Abstract
OBJECTIVE
The amount of visceral adipose tissue is a risk factor for the metabolic syndrome. It is unclear how BMI changes during childhood and adolescence predict adult fat distribution. We hypothesized that there are critical periods during development for the prediction of adult subcutaneous and visceral fat mass by BMI changes during childhood and adolescence.
RESEARCH DESIGN AND METHODS
Detailed growth charts were retrieved for the men participating in the population-based Gothenburg Osteoporosis and Obesity Determinants (GOOD) Study (n = 612). Body composition was analyzed using dual-energy X-ray absorptiometry and adipose tissue areas using abdominal computed tomography at 18 to 20 years of age.
RESULTS
The main finding in the present study was that subjects with increases in BMI Z score of more than 1 SD during adolescence had, independent of prepubertal BMI, both larger subcutaneous (+138%; P < 0.001) and visceral adipose tissue areas (+91%; P < 0.001) than subjects with unchanged BMI Z-score. In contrast, subjects with increases in BMI Z score of more than 1 SD during late childhood had a larger amount of adult subcutaneous adipose tissue (+83%; P < 0.001) than subjects with unchanged BMI Z score but an unaffected amount of visceral adipose tissue. BMI changes during adolescence predict both visceral and subcutaneous adipose tissue of the abdomen, whereas BMI changes during late childhood predict only the subcutaneous adipose tissue.
CONCLUSIONS
The amount of visceral adipose tissue in young adult men was associated with BMI changes specifically during adolescence, whereas the amount of subcutaneous adipose tissue was associated with BMI changes during both late childhood and adolescence.
Childhood obesity has developed into an epidemic in the Western world and not only adults but also children are now treated for metabolic syndrome disorders (1). Obesity is an important risk factor for the development of diabetes and cardiovascular disease, and in the third U.S. National Health and Nutrition Examination Survey (1999–2002), 16.8% of boys aged 6 to 19 years were overweight and 31.8% were overweight or at risk for overweight (1,2).
A large amount of visceral fat is a well-known risk factor for the metabolic syndrome and for cardiovascular disease (3,4). A recent study demonstrated that although the amount of subcutaneous adipose tissue is associated with the metabolic syndrome, the amount of visceral adipose tissue remains more strongly related and is therefore regarded as an independent risk factor (5).
Anthropometric measures of obesity in childhood such as BMI have previously been shown to be associated with measures of obesity in adulthood (6). The concept of childhood and adolescent BMI changes as predictors for adult obesity as well as adult fat mass and distribution is attractive because BMI is easily obtained from standardized growth charts, including height and weight. It has been reported that both BMI and obesity track from childhood to adulthood; the closer to adulthood, the stronger the tracking (7–9). Recent studies have also linked rapid alterations in BMI during childhood to an increased number of coronary events and impaired glucose tolerance (10–12).
In the New Delhi birth cohort study by Sachdev et al., children were followed with anthropometric measurements from birth until 21 years of age with the objective to identify indicators of adult body composition (13). The authors reported that BMI during early childhood predicted anthropometric indexes of adult lean mass more strongly than anthropometric indexes of adult adiposity in Indian children. In contrast, BMI at 8 years of age was a clear predictor of indicators of adiposity in these children. Analyses of visceral fat depots were not performed in the New Delhi birth cohort study.
Dietz has previously identified both “adiposity rebound” (∼5 years of age after which BMI starts to incline) and adolescence as critical periods for the onset of obesity (14,15). We and others have found support for the association between puberty and fat accumulation (16–20). We demonstrated an association between early puberty and high BMI at adult age in males and showed that pubertal timing, independently of prepubertal BMI, predicts a central pattern of fat distribution and visceral fat depots (16). Fox et al. (21) reported that the intra-abdominal–to–subcutaneous fat ratio increased during early puberty in boys (n = 25). In contrast, in a small study by Brambilla et al. investigating eight obese children, it was found that the amount of intra-abdominal fat was stable during pubertal development (22). It is therefore unclear if BMI changes during childhood and adolescence predict the amount of subcutaneous and visceral fat differentially. We used a well-characterized population-based cohort of Swedish young adult males and hypothesized that there are critical periods during development for the prediction of adult subcutaneous and visceral fat mass by BMI changes during childhood and adolescence. The main objective with the current study was, therefore, to investigate the role of BMI changes during childhood and adolescence for young adult subcutaneous and visceral fat depots.
