 89.Munch G, Robinson SR. Alzheimer's vaccine: a cure as dangerous as the disease? J Neural Transm. 2002;109:537–539. doi: 10.1007/s007020200044. [DOI] [PubMed] [Google Scholar]
- 90.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olsson T, Diener P, Ljungdahl A, Hojeberg B, van der Meide PH, Kristensson K. Facial nerve transection causes expansion of myelin autoreactive T cells in regional lymph nodes and T cell homing to the facial nucleus. Autoimmun. 1992;13:117–126. doi: 10.3109/08916939209001912. [DOI] [PubMed] [Google Scholar]
- 92.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 93.Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier I. Permeability changes after experimental spinal contusion injury. Exp Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- 94.Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: Possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45:349–363. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 95.Popovich PG, Streit WJ, Stokes BT. Differential expression of MHC Class II antigen in the contused rat spinal cord. J Neurotrauma. 1993;10:37–46. doi: 10.1089/neu.1993.10.37. [DOI] [PubMed] [Google Scholar]
- 96.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 97.Poser CM. The role of trauma in the pathogenesis of multiple sclerosis: a review. Clin Neurol Neurosurg. 1994;96:103–110. doi: 10.1016/0303-8467(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 98.Potas JR, Zheng Y, Moussa C, Venn M, Gorrie CA, Deng C, Waite PM. Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J Neurotrauma. 2006;23:660–673. doi: 10.1089/neu.2006.23.660. [DOI] [PubMed] [Google Scholar]
- 99.Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, Volk HD, Meisel A. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raivich G, Jones LL, Kloss CUA, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 102.Reinherz EL, Weiner HL, Hauser SL, Cohen JA, Distaso JA, Schlossman SF. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980;303:125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez M. Immunoglobulins stimulate central nervous system remyelination: electron microscopic and morphometric analysis of proliferating cells. Lab Invest. 1991;64:358–370. [PubMed] [Google Scholar]
- 104.Rodriguez M, Lennon VA, Benveniste EN, Merrill JE. Remyelination by oligodendrocytes stimulated by antiserum to spinal cord. J Neuropathol Exp Neurol. 1987;46:84–95. doi: 10.1097/00005072-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez M, Miller DJ, Lennon VA. Immunoglobulins reactive with myelin basic protein promote CNS remyelination. Neurology. 1996;46:538–545. doi: 10.1212/wnl.46.2.538. [DOI] [PubMed] [Google Scholar]
- 106.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 107.Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol. 2005;24:211–226. doi: 10.1080/08830180590934976. [DOI] [PubMed] [Google Scholar]
- 108.Schmitt AB, Buss A, Breuer S, Brook GA, Pech K, Martin D, Schoenen J, Noth J, Love S, Schroder JM, Kreutzberg GW, Nacimiento W. Major histocompatibility complex class II expression by activated microglia caudal to lesions of descending tracts in the human spinal cord is not associated with a T cell response. Acta Neuropathol (Berl) 2000;100:528–536. doi: 10.1007/s004010000221. [DOI] [PubMed] [Google Scholar]
- 109.Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 110.Schnell L, Schneider R, Berman MA, Perry VH, Schwab ME. Lymphocyte recruitment following spinal cord injury in mice is altered by prior viral exposure. Eur J Neurosci. 1997;9:1000–1007. doi: 10.1111/j.1460-9568.1997.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 112.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwartz M, Kipnis J. A common vaccine for fighting neurodegenerative disorders: recharging immunity for homeostasis. Trends Pharmacol Sci. 2004;25:407–412. doi: 10.1016/j.tips.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 114.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sercarz E, Maverakis E, van den EP, Madakamutil L, Kumar V. Seven surprises in the TCR-centred regulation of immune responsiveness in an autoimmune system. Novartis Found Symp. 2003;252:165–171. doi: 10.1002/0470871628.ch12. [DOI] [PubMed] [Google Scholar]
- 116.Serpe CJ, Byram SC, Sanders VM, Jones KJ. Brain-derived neurotrophic factor supports facial motoneuron survival after facial nerve transection in immunodeficient mice. Brain Behav Immun. 2005;19:173–180. doi: 10.1016/j.bbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 117.Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 118.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 119.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 121.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 122.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 123.Taranova NP, Makarov AI, Amelina OA, Luchakova OS, Loboda EB, Leikin IB. The production of autoantibodies to nerve tissue glycolipid antigens in patients with traumatic spinal cord injuries. Zh Vopr Neirokhir Im N N Burdenko. 1992:21–24. [PubMed] [Google Scholar]
- 124.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53:189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 125.Tojo T, Friou GJ. Lupus nephritis: varying complement-fixing properties of immunoglobulin G antibodies to antigens of cell nuclei. Science. 1968;161:904–906. doi: 10.1126/science.161.3844.904. [DOI] [PubMed] [Google Scholar]
- 126.Tornqvist E, Liu L, Aldskogius H, Holst HV, Svensson M. Complement and clusterin in the injured nervous system. Neurobiol Aging. 1996;17:695–705. doi: 10.1016/0197-4580(96)00120-0. [DOI] [PubMed] [Google Scholar]
- 127.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 128.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilson HA, Winfield JB, Lahita RG, Koffler D. Association of IgG anti-brain antibodies with central nervous system dysfunction in systemic lupus erythematosus. Arthritis Rheum. 1979;22:458–462. doi: 10.1002/art.1780220504. [DOI] [PubMed] [Google Scholar]
- 131.Wisniewski HM, Bloom BR. Primary demyelination as a nonspecific consequence of a cell- mediated immune reaction. J Exp Med. 1975;141:346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wucherpfennig KW, Allen PM, Celada F, Cohen IR, De Boer R, Garcia KC, Goldstein B, Greenspan R, Hafler D, Hodgkin P, Huseby ES, Krakauer DC, Nemazee D, Perelson AS, Pinilla C, Strong RK, Sercarz EE. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19:216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2008;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 136.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 137.Zirger JM, Liu C, Barcia C, Castro MG, Lowenstein PR. Immune regulation of transgene expression in the brain: B cells regulate an early phase of elimination of transgene expression from adenoviral vectors. Viral Immunol. 2006;19:508–517. doi: 10.1089/vim.2006.19.508. [DOI] [PMC free article] [PubMed] [Google Scholar]


