Abstract
Repetitive strain injuries (RSI), which include several musculoskeletal disorders and nerve compression injuries, are associated with performance of repetitive and forceful tasks. In this study, we examined the effects of performing a voluntary, moderate repetition, high force (MRHF; 9 reaches/min; 60% maximum pulling force) task for 12 weeks on motor behavior and nerve function, inflammatory responses in forearm musculoskeletal and nerve tissues and serum, and neurochemical immunoexpression in cervical spinal cord dorsal horns. We observed no change in reach rate, but reduced voluntary participation and grip strength in week 12, and increased cutaneous sensitivity in weeks 6 and 12, the latter indicative of mechanical allodynia. Nerve conduction velocity (NCV) decreased 15% in the median nerve in week 12, indicative of low-grade nerve compression. ED-1 cells increased in distal radius and ulna in week 12, and in the median nerve and forearm muscles and tendons in weeks 6 and 12. Cytokines IL-1α, IL-1β, TNF-α, and IL-10 increased in distal forearm bones in week 12, while IL-6 increased in tendon in week 12. However, serum analysis revealed only increased TNF-α in week 6 and macrophage inflammatory protein 3a (MIP3a) in weeks 6 and 12. Lastly, Substance P and neurokinin-1 were both increased in weeks 6 and 12 in the dorsal horns of cervical spinal cord segments. These results show that a high force, but moderate repetition task, induced declines in motor and nerve function as well as peripheral and systemic inflammatory responses (albeit the latter was mild). The peripheral inflammatory responses were associated with signs of central sensitization (mechanical allodynia and increased neurochemicals in spinal cord dorsal horns).
Keywords: spinal cord, macrophages, cytokines, musculoskeletal disorder, nerve injury, repetitive strain injury
Introduction
Repetitive strain injuries (RSIs) are associated with several common pain conditions including back pain, arthritis and musculoskeletal pain. The estimated cost of these disorders is high ($61.2 billion annually) when considering the cost of health care to treat these disorders and lost productivity (Stewart et al., 2003). Epidemiological evidence suggests that nerve compression injury of the upper extremity is associated with the performance of repetitive and forceful tasks (See Barr et al. 2004 for review). In fact, repetitive motion such as typing and repeated grasping was the exposure that resulted in the longest absences from work in 2005 and 2006 (BLS, 2007). One of the most common compressive neuropathies affects the median nerve and is clinically referred to as carpel tunnel syndrome. In 2005 and 2006, carpel tunnel syndrome was listed as one of the most severe of all disabling injuries and illnesses having the highest median days away from work (BLS, 2006, 2007). Patients with this syndrome have symptoms such as pain in the hand and wrist that may travel into the forearm, elbow, and shoulder, as well as paresthesias, numbness and weakness.
Investigations of peripheral nerve compressive injury induced by repetitive motion report reduced nerve conduction velocity, decreased grip strength, performance declines, inflammation and fibrosis as a result of task performance (Clark et al., 2003;2004;Sommerich et al., 2007). There are also laboratories studying nerve compression using invasive, surgically induced injuries to the sciatic nerve (Winkelstein et al., 2001a;Gupta and Steward, 2003;Pitcher and Henry, 2004;Hu et al., 2007) and median nerve (Diao et al., 2005). These latter studies have found tactile allodynia, reduced nerve conduction velocity, endoneurial macrophage infiltration, spinal cord neuroplasticity and augmented neuronal excitation, as well as spinal cord inflammatory responses after peripheral nerve injury. In addition to the effects on peripheral nerve, several laboratories, including our own, have documented the effects of repetitive motion on musculoskeletal tissues, including inflammatory cell infiltrates, tendinopathy, degenerative changes and tissue necrosis (Soslowsky et al., 1996;Willems and Stauber, 1999;Barbe et al., 2003;Barr et al., 2003;Geronilla et al., 2003;Diao et al., 2005;Nakama et al., 2005;Perry et al., 2005;Baker et al., 2007;Sommerich et al., 2007).
Our laboratory has developed a rat model of RSI in which rats perform a voluntary, repetitive, upper extremity task. We have examined the effects of a high repetition, negligible force (HRNF) task and found that performance of this task for 8–12 weeks induces motor declines, local and systemic inflammatory responses in forearm nerve and musculoskeletal tissues, fibrotic compression of the median nerve and a modest, yet significant, 9% decline in nerve conduction velocity (Barbe et al., 2003;Clark et al., 2003;Barr et al., 2004;Al-Shatti et al., 2005;Barbe et al., 2008;Elliott et al., 2008). The inflammatory response began in week 3, peaked between 5 and 8 weeks, and included increased macrophages and proinflammatory cytokines in the involved nerves, muscles, tendons, bones, and synovial tissues (Barbe et al., 2003;Barr et al., 2003;Al-Shatti et al., 2005;Barbe et al., 2008). We also observed increased levels of pro-inflammatory cytokines and chemokines in serum (Barbe et al., 2003;Barbe et al., 2008). Investigation of a high repetition, high force (HRHF) task by our laboratory found motor deficits, cutaneous hyposensitivity, a 17% decline in NCV, and inflammatory and fibrotic changes in the median nerve by 12 weeks (Clark et al., 2004). We recently examined the performance of a low repetition, negligible force (LRNF) task and found that performance of this low demand task induced mild tissue inflammation in nerve and bone, increased neurochemicals (Substance P and Neurokinin-1) in spinal cord dorsal horns and declines in fine motor control but no declines in gross motor function (Elliott et al., 2008).
Our finding of a neurochemical response in the spinal cord after performance of a low demand task prompted us to explore the relationship between pain behaviors and spinal cord neurochemical changes in animals with higher levels of task-induced inflammation, since Woolf and colleagues proposed a mechanism of inflammation-induced sensitization that drives plasticity of sensory afferents and spinal cord neurons (Woolf and Salter, 2000). Only recently has evidence for involvement of the spinal cord in the pathology associated with peripheral nerve compression injuries and pain been provided (Pitcher and Henry, 2004;Hubbard and Winkelstein, 2005;Rothman et al., 2005;Chao et al., 2008). The purpose of the present study was to examine whether pain behaviors and declines in motor function are associated temporally with peripheral inflammation and spinal cord neuroplasticity in rats performing a moderately repetitive and highly forceful (MRHF) task, a task exposure that we have not yet explored. Specifically, mechanical allodynia, grip strength and other motor behaviors, nerve conduction velocity, peripheral tissue (forelimb muscle, tendon, distal bone, and nerve) and systemic inflammation, and spinal cord neurochemicals were examined.