RESEARCH DESIGN AND METHODS
The Gothenburg Osteoporosis and Obesity Determinants (GOOD) Study was initiated with the aim to determine both environmental and genetic factors involved in the regulation of fat mass as previously described (23). To be included in the GOOD Study, subjects had to be male, 18 to 20 years of age, and willing to participate in the study. There were no other exclusion criteria. The study population was homogenous with respect to ethnicity. A total of 1,068 subjects, representative of the general young male population of Gothenburg (23) (data not shown), were enrolled in the study. Of the 1,068 subjects, complete growth and weight charts for determination of BMI between 1 and 10 years of age were available for 612 subjects (growth chart subsample). The results presented here were obtained from a subsample of the original GOOD cohort. Dual-energy X-ray absorptiometry analyses were performed on 610 subjects of the growth chart subsample, whereas abdominal CT scans were performed on 201 (the CT subsample) of the 612 study subjects in the growth chart subsample. The growth chart subsample is representative of the entire GOOD cohort in terms of age (GOOD 18.9 ± 0.6 years, growth chart subsample 18.9 ± 0.5 years, nonsignificant), weight (GOOD 73.9 ± 11.9 kg, growth chart subsample 73.3 ± 11.5 kg, nonsignificant), height (GOOD 181.4 ± 6.8 cm, growth chart subsample 182.0 ± 6.8 cm, nonsignificant), and BMI (GOOD 22.4 ± 3.2 kg/m2, growth chart subsample 22.1 ± 3.1 kg/m2, nonsignificant). The CT subsample is representative of both the growth chart subsample and the entire GOOD cohort in terms of weight, height, and BMI (CT subsample weight 72.7 ± 11.2 kg, height 181.9 ± 7.0 cm, BMI 21.9 ± 3.0 kg/m2, nonsignificant versus both the entire GOOD cohort and the growth chart subsample). However, the subjects of the CT subsample were slightly younger (CT subsample 18.7 ± 0.5 years) than both the subjects of the entire GOOD cohort and the subjects of the growth chart subsample (P < 0.05).
The GOOD Study was approved by the local ethics committee at Gothenburg University. Written and oral informed consent was obtained from all the study participants.
Anthropometric measurements.
Height was measured using a wall-mounted stadiometer and weight was measured to the nearest 0.1 kg as previously described (16,23).
Dual-energy X-ray absorptiometry.
Total body fat mass, percentage body fat, total body lean mass, and fat mass of the trunk were assessed using the Lunar Prodigy DXA (GE Lunar, Madison, WI).
Abdominal CT analyses of cross-sectional adipose tissue areas.
A previously described CT technique was used to measure the cross-sectional adipose area of the abdomen (16,24,25). A single slice at the level of the fourth lumbar vertebra was acquired with a General Electric High Speed Advantage CT system (version RP2; GE Medical Systems, Milwaukee, WI). Subcutaneous and visceral adipose tissue areas were measured, and then the visceral adipose tissue area was divided into an intraperitoneal and a retroperitoneal adipose tissue area. This enabled both subcutaneous and intra/retroperitoneal adipose tissue areas to be determined.
Estimation of childhood BMI.
Growth and weight charts were collected for the subjects of the GOOD Study and longitudinal growth was curve fitted according to the infancy-childhood–puberty (ICP) model (26) as previously described (16,27). The ICP model represents a widely used model for fitting of human growth data to mathematical functions (26). The infancy part is represented by an exponential function, the childhood part by a quadratic function, and the pubertal part by a logistic function. The model also includes the transformations between the different phases. By using this model, we computed values of height at exact ages.