Experimental Procedures
Subjects
Sprague-Dawley rats (adult females, 3.5 months of age at onset of experiments) were obtained from ACE, PA. The rats were housed in the Central Animal Facility on the Health Sciences Campus at Temple University. Animal care and use was in compliance with the provisions of Federal Regulations and the NIH "Guide for the Care and Use of Laboratory Animals" monitored by the University Animal Care and Use Committee. Rats were allowed free access to water. Experimental and trained control rats learned to reach for the food during an initial 7–10 day shaping period in which access to food was restricted in order to motivate them to learn the task. Some animals may have undergone a short period (no more than 7 days) of weight reduction to 80% of the weights of the age-matched control group that did not undergo food restriction. Once the animals learned the task, they rapidly gained weight and were maintained at ± 5% of age-matched control rats’ weights. Rats were weighed twice weekly and food adjusted accordingly.
Repetitive Movement Task
Fifty-one rats were randomized into one of three groups: moderate repetition, high force group (MRHF; n=20), a normal control group (NC; n=21) and a trained only control group (TC; n=10). The MRHF rats and the trained control rats (the latter undergoing the initial training (shaping) only), learned to reach for a food pellet (45 mg purified formula pellet, Bioserve, Frenchtown, NJ) for a 2–3 week shaping period, as described earlier in Barbe et al, 2003; Elliott et al., 2008; Clark et al, 2003, 2004. Rats were allowed to use their preferred limb to reach, hereafter referred to as the “preferred limb”.
The task rats then performed a MRHF reaching and grasping task for a food pellet for 2 hours/day, 3 days/week for 6 (n=4) or 12 (n=16) weeks using operant test chambers for rodents (Med. Associates, VT) and a custom designed handle pulling apparatus (Custom Medical Equipment, NJ), as described in Clark et al., 2004.The daily task was divided into 4, 0.5-hour training sessions separated by 1.5 hours. The defined target rate was 4 reaches/minute, although the rats reached above this target maintaining an average, actual reach rate of 9.4 reaches/minute. Pull force for this study was set at 60 ± 5 percent of maximum voluntary pulling force. Maximum pulling force was determined on the last day of the initial training period during a 5 min session in which the force criterion for a food reward was gradually increased. Animals were observed carefully for their maximum force generating ability during this 5 min session, and maximum voluntary pulling force of the force lever handle was selected as the highest force resulting in a successful reach (i.e., food pellet reward) that could be repeated 3 times. Force threshold criteria established a window in which force was maintained for at least 50 msec for animals to obtain a food reward.
Sensorimotor Behavioral Testing
The effects of the task on motor performance were evaluated in experimental rats (n=20), normal controls (n=21) and trained controls (n=10) by examining task duration (minutes/day), reach rate (reaches/min), mechanical cutaneous sensation of the forepaws using the Von Frey test (Chaplan et al., 1994) and median grip strength as described previously (Barbe et al., 2003;Clark et al., 2003;Clark et al., 2004). Cutaneous sensation and grip strength are reported as mean maximum force in grams and SEM for preferred limbs.
Nerve conduction velocity (NCV)
In order to test focal slowing of conduction (Kimura, 1979;Walters and Murray, 2001), NCV was determined for the segment of the median nerve that passes beneath the transcarpal ligament. NCV was measured in terminal surgical experiments in 10 normal control rats (20 limbs) and in 8 rats that had performed the MRLF task for 12 weeks. The method for measurement of NCV of the median nerve in rats is as described previously in Clark et al, 2003 and 2004. All euthanasia methods are consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Euthanasia was implemented using a lethal overdose (Nembutal, 120 mg/kg body weight). Tissues or serum were not collected from rats that underwent the NCV testing to avoid confounding interpretation of results by changes induced by this surgical procedure.
Immunohistochemistry and quantification of ED1-Positive macrophages and neurochemicals
Tissues were examined immunohistochemically in normal control rats (n=4), trained control rats (n=4), and in the preferred limbs of rats performing the MRHF task for 6 (n=4), or 12 (n=4) weeks. Rats were euthanized with an overdose of sodium pentobarbital (Nembutal; 120 mg/kg body weight), serum was collected, and then the rats received transcardial perfusion with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Forelimb tissues from the preferred limbs were collected and postfixed “en bloc” by immersion overnight, sectioned and immunostained for ED1-positive (ED1+) cells as described previously (Barbe et al., 2003;Barr et al., 2003;Elliott et al., 2008). Numbers of ED1+ macrophages were quantified in the median nerve, forearm muscles and tendons using methods described previously (Barbe et al., 2003;Clark et al., 2003;Elliott et al., 2008). Numbers of ED1+ cells in bones (osteoclasts, macrophages and their progenitors) were quantified in forearm bones using methods described previously (Barr et al., 2003;Elliott et al., 2008). Group means and SEM were plotted against week of task performance and are expressed as the mean number of ED1+ cells/mm2. Additional sections were stained with hematoxylin and eosin (H&E) and examined for other pathological changes such as increased collagen deposition in experimental rats.
The cervical and upper thoracic spinal cord tissues were also collected and postfixed “en bloc” by immersion overnight in fixative from normal control rats (n=4), trained control rats (n=4), and rats performing the MRHF task for 6 (n=4), or 12 (n=4) weeks. Spinal cord tissues were prepared and stained for presence of Substance P and Neurokinin-1, as described previously (Elliott et al., 2008). Group means and SEM were plotted against week of task performance and are expressed as mean percent area immunoreactivity. All assessments and image analyses for all experiments were carried out in a blinded fashion.
Measurement of musculoskeletal tissue cytokines
Rats were euthanized with an overdose of sodium pentobarbital (Nembutal; 120 mg/kg body weight). After collection of serum, forearm bones and flexor muscles and tendons were collected bilaterally from normal controls (n=5) and rats that had performed the MRHF task for 12 (n=5) weeks. Distal forearm bones consisted of radial and ulnar metaphyses and epiphyses and first row of carpal bones. Tissues were flash-frozen and stored at −80°C until homogenization, and then prepared for ELISA protein analysis as described previously (Barbe et al., 2008). Total protein was determined using BCA-200 protein assays (Bicin Choninic Acid, Pierce). Tissue lysates were analyzed for IL-1α, IL-1β, IL-6, IL-10 and TNF-α, using commercially available ELISA kits according to manufacturer’s protocols (BioSource™, Invitrogen Life Sciences). Each sample was run in duplicate. ELISA assay data (pg cytokine protein) were normalized to μg total protein. Group means and SEM are reported for each tissue type and preferred limbs are plotted against week of task performance and expressed as pg of cytokine per μg total protein.
Measurement of serum cytokines and chemokines
Serum was collected from normal control rats (n=11), trained control rats (n=10), and rats performing the MRHF task for 6 (n=4) or 12 weeks (n=4). Following euthanasia (Nembutal, 120 mg/kg body weight), 18–36 hours after completion of the final task session, blood was collected by cardiac puncture using a 23-gauge needle and centrifuged immediately at 1000 g for 20 min at 4°C. Serum was collected, flash-frozen, and stored at −80°C until analyzed. The following cytokines and chemokines were analyzed in serum using a customized multiplexed sandwich ELISA system (SearchLight, Pierce): IL-1α, IL-1-β, IL-6, IL-10, TNFα, interferon gamma (IFNγ), macrophage inflammatory protein 2 (MIP2), and macrophage inflammatory protein 3a (MIP3a). All samples were analyzed in duplicate in a blinded fashion, and batched to reduce potential inter-assay variability. Search Light protein assays have lower detection limits of 1.5 mg/ml for IL-1α, 6.2 pg/ml for IL-1β, 6.3 mg/ml for IL-6, 8 mg/ml for IL-10; 3.1 pg/ml for TNFα, 6.2 pg/ml for IFNγ, 0.2 pg/ml for MIP2, and 1.6 pg/ml for MIP3a. Group means and SEM were plotted against week of task performance and are expressed as pg/ml serum.
Statistical analyses
To test for differences in ED1+ cells, serum or tissue cytokines/chemokines between the 2 control groups (NC and TC), a two tailed, unpaired, Student’s t test was used. Since there were no significant differences between the 2 control groups (see results section below), all control rats were combined into 1 group for the subsequent comparisons to experimental rats. For the reach rate and task duration, repeated measures ANOVAs were used to compare baseline (week 1) with weeks 6 and 12. For cutaneous sensitivity and grip strength, mixed model univariate ANOVAs were used to determine if week of task performance (6 or 12 week) had any effect compared to normal controls. For NCV data, a two tailed, unpaired, Student’s t test was used to determine differences between controls and MRHF 12 week rats. For numbers of ED1 positive cells in tissues, two-way ANOVAs were used with the factors week (control, 6 or 12 week) and tissue type (nerve, muscle, tendon, distal bone), with microscopic field (5–6 observations per rat) used as a blocking factor. For levels of tissue cytokines, two-way ANOVAs were used with the factors week (control or 12 week) and tissue type (nerve, muscle, tendon, distal bone). For serum levels of cytokines and chemokines, univariate ANOVAs were used to determine if week of task performance (control, 6 or 12 week) had any effect. For analysis of the percent area of immunoreactivity of substance P and neurokinin-1 in the spinal cord, univariate ANOVAs were used to determine if week of task performance (control, 6 or 12 week) had any effect, with microscopic field (3–6 observations per dorsal horn) used as a blocking factor. Post-hoc analyses were carried out by the Bonferroni method for multiple comparisons, with results compared to baseline (week 1) or control levels, and adjusted p values reported. A p value of ≤ 0.05 was considered significant for all analyses.
Results
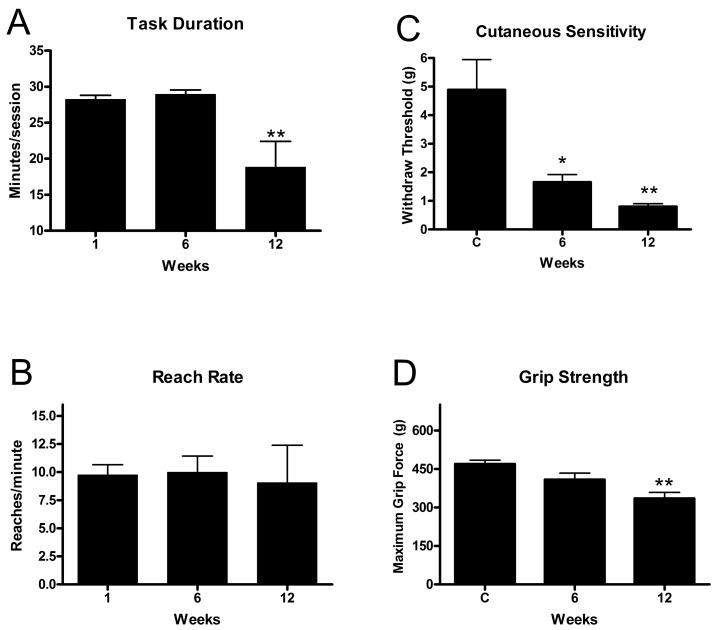
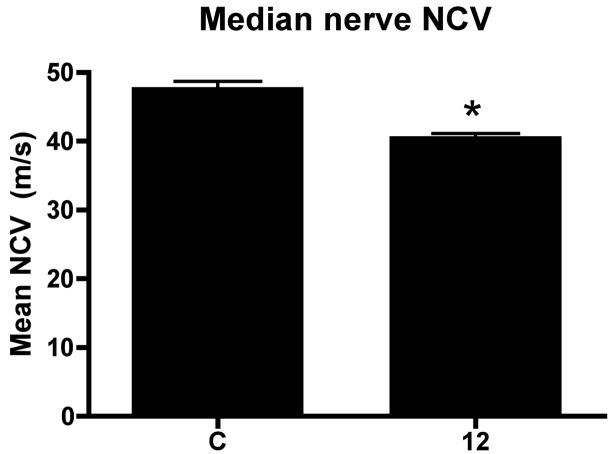
We first examined behavioral results for changes in sensorimotor function. Task duration, an indicator of task participation, was significantly decreased in MRHF rats in week 12 compared to baseline (week 1) (p<0.0001; Fig 1A). In contrast, reach rate was maintained over the 12 weeks of task performance at an overall mean of 9.4 ± 0.5 reaches per minute (Fig. 1B, Table 1). Cutaneous sensitivity was increased (reduced withdraw threshold) in weeks 6 and 12 (p=0.001 each), while grip strength was significantly decreased in MRHF rats compared to controls in week 12 (p<0.0002; Fig. 1C,D). Nerve conduction velocity was significantly decreased in the reach limbs of MRHF rats at 12 weeks compared to controls (p <0.0001; Fig. 2).