Body composition, including BMI, is greatly influenced by pubertal stage and thereby pubertal timing (28). Complete growth charts with enough data to determine pubertal timing, requiring several height and weight measurements around the pubertal growth spurt, were not available for the complete growth chart subsample used in the present study. We have therefore chosen to use measurements of BMI before and after, but not during, puberty. Collection and processing of childhood data took place between 2003 and 2008. Body weights between 1 and 10 years of age were estimated through fitting of the weight curve for each child using smooth splines (smooth.spline in the R package statistics, the R foundation for statistic computing, Vienna, Austria; http://www.r-project.org). BMI values between 1 and 10 years of age were then calculated from the estimated values of weight and height.
The Z score, the standardization of a variable relative to the investigated population expressed in SDs, was calculated using the software SPSS 15.0 (SPSS, Chicago, IL). For each subject (n = 612) in this cohort and for each age, the height was calculated using the ICP model. These values were then used to calculate the Z score for each subject at each age based on the variation (SD) in calculated height within the presently investigated population at that specific age. The variance of young adult body composition parameters (R2) explained by BMI or BMI Z score changes and the corresponding β coefficients during childhood and adolescence was calculated using linear regression analyses with age-adjusted adult body composition variables as the dependent variable. Age adjustments were performed because the subjects differed somewhat in age at body composition analysis (18–20 years of age, mean ± SD 18.9 ± 0.5 years) and were performed between age at fat analysis and the body composition variables. All subsequent statistical calculations were performed using the age-adjusted body composition variables. For the calculations in Fig. 2, adjustments for baseline BMI were performed. BMI and fat parameters derived from body composition analyses have been log transformed. For all the statistical analyses, the software SPSS (version 15.0) was used. Values are given as means ± SD unless otherwise stated.
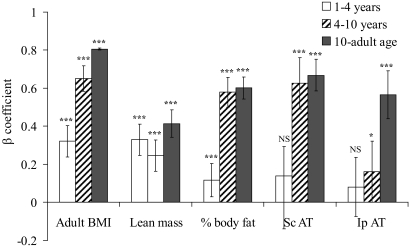
FIG. 2.
Correlation between change in standardized BMI during different developmental time periods and adult body composition and fat distribution parameters (early childhood defined as 1–4 years, late childhood defined as 4–10 years, and adolescence defined as 10 years to young adult). Bars indicate β coefficient (95% CIs) expressed as SD in investigated adult body composition parameter per SD change in BMI during that growth period. Variables of adult body composition and fat distribution have been age adjusted. ***P < 0.01, *P < 0.05 significant association between the developmental time period and the investigated body composition parameter. 95% CIs are given for the β coefficients, making it possible to evaluate the differences in relative contribution of the three time periods for each dependent parameter. AT, adipose tissue; Ip, intraperitoneal; NS, not significant; Sc, subcutaneous.
RESULTS
The population-based GOOD Study has been thoroughly described earlier (23). The present study represents a subsample (n = 612 [growth chart subsample]) of the original GOOD cohort (n = 1,068) in which subjects with available detailed growth charts from 1 to 10 years of age were included.
Anthropometrics and measurements of fat (DXA)/adipose tissue areas (abdominal CT) of the subjects participating in the present study are presented in Table 1.
TABLE 1.
Anthropometrics and fat variables
| Mean ± SD | Median (range) | |
|---|---|---|
| Adult anthropometrics (n = 612) | ||
| Age (years) | 18.9 ± 0.5 | 18.9 (18.0–20.1) |
| Height (cm) | 182.0 ± 6.8 | 181.9 (161.0–202.8) |
| Weight (kg) | 73.3 ± 11.5 | 71.7 (51.3–122.8) |
| Young adult BMI (kg/m2) | 22.1 ± 3.1 | 21.6 (16.1–41.6) |
| Childhood anthropometrics (n = 612) | ||
| 1 year of age | ||
| Length (cm) | 76.6 ± 2.2 | 76.6 (68.6–82.8) |
| Weight (kg) | 10.5 ± 0.97 | 10.4 (7.9–13.9) |
| BMI (kg/m2) | 17.8 ± 1.2 | 17.8 (14.3–22.8) |
| 4 years of age | ||
| Height (cm) | 105.0 ± 3.7 | 104.9 (94.6–116.6) |
| Weight (kg) | 17.7 ± 1.9 | 17.5 (12.7–24.1) |
| BMI (kg/m2) | 16.0 ± 1.2 | 15.9 (12.8–21.3) |
| 10 years of age | ||
| Height (cm) | 142.3 ± 6.2 | 142.1 (125.5–167.1) |
| Weight (kg) | 34.5 ± 5.7 | 33.5 (23.3–59.1) |
| BMI (kg/m2) | 17.0 ± 2.0 | 16.5 (13.2–26.9) |
| Adult DXA measurements (n = 610) | ||
| Whole body fat (kg) | 12.9 ± 7.5 | 11.1 (3.1–54.3) |
| Percentage body fat (%) | 16.8 ± 7.1 | 15.5 (5.2–44.9) |
| Fat mass trunk (kg) | 6.5 ± 4.1 | 5.5 (1.4–30.9) |
| Whole body lean mass (kg) | 57.4 ± 6.2 | 57.0 (40.7–77.3) |
| Adult abdominal CT measurements (n = 201) | ||
| Subcutaneous fat (cm2) | 99 ± 80 | 76 (15–488) |
| Intraperitoneal fat (cm2) | 22 ± 13 | 20 (3–84) |
CT, computed tomography.
BMI changes during both early and late childhood as well as during adolescence predicted young adult BMI.
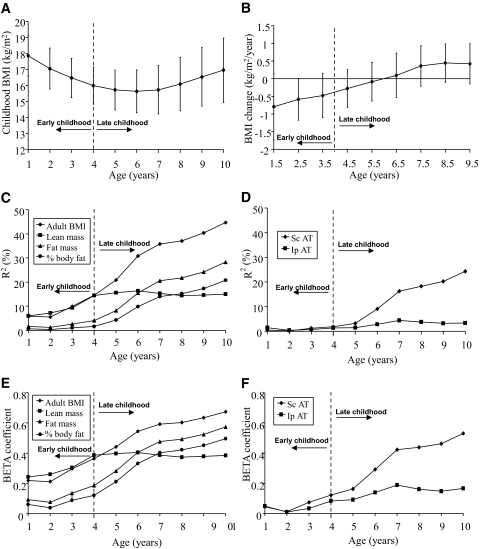
Average BMI in this cohort declined during early childhood years, and adiposity rebound (the lowest BMI) was reached slightly before 6 years of age, after which it began to increase (Fig. 1A and B). BMI (Fig. 1C and E) as well as BMI changes (Fig. 2) during both early (age 1–4 years) and late (age 4–10 years) childhood were, as expected, clear positive predictors of adult BMI. During adolescence, pubertal stage influences body composition (29). Because interindividual variations in pubertal onset will confound the calculations during adolescence, the predictive role of BMI for each individual year during adolescence for adult BMI and body composition is not shown. Instead, the β coefficient for the correlation of the entire adolescence (age 10–19 years) is shown in Fig. 2, demonstrating that, as expected, BMI changes during adolescence also predicted adult BMI.
FIG. 1.
Childhood BMI and variance in body composition and fat variables (BMI [A] and BMI change [B]) during early and late childhood. Data are means ± SD (C–F). Association between childhood BMI between 1 and 10 years of age and young-adult body composition parameters is expressed as accumulated R2 (%, C and D) or β coefficients (SD in adult body composition parameter per SD in BMI at that age, E and F). Variables of adult body composition and fat distribution have been age adjusted. AT, adipose tissue; Ip, intraperitoneal; Sc, subcutaneous.
Adult total body lean mass was predicted by changes in BMI during early childhood and adolescence.
BMI alterations during development reflect changes in fat mass, lean mass, or both. To study the role of childhood BMI and BMI changes for the prediction of different adult body compartments, the associations between BMI during development and age-adjusted adult body composition variables were analyzed. The variance (R2) in adult total body lean mass explained by BMI as well as the corresponding β coefficients for the correlations increased during early childhood, leveled off during late childhood, and increased again during adolescence (Figs. 1C and E and 2). The variance in adult BMI and the variance in adult lean mass explained by childhood BMI and the corresponding β coefficients for the correlations were similar until 4 years of age, when they separated distinctly (Fig. 1C and E). The β coefficient for the correlation between change in BMI and adult lean mass was higher during adolescence than during late childhood (Fig. 2). Thus, BMI changes during early childhood and adolescence but not during late childhood were major determinants of young adult lean mass.