Figure 1.
Means (+ SEM) for behavioral parameters over weeks of task performance. A) Task duration and B) Reach rate after 1 (baseline), 6 and 12 weeks of MRHF task performance. C) Cutaneous sensitivity (withdrawal threshold) and D) grip strength are shown for controls (C) and after 6 and 12 weeks of task performance. Significant declines from week 1 or control levels are denoted by symbols (*p<0.01, **p<0.001). The number of animals quantified per group: controls (n=39), week 1 (n=11), week 6 (n=17) and week 12 (n=13).
Table 1.
Repetitive task group parameters
| Group | Target Reach Rate (reaches/min) | Actual Reach Rate(reaches/min) | Reach Force (% of Maximum Pull Force) |
|---|---|---|---|
| HRHF | 8 | 12 | 60 ± 5 |
| MRHF | 4 | 9.4 | 60 ± 5 |
| HRNF = MRNF | 4 | 8 | <5a |
| LRNF | 2 | 3.3 | <5a |
HRHF = high repetition high force; MRHF = moderate repetition high force; HRNF = high repetition negligible force; we will be calling this group MRNF in future studies based on the repetition rate; LRNF = Low repetition negligible force.
The negligible force rats retrieved a 45 mg food pellet which we estimate to be <5% maximum pulling force.
Figure 2.
Mean (+ SEM) for nerve conduction velocity (NCV) after 12 weeks of MRHF task performance compared to normal controls (C). The significant difference from controls are denoted by a symbol (**p<0.001). The number of animals quantified per group: controls (n=20) and week 12 (n=8).
We next compared numbers of ED1+ cells and levels of cytokines in tissues and serum in the 2 control groups (age-matched normal controls, and age- and weight-matched trained controls). We found no significant differences in numbers of ED1+ cells, levels of tissue cytokines, or levels of serum cytokines or chemokines in trained controls compared to normal controls (p>0.05 for each). Therefore, controls were combined where we had both and termed “C” hereafter.
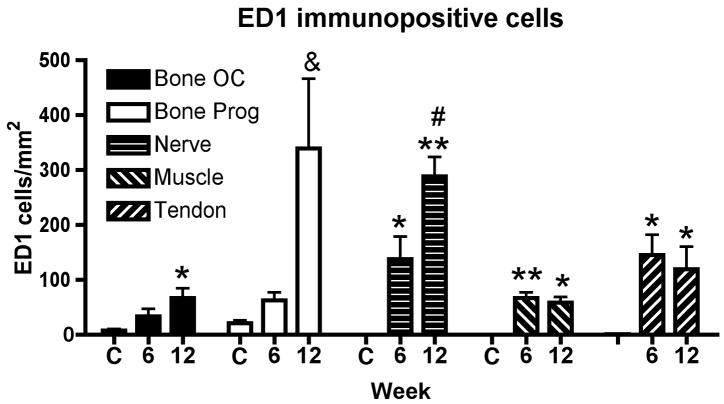
We then examined forearm tissues of control and task rats for evidence of inflammation. Numbers of infiltrating ED1+ cells (macrophages) were examined in the median nerve at the level of the wrist and in forelimb flexor muscles and tendons. Also, numbers of ED1+ osteoclasts (multinucleated) and progenitors (mononucleated) were examined in distal radius and ulna at the periosteal-bone interface. A two way ANOVA revealed a significant difference in ED1+ cells by week (p<0.001), tissue type (p=0.0002), and their interaction (p=0.0001). Figure 3 shows the results of the post hoc analysis. Significant increases of ED1+ osteoclasts were seen in distal radius and ulna in week 12 (p=0.04), in the median nerve at the wrist in weeks 6 and 12 (p<0.01 and p<0.001, respectively), in flexor forelimb muscles in weeks 6 and 12 (p<0.001 and p<0.01, respectively), and in flexor forelimb tendons in weeks 6 and 12 (p=0.04 for each), compared to control rats. The greatest increases were seen in distal radius and ulna (ED1+ progenitors) and median nerve (ED1+ macrophages) compared to the other tissues (Fig. 3). Adjacent hematoxylin and eosin (H&E) stained sections were examined for increased collagen in or around the median nerve, which would be suggestive of fibrosis. No increases over control amounts were observed (data not shown).
Figure 3.
Mean (+ SEM) ED1-positive cells (a marker of macrophages, osteoclasts (OC) or their progenitors (Prog)) was examined in distal radius and ulna bones, median nerves, forelimb flexor muscles or forelimb flexor tendons. Normal controls (C, n=4) and rats that had performed the MRHF task for 6 weeks (n=4) or 12 weeks (n=4) were evaluated. Significant increases from control levels are denoted by symbols (*p<0.01 and **p<0.001 compared to controls; # p<0.001 compared to bone osteoclasts, muscle or tendon).
To further examine tissues for evidence of inflammation, cytokine levels were examined in musculoskeletal tissues using single plex ELISA. As shown in Figure 4, TNF-α, IL-1α, IL-1β, and IL-10 were significantly increased in 12 week MRHF rats in distal bone (distal radius and ulna, and first row of carpal bones) compared to normal controls (p<0.001 for each; Fig 4A,B,D,E). IL-6 was not tested in distal forelimb bones, but was significantly elevated in flexor forelimb tendons in 12 week MRHF rats compared to normal controls (p<0.01; Fig. 4C). For each ANOVA, there were significant differences by tissue type (p<0.0001). There were also significant differences by week of task performance and the interaction of week and tissue type: TNFα: week p=0.0031, interaction p=0.0003; IL-1α: week p=0.0320, interaction p=0.04; IL-1β: week p=0.0019, interaction p=0.0003; IL-10: week p=0.0001; interaction p<0.0001. However, for IL-6, only the factor week was significant (p=0.001).
Figure 4.
Mean (+ SEM) for cytokine levels in forelimb flexor muscles, forelimb flexor tendons, and distal bone (which included distal radius and ulna, and first row of carpal bones). Controls (white bars; n=6, which included 3 normal controls and 3 trained controls) and rats that had performed the MRHF for 6 weeks (n=4) or 12 weeks (n=4) were tested using ELISA. IL-6 was not tested in distal bone (N/A). Significant increases from control levels are denoted by symbols (**p<0.001).
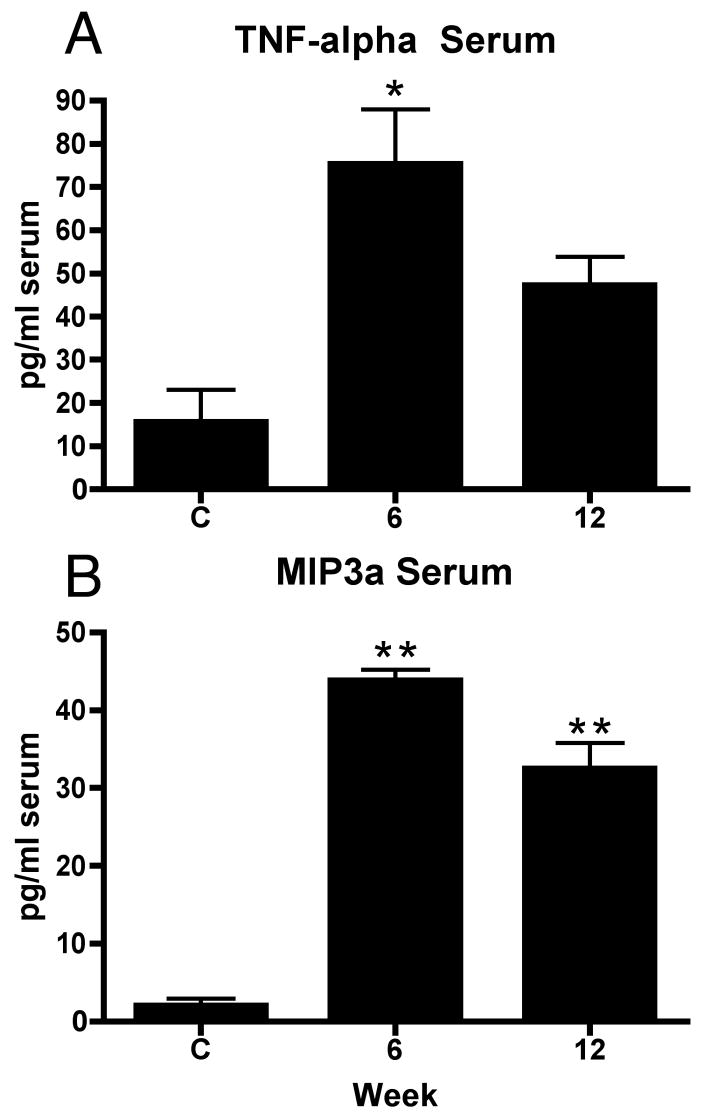
To examine for evidence of systemic inflammation, 8 cytokine and chemokine levels were tested in serum using multiplex ELISA. As shown in Figure 5, significant increases of TNF-α was observed in week 6 (ANOVA: p=0.0038; post hoc for week 6: p<0.01) and for MIP3a in weeks 6 and 12 (ANOVA: p<0.001; post hoc for each: p<0.001), compared to controls. No significant increases were observed for serum IL-1α, IL-1-β, IL-6, IL-10, IFNγ, or MIP2 (data not shown).
Figure 5.
Mean (+ SEM) level of TNF-alpha or MIP3a in serum. Normal and trained controls (C, n=21, 11 of which were normal controls) and rats that had performed the MRHF task for 6 weeks (n=4) or 12 weeks (n=4) were evaluated. Significant increases from control levels are denoted by symbols (*p<0.01 and **p<0.001 compared to controls).
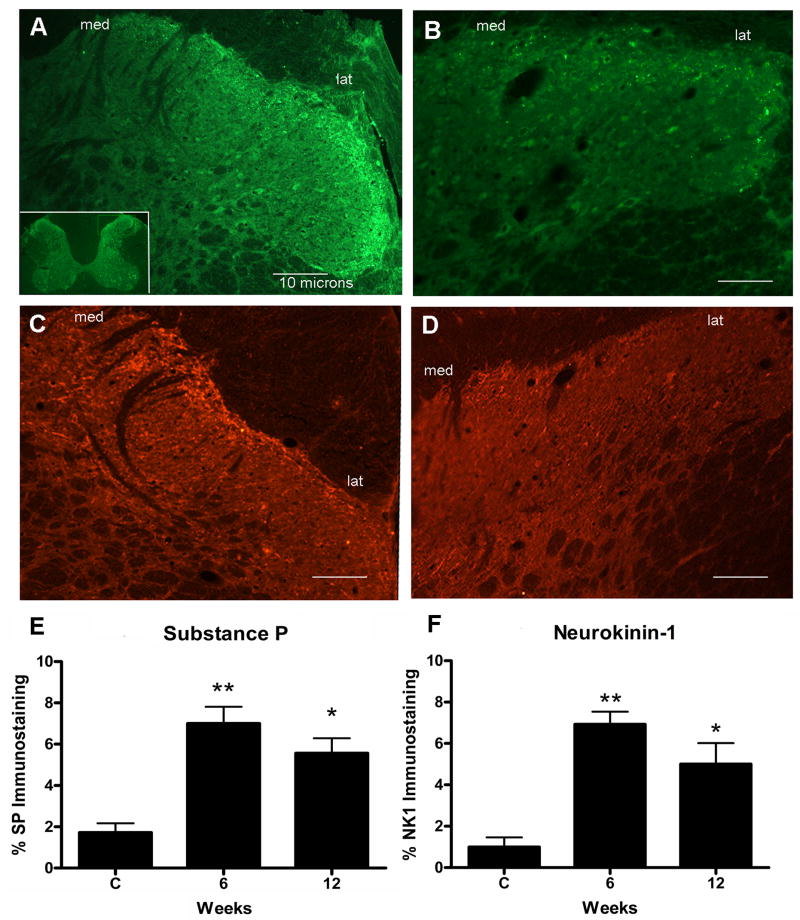
Lastly, we determined if the peripheral inflammatory changes were associated with central spinal cord neurochemical responses. We observed increased immunoexpression of substance P and neurokinin-1 in weeks 6 and 12. Substance P expression was significantly increased at 6 weeks (p<0.001) and 12 weeks (p<0.01) of MRHF task performance (Fig 5A,E) compared to controls (ANOVA p<0.0001)(Fig. 5B,E). Neurokinin-1 expression was also significantly increased at 6 weeks (p<0.001) and 12 weeks (p<0.01) of MRHF task performance (Fig. 5C,F) compared to controls (ANOVA p < 0.0001) (Fig. 5D,F).