Adult total body fat mass and subcutaneous adipose tissue area were predicted by changes in BMI during late childhood and adolescence.
The prediction of young-adult total body fat mass from BMI and BMI changes during different developmental periods followed a different pattern from that of lean mass. The variances in total body fat mass and percentage total body fat explained by childhood BMI and the corresponding β coefficients for the correlation were low before 4 years of age, but after 4 years of age, the plotted cumulated variance (R2) and the corresponding β coefficients for the correlation for young- adult total body fat mass and percentage body fat tightly followed the curve displaying the variance of adult BMI explained (Fig. 1C and E). The predictive value of childhood BMI for young-adult percentage total body fat increased substantially during late childhood years, as demonstrated by the fact that the variance in percentage total body fat explained by BMI at 4 years of age was only 1.7%, whereas it was 20.8% at 10 years of age (Fig. 1C).
To analyze fat distribution in detail, we performed abdominal CT scans (on a subset of the present GOOD sample [CT subsample], n = 201) for measurements of abdominal subcutaneous and visceral adipose tissue areas. The abdominal CT measurements revealed that the contribution of BMI during development for the explanation of the variance in young-adult subcutaneous adipose tissue area and the corresponding β coefficients for the correlations followed a pattern similar to that of total body fat mass and percentage total body fat, with a distinct increase after 4 years of age (Fig. 1D and F). For both young-adult percentage fat mass and subcutaneous adipose tissue area, the β coefficients for the correlations were of similar magnitude during late childhood and adolescence but clearly higher than seen during early childhood (Fig. 2).
BMI changes during late childhood and adolescence, but not during early childhood, clearly predicted young-adult total fat mass and the amount of subcutaneous adipose tissue.
Adult visceral adipose tissue areas were predicted by changes in BMI, specifically during adolescence.
The contribution of BMI during the different developmental periods for the explanation of the variance in amount of young-adult visceral adipose tissue (intraperitoneal + retroperitoneal adipose tissue areas) and the corresponding β coefficients for the correlations followed a pattern different and clearly distinct from that of total body fat mass and subcutaneous adipose tissue area. BMI and BMI changes during early and late childhood contributed only marginally to the explanation of the variance in intraperitoneal adipose tissue area, and the corresponding β coefficient for the correlation between change in BMI and amount of young-adult intraperitoneal adipose tissue was low (Figs. 1D and F and 2). Instead, adolescence was the developmental period when BMI changes started to contribute substantially to the explanation of the variance in amount of adult intraperitoneal adipose tissue, and the corresponding β coefficients for the correlations between change in BMI and amount of young-adult intraperitoneal adipose tissue were then higher than seen during early and late childhood (Fig. 2). The results for retroperitoneal adipose tissue were similar as seen for intraperitoneal adipose tissue (data not shown). Analyses using weight for age during childhood and adolescence showed, in a manner similar to that seen for BMI for age, clear age-dependent associations with adult fat parameters (data not shown). In contrast, analyses of height for age did not show any obvious age-dependent association with fat parameters (data not shown). Changes in BMI during adolescence, but not during early or late childhood, predicted the amount of adult visceral adipose tissue.
Changes in BMI Z score and adult fat mass.