Discussion
These results show that a moderate repetition, high force task produces motor declines and mechanical allodynia as well as a small decline in nerve conduction velocity suggestive of a low level chronic constriction injury of the median nerve. These behavioral changes were accompanied by local tissue (musculoskeletal and peripheral nerve) and systemic inflammatory responses, as well as increased neurochemicals in the cervical spinal cord that receives input from the involved tissue regions.
Motor Behavior and Nerve Function
Task duration, an indicator of task participation, was significantly reduced at the end of 12 weeks of the MRHF task. This reduction may have been due to discomfort occurring from tissue inflammation or from sensitization of nociceptors (see below). This is consistent with our previous findings that high repetition tasks result in transient reductions in task duration at time points coinciding with tissue inflammation (Clark et al., 2004;Barbe et al., 2008). However, we did not observe a reduction in reach rate, in contrast to our previous HRHF study (Clark et al., 2004), but similar to our study on rats performing a low repetition, negligible force (LRNF) task (Elliott et al., 2008). In the latter study, we found little to no inflammatory response. We hypothesize that the ability to maintain reach rate and task duration is dependent on the level of underlying developing tissue pathology produced by the demands of a particular task. This hypothesis is substantiated by a recent study using a pinching task in primates in which performance declined with increased force level and pinch hold time, findings that coincided with a diagnosis of carpel tunnel syndrome (Sommerich et al., 2007).
Reduced grip strength as was found in this study is a sign of neuropathic injury, although it can also decline as a result of muscle inflammation (Kehl and Fairbanks, 2003). Kehl et al. reported reduced forelimb grip force following intramuscular injection of carrageenan, an agent used to stimulate cutaneous inflammation and activate muscle nociceptors. This chemically induced reduction in grip strength was reversed using several drugs used for pain management. Baker and colleagues have also reported declines in isometric force production with inflammation and damage to myofibrils postulated as injury mechanisms (Baker et al., 2007).
The reduction in NCV induced by this MRHF task is similar to results from our high repetition, negligible force task (9%) (Clark et al., 2003), but less than that induced by a HRHF task (17%) (Clark et al., 2004). Significant impairments to nerve conduction have been documented in studies of median nerve compression injury (Diao et al., 2005;Sommerich et al., 2007). In Sommerich’s study, the significant decline in NCV (25–31%) induced by their repetitive task recovered to normal levels after cessation of the task. In contrast, Gupta and colleagues found no recovery after a 65% reduction in NCV even after 12 months of rest (Gupta et al., 2003). We have not yet examined the effects of rest in our model.
Peripheral Inflammation and Sensitization
Peripheral inflammatory responses in the present study are in line with our previous findings of widespread musculoskeletal and neural macrophage infiltration and cytokine increases after performance of a negligible force, repetitive task (Barbe et al., 2003;Clark et al., 2003;Al-Shatti et al., 2005;Barbe et al., 2008;Elliott et al., 2008). Increased endoneurial macrophage infiltration has been documented in studies of nerve constriction and ligation injuries (Wagner et al., 1998;Ma and Eisenach, 2003;Gupta et al., 2006). The presence of macrophages in tissues has been linked to inflammation-induced mechanical sensitivity of axons (Bove et al., 2003;Twining et al., 2004). In this study, we found a temporal association between peripheral inflammation and the presence of mechanical allodynia, a novel finding for our model. Several studies using nerve or nerve root ligation or clamping to induce compression injuries also report significant mechanical allodynia (Hunt et al., 2001;Schafers et al., 2001;Hubbard and Winkelstein, 2005). There is a wealth of evidence showing that inflammatory mediators released as a result of tissue injury contribute to increased afferent nociceptor sensitivity and excitability (Schafers et al., 2001;Sweitzer et al., 2001;Winkelstein et al., 2001a;Leem and Bove, 2002;Sachs et al., 2002;Pitcher and Henry, 2004;Twining et al., 2004;Gupta et al., 2006;Chao et al., 2008). For example, Schafers et al, (2001) reports increased endoneurial TNF-α and IL-1β expression accompanied by mechanical allodynia and thermal hyperalgesia following chronic constriction nerve injury. Mechanical allodynia can be ameliorated by anti-inflammatory cytokine therapy, suggesting an inflammation-induced mechanism (Wagner et al., 1998;Schafers et al., 2001). We believe it is the widespread tissue and systemic inflammatory response to our repetitive task that contributes to sensitization of nociceptors as opposed to the response of a single tissue type or system. This is the first study to document that inflammation in multiple tissues including nerve are present simultaneously with hypersensitivity/behaviors, findings that are indicative of neuropathic pain mechanisms with repetitive strain injuries.
Central neurochemicals and sensitization
This study extends our previous findings that injury-induced inflammation in peripheral nerve invokes a central neuroplastic response (Elliott et al., 2008). In the present MRHF study, increased Substance P and neurokinin-1 expression were observed in week 6 followed by a decline, although levels remained well above baseline in week 12. In the LRNF study, Substance P immunoexpression levels dropped toward baseline by week 12 (Elliott et al.). In addition, immunoexpression of substance P and neurokinin-1 occurred earlier with the MRHF task than the LRNF task (Elliott et al.). These results further support our hypothesis of tissue responses that match the level of exposure to repetition and force. Likewise, the severity of injury (i.e., ligation tightness) appears to influence the timing for spinal neuropeptide responses. Wallin et al. found that substance P expression was increased in spinal cord dorsal horns at 60 days, but not at 3 or 14 days following partial ligation of the sciatic nerve (Wallin and Schott, 2002). In another study, partial nerve ligation resulted in a cyclical pattern of elevated spinal substance P mRNA in which levels were up at 1 and 2 days, down from 3 days to 3 weeks, followed by another increase at 4 weeks (Delander et al., 1997). In a study of chronic constriction injury of the sciatic nerve, Neurokinin-1 immunoreactivity was increased by 7 days followed by a steady increase over time until the end of the 40 day study (Cruce et al., 2001).
Numerous studies have documented central responses to peripheral nerve injury or inflammation. For example, substance P and neurokinin-1 are significantly elevated in the spinal cord dorsal horns after nerve ligation and chronic compression, and in models of peripheral inflammatory pain (Abbadie et al., 1996;Delander et al., 1997;Allen et al., 1999;Honor et al., 1999;McCarson, 1999;Cruce et al., 2001;Rothman et al., 2005). The increase in neurokinin-1, a receptor for substance P, appears to occur as a result of increased release of substance P from nociceptive afferent terminals (Pitcher and Henry, 2004). There are also phenotypic changes in dorsal root ganglion neurons following chronic nerve compression or ligation injury in which neurons alter their expression of proteins, receptors, neurotransmitters, and neurotrophic factors (Hammond et al., 2004;Chao et al., 2008). The afferent barrage of excitatory transmitters into the spinal cord dorsal horn is considered to be the presynaptic component of central sensitization (Schaible et al., 2002). Such increases are temporally associated with allodynia (Sweitzer et al., 2001;Winkelstein et al., 2001b;Rothman et al., 2005). These studies provide evidence that spinal cord plasticity under injury and inflammatory conditions is involved in central sensitization, in turn contributing to chronic pain conditions, such as mechanical allodynia.
Conclusion
Our findings show that performance of a repetitive and forceful task, in this case a moderate repetition task with high force, leads to pain behaviors and motor declines. These behavioral changes were associated temporally with tissue inflammation and inflammation-induced spinal cord neurochemical increases. Furthermore, these results combined with our previous findings indicate that the level and timing of behavioral and tissue responses varies depending on the exposure to repetition and force.
Figure 6.
Substance P and NK-1 immunostaining in the superficial lamina of dorsal horns in cervical spinal cord segments. (A) Dorsal horns of week 6 MRHF rats have punctuate substance P immunofluorescence (green) staining that is distributed across the entire zone, medial to lateral, of the superficial lamina with increased expression more laterally. Medial (med) and lateral (lat) regions of the superficial lamina are indicated. Inset in panel (A) shows a C7 spinal cord cross-section at low power. (B) Dorsal horns of control rats have low levels of substance P immunofluorescence staining in the superficial lamina. (C) Dorsal horns of week 6 MRHF rats have NK-1 immunofluorescence (red) staining on plasma membranes and dendrites with some endosome swellings that spans the entire zone of the superficial lamina in which increased expression is observed more medially. (D) Dorsal horns of control rats have low levels of NK-1 immunofluorescence staining in the dorsal horn superficial lamina. Bar = 10 μm. D and E) Mean (+ SEM) percent immunofluorescent staining for substance P and neurokinin1 in the spinal cord dorsal horn superficial lamina. Normal and trained controls (C, n=4 each group for a total of 8 control rats) and rats that had performed the MRHF task for 6 weeks (n=4) or 12 weeks (n=4) were quantified. Significant increases from control levels are denoted by symbols (*p<0.01 and **p<0.001 compared to controls).
Acknowledgments
This project was supported by Grant OH 03970 from CDC-NIOSH to M.F.B. and Grant AR051212 from NIH-NIAMS to A.E.B. We would like to thank Michelle Harris for technical assistance on this project.
List of Abbreviations
- RSI
repetitive strain injury
- MRHF
moderate repetition high force
- NCV
nerve conduction velocity
- HRNF
high repetition negligible force
- LRLF
low repetition low force
- LRNF
low repetition negligible force
- NC
normal control
- TC
trained control
- C
normal and trained control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J Neuroimmunol. 2005;167:13–22. doi: 10.1016/j.jneuroim.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Baker BA, Mercer RR, Geronilla KB, Kashon ML, Miller GR, Cutlip RG. Impact of repetition number on muscle performance and histological response. Med Sci Sports Exerc. 2007;39:1275–1281. doi: 10.1249/mss.0b013e3180686dc7. [DOI] [PubMed] [Google Scholar]
- Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe MF, Elliott MB, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J Orthop Res. 2008 doi: 10.1002/jor.20674. [DOI] [PubMed] [Google Scholar]
- Barr AE, Barbe MF, Clark BD. Systemic inflammatory mediators contribute to widespread effects in work-related musculoskeletal disorders. Exerc Sport Sci Rev. 2004;32:135–142. doi: 10.1097/00003677-200410000-00003. [DOI] [PubMed] [Google Scholar]
- Barr AE, Safadi FF, Gorzelany I, Amin M, Popoff SN, Barbe MF. Repetitive, negligible force reaching in rats induces pathological overloading of upper extremity bones. J Bone Miner Res. 2003;18:2023–2032. doi: 10.1359/jbmr.2003.18.11.2023. [DOI] [PubMed] [Google Scholar]
- Labor USDo., editor. BLS. Nonfatal occupational injuries and illnesses requiring days away from work, 2005. Washington, D.C.: United States Department of Labor; 2006. [Google Scholar]