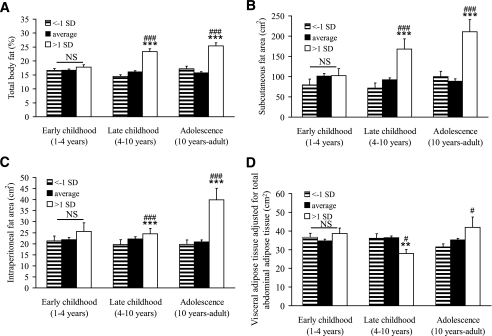
To further describe the association between developmental changes in BMI and adult fat mass/adipose tissue areas, subjects were divided into groups based on change in BMI Z score (>1 SD, less than −1 SD, and average BMI Z score change) during the three different developmental time periods (Fig. 3). Subjects with more than 1 SD increase in BMI Z score during late childhood and during adolescence had a clearly higher percentage total body fat mass (late childhood, +44%, P < 0.001; adolescence, +60%, P < 0.001, Fig. 3A) and larger subcutaneous adipose tissue area (late childhood +83%, P < 0.001; adolescence, +138%, P < 0.001, Fig. 3B) than subjects with average change in BMI. In contrast, subjects with more than 1 SD increase in BMI Z score during late childhood had unaffected intraperitoneal adipose tissue area compared with subjects with average change in BMI (Fig. 3C). However, for adolescence, subjects with more than 1 SD increase in BMI Z score had larger intraperitoneal adipose tissue area (+91%, P < 0.001) than subjects with unchanged BMI Z score (Fig. 3C). A specific association between high young-adult subcutaneous, but not visceral (intraperitoneal + retroperitoneal), adipose tissue area and increased BMI Z score during late childhood is supported by the finding that young-adult visceral adipose tissue area adjusted for total adipose tissue area was clearly reduced in subjects with more than 1 SD increase in BMI Z score during late childhood (Fig. 3D). In contrast, an increase in BMI Z score of more than 1 SD during adolescence was associated with an increased visceral adipose tissue area adjusted for total adipose tissue area (Fig. 3D). Subjects who decreased their BMI with more than 1 SD did not differ from those with no change in BMI Z score for any of the investigated fat parameters (Fig. 3A–D). Thus, the amount of visceral adipose tissue was predicted by large increases in standardized BMI specifically during adolescence, whereas a high amount of subcutaneous adipose tissue area was predicted by increases in BMI during both late childhood and adolescence.
FIG. 3.
The impact of developmental BMI changes for percentage total body fat (A), subcutaneous adipose tissue area (B), intraperitoneal adipose tissue area (C), and visceral (intraperitoneal + retroperitoneal) adipose tissue adjusted for total abdominal (visceral + subcutaneous) adipose tissue area (D). Subjects with more than 1 SD increase were compared with subjects with average change or less than −1 SD decrease in standardized BMI during early childhood (1–4 years of age), late childhood (4–10 years of age), and adolescence (10–19 years of age). Variables of adult body composition and fat distribution have been age adjusted and are expressed as means ± SEM and were analyzed using ANOVA followed by Tukey's post hoc analysis. ***P < 0.001 versus average, **P < 0.01 versus average, ###P < 0.001 versus less than −1 SD decrease, and #P < 0.05 versus less than −1 SD. NS, not significant. A: Early childhood less than −1 SD, n = 72, average n = 471; more than 1 SD, n = 67; late childhood less than −1 SD, n = 75, average n = 462; more than 1 SD, n = 73; adolescence less than −1 SD, n = 55, average n = 500; more than 1 SD, n = 55. B–D: Early childhood less than −1 SD, n = 21, average n = 160; more than 1 SD, n = 20; late childhood less than −1 SD, n = 22, average n = 154; more than 1 SD, n = 25; adolescence less than −1 SD, n = 22, average n = 164; more than 1 SD, n = 15.
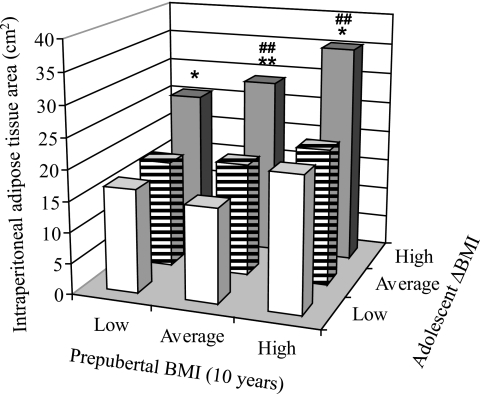
BMI increases during adolescence predict the amount of adult visceral adipose tissue independently of prepubertal BMI.
We next investigated if BMI increases in subjects with low prepubertal BMI had a similar impact on the amount of adult visceral adipose tissue as seen in subjects with a high prepubertal BMI. Subjects were divided into tertiles according to prepubertal (10 years of age) BMI (low 15.2 ± 0.8 kg/m2, average 16.7 ± 0.4 kg/m2, and high 19.3 ± 1.5 kg/m2) and tertiles of BMI Z score change (low −1.0 ± 0.6 SD, average −0.1 ± 0.2 SD, and high 0.7 ± 0.6 SD) during adolescence (Fig. 4). For all three prepubertal BMI groups, subjects with greater increase in adolescence BMI had significantly larger visceral adipose tissue areas (low prepubertal BMI +56%, average prepubertal BMI +53%, and high prepubertal BMI +57%; P < 0.05 for all) than subjects with average adolescence BMI increase (Fig. 4). BMI increases during adolescence predicted the amount of adult visceral adipose tissue independently of prepubertal BMI.
FIG. 4.
BMI increases during adolescence are associated with high adult visceral fat mass in subjects with low, average, and high prepubertal BMI. Intraperitoneal fat area (y-axis) in subjects divided into tertiles according to (i) prepubertal BMI (x-axis; low, average, and high) and (ii) BMI-Z score increase during adolescence (z-axis; low, average, and high). The intraperitoneal adipose tissue area has been age adjusted. Data are expressed as means and were analyzed using ANOVA followed by Tukey's post hoc analysis. *P < 0.05, **P < 0.01 versus average BMI Z score change during adolescence (▤); ##P < 0.01 versus low (□). N for lowest ΔBMI during adolescence (□) from low prepubertal BMI to high (left to right) 9, 19, and 39; for average ΔBMI during adolescence (▤) low prepubertal BMI to high (left to right) 26, 25, and 16; and for high ΔBMI during adolescence (▩) from low prepubertal BMI to high (left to right) 32, 23, and 12. N for all = 201.
DISCUSSION
Both BMI and obesity track strongly from childhood to adulthood (7–9,30,31), but the role for BMI changes during development as predictors of adult body composition and fat distribution is unclear. The main objective of the present study was to investigate the association between BMI changes during different developmental periods and young-adult fat distribution in a well-characterized cohort of young-adult men. We demonstrate that the amount of adult subcutaneous adipose tissue and visceral adipose tissue was associated with BMI changes during distinct developmental periods. We also identified the childhood age after which BMI increases had a clear impact on adult total body fat mass.
Our main finding was that the amount of adult visceral adipose tissue was associated with BMI changes specifically during adolescence, whereas the amount of subcutaneous adipose tissue was associated with BMI changes during both late childhood and adolescence. Subjects with more than 1 SD increase in BMI Z score during adolescence had >90% greater visceral adipose tissue areas than subjects with average change in BMI Z score. These data, hence, demonstrate that BMI increases during adolescence were associated with a larger amount of visceral adipose tissue at young adult age. A large amount of visceral fat is a well-known risk factor for cardiovascular disease. A recent study demonstrated that although the amount of subcutaneous adipose tissue is associated with the metabolic syndrome, the amount of visceral adipose tissue remains more strongly related and is therefore regarded as an independent risk factor (5). Increased understanding of how and when subcutaneous and visceral fat compartments are predicted might, therefore, add important knowledge for the prevention of cardiovascular disease. In the present study, BMI increases during adolescence in subjects with a low prepubertal BMI had a similar impact on the amount of adult visceral adipose tissue as in subjects with a high prepubertal BMI. BMI increases during adolescence predicted the amount of adult visceral adipose tissue independently of prepubertal BMI.