- Labor USDo., editor. BLS. Nonfatal occupational injuries and illnesses requiring days away from work, 2006. Washington, D.C.: United States Department of Labor; 2007. [Google Scholar]
- Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–1955. doi: 10.1152/jn.00175.2003. [DOI] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clark BD, Al-Shatti TA, Barr AE, Amin M, Barbe MF. Performance of a high-repetition, high-force task induces carpal tunnel syndrome in rats. J Orthop Sports Phys Ther. 2004;34:244–253. doi: 10.2519/jospt.2004.34.5.244. [DOI] [PubMed] [Google Scholar]
- Clark BD, Barr AE, Safadi FF, Beitman L, Al-Shatti T, Amin M, Gaughan JP, Barbe MF. Median nerve trauma in a rat model of work-related musculoskeletal disorder. J Neurotrauma. 2003;20:681–695. doi: 10.1089/089771503322144590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruce WL, Lovell JA, Crisp T, Stuesse SL. Effect of aging on the substance P receptor, NK-1, in the spinal cord of rats with peripheral nerve injury. Somatosens Mot Res. 2001;18:66–75. doi: 10.1080/08990220020021366. [DOI] [PubMed] [Google Scholar]
- Delander GE, Schott E, Brodin E, Fredholm BB. Temporal changes in spinal cord expression of mRNA for substance P, dynorphin and enkephalin in a model of chronic pain. Acta Physiol Scand. 1997;161:509–516. doi: 10.1046/j.1365-201X.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Diao E, Shao F, Liebenberg E, Rempel D, Lotz JC. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–223. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Barr AE, Kietrys DM, Al-Shatti T, Amin M, Barbe MF. Peripheral neuritis and increased spinal cord neurochemicals are induced in a model of repetitive motion injury with low force and repetition exposure. Brain Res. 2008;1218:103–113. doi: 10.1016/j.brainres.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronilla KB, Miller GR, Mowrey KF, Wu JZ, Kashon ML, Brumbaugh K, Reynolds J, Hubbs A, Cutlip RG. Dynamic force responses of skeletal muscle during stretch-shortening cycles. Eur J Appl Physiol. 2003;90:144–153. doi: 10.1007/s00421-003-0849-8. [DOI] [PubMed] [Google Scholar]
- Gupta R, Lin YM, Bui P, Chao T, Preston C, Mozaffar T. Macrophage recruitment follows the pattern of inducible nitric oxide synthase expression in a model for carpal tunnel syndrome. J Neurotrauma. 2003;20:671–680. doi: 10.1089/089771503322144581. [DOI] [PubMed] [Google Scholar]
- Gupta R, Rummler LS, Palispis W, Truong L, Chao T, Rowshan K, Mozaffar T, Steward O. Local down-regulation of myelin-associated glycoprotein permits axonal sprouting with chronic nerve compression injury. Exp Neurol. 2006;200:418–429. doi: 10.1016/j.expneurol.2006.02.134. [DOI] [PubMed] [Google Scholar]
- Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–1932. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine. 2001;26:2073–2079. doi: 10.1097/00007632-200110010-00005. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Fairbanks CA. Experimental animal models of muscle pain and analgesia. Exerc Sport Sci Rev. 2003;31:188–194. doi: 10.1097/00003677-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Kimura J. The carpal tunnel syndrome: localization of conduction abnormalities within the distal segment of the median nerve. Brain. 1979;102:619–635. doi: 10.1093/brain/102.3.619. [DOI] [PubMed] [Google Scholar]
- Leem JG, Bove GM. Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain. 2002;3:45–49. doi: 10.1054/jpai.2002.27138. [DOI] [PubMed] [Google Scholar]
- Ma W, Eisenach JC. Cyclooxygenase 2 in infiltrating inflammatory cells in injured nerve is universally up-regulated following various types of peripheral nerve injury. Neuroscience. 2003;121:691–704. doi: 10.1016/s0306-4522(03)00495-0. [DOI] [PubMed] [Google Scholar]
- McCarson KE. Central and peripheral expression of neurokinin-1 and neurokinin-3 receptor and substance P-encoding messenger RNAs: peripheral regulation during formalin-induced inflammation and lack of neurokinin receptor expression in primary afferent sensory neurons. Neuroscience. 1999;93:361–370. doi: 10.1016/s0306-4522(99)00102-5. [DOI] [PubMed] [Google Scholar]
- Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Henry JL. Nociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic pain. Exp Neurol. 2004;186:173–197. doi: 10.1016/j.expneurol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Kreider RA, Winkelstein BA. Spinal neuropeptide responses in persistent and transient pain following cervical nerve root injury. Spine. 2005;30:2491–2496. doi: 10.1097/01.brs.0000186316.38111.4b. [DOI] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Poole S, Ferreira SH. Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain. 2002;96:89–97. doi: 10.1016/s0304-3959(01)00433-x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Brinkhoff J, Neukirchen S, Marziniak M, Sommer C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci Lett. 2001;310:113–116. doi: 10.1016/s0304-3940(01)02077-8. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Sommerich CM, Lavender SA, Buford JA, J JB, Korkmaz SV, Pease WS. Towards development of a nonhuman primate model of carpal tunnel syndrome: performance of a voluntary, repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J Orthop Res. 2007;25:713–724. doi: 10.1002/jor.20363. [DOI] [PubMed] [Google Scholar]
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004;110:299–309. doi: 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Wagner R, Janjigian M, Myers RR. Anti-inflammatory interleukin-10 therapy in CCI neuropathy decreases thermal hyperalgesia, macrophage recruitment, and endoneurial TNF-alpha expression. Pain. 1998;74:35–42. doi: 10.1016/S0304-3959(97)00148-6. [DOI] [PubMed] [Google Scholar]
- Wallin J, Schott E. Substance P release in the spinal dorsal horn following peripheral nerve injury. Neuropeptides. 2002;36:252–256. doi: 10.1016/s0143-4179(02)00024-0. [DOI] [PubMed] [Google Scholar]
- Walters RJ, Murray NM. Transcarpal motor conduction velocity in carpal tunnel syndrome. Muscle Nerve. 2001;24:966–968. doi: 10.1002/mus.1096. [DOI] [PubMed] [Google Scholar]
- Willems ME, Stauber WT. Isometric and concentric performance of electrically stimulated ankle plantar flexor muscles in intact rat. Exp Physiol. 1999;84:379–389. [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001a;439:127–139. [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Quantification of neural tissue injury in a rat radiculopathy model: comparison of local deformation, behavioral outcomes, and spinal cytokine mRNA for two surgeons. J Neurosci Methods. 2001b;111:49–57. doi: 10.1016/s0165-0270(01)00445-9. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]