An increase by more than 1 SD in standardized BMI both during late childhood and during adolescence was, in the present study, associated with ∼50% more adult percentage total body fat. The New Delhi birth cohort study also demonstrated that BMI changes during late childhood were associated with anthropometric measures of adult fat mass (13). The New Delhi study represents a large longitudinally followed cohort, but indicators of fat mass and lean mass were only derived from anthropometric measurements and, consequently, the visceral fat could not be determined in that study. The differences both in height and weight for age and in nutritional status between the Indian children in the New Delhi birth cohort and children in the Western world (13,32–34) might also limit the interpretations of the data from the New Delhi birth cohort into the Western world. A major strength of the present study is that both the subcutaneous and the visceral adipose tissue areas were measured using abdominal CT scans, giving us the opportunity to determine the predictive value of developmental BMI changes for these two fat depots separately. In a Danish study, including men born between 1930 and 1956, it was shown that subjects with both increases and decreases in BMI between 7 and 13 years of age had a higher risk of obesity in adulthood compared with those who maintained their BMI level (35). In contrast, in the present cohort of boys born between 1983 and 1985, increases but not decreases in BMI during growth were associated with increased adult fat mass and BMI. Because the subjects in the Danish study were born between 1930 and 1956, it is possible that the decline in BMI during growth is related to environmental factors such as temporary undernourishment among certain subjects during this period (including World War II). Although we in the present study have characterized the associations between childhood BMI and adult obesity, it is clear that a substantial part of adult obesity cannot be explained by childhood obesity (6).
BMI changes during early childhood and adolescence were, in the present study, predicting adult lean mass, whereas BMI changes during late childhood and adolescence were predicting adult total body fat mass. These findings are in accordance with the findings in the New Delhi birth cohort study, demonstrating that BMI during early childhood predicts anthropometric measures of adult lean mass more strongly than anthropometric measures of adult fat mass in Indian children (13). Both the present study and the New Delhi birth cohort study thus support the notion that BMI changes during early childhood are indicators of adult lean mass, whereas BMI changes during late childhood and adolescence are indicators of adult total body fat mass. Our data demonstrate that 4 years of age was the threshold age for Swedish boys after which BMI increases were clearly associated with increased adult total body fat mass. A high BMI before this age was an indicator of high adult lean mass. It should be emphasized that the present findings are based on association studies and, therefore, should be interpreted with caution.
The present study demonstrates that the amount of young-adult subcutaneous adipose tissue was associated with BMI changes both during late childhood and adolescence, although young-adult visceral adipose tissue areas were associated with BMI changes specifically during adolescence. These findings suggest that avoiding substantial BMI increases during adolescence might, independent of prepubertal BMI, result in lower adult visceral fat mass.
Acknowledgments
This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, The ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Emil and Vera Cornell Foundation, the Torsten and Ragnar Söderberg's Foundation, Petrus and Augusta Hedlunds Foundation, the Västra Götaland Foundation, the Göteborg Medical Society, and the Tore Nilson Foundation for Medical Research.
This study was partly supported by the Novo Nordisk Foundation. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362– 2374, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM: Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847– 2850, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arsenault BJ, Lachance D, Lemieux I, Almeras N, Tremblay A, Bouchard C, Perusse L, Despres JP: Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med 167: 1518– 1525, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB: Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165: 777– 783, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ: Abdominal visceral and subcutaneous adipose tissue compartments. Association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39– 48, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T: Do obese children become obese adults? A review of the literature. Prev Med 22: 167– 177, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Campbell PT, Katzmarzyk PT, Malina RM, Rao DC, Perusse L, Bouchard C: Stability of adiposity phenotypes from childhood and adolescence into young adulthood with contribution of parental measures. Obes Res 9: 394– 400, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS: The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics 115: 22– 27, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Guo SS, Wu W, Chumlea WC, Roche AF: Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 76: 653– 658, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Baker JL, Olsen LW, Sorensen TI: Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 357: 2329– 2337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG: Trajectories of growth among children who have coronary events as adults. N Engl J Med 353: 1802– 1809, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS: Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350: 865– 875, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdev HS, Fall CH, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, Reddy KS, Barker DJ, Bhargava SK: Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr 82: 456– 466, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dietz WH: Critical periods in childhood for the development of obesity. Am J Clin Nutr 59: 955– 959, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dietz WH: Overweight in childhood and adolescence. N Engl J Med 350: 855– 857, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kindblom JM, Lorentzon M, Norjavaara E, Lonn L, Brandberg J, Angelhed JE, Hellqvist A, Nilsson S, Ohlsson C: Pubertal timing is an independent predictor of central adiposity in young adult males: the Gothenburg Osteoporosis and Obesity Determinants Study. Diabetes 55: 3047– 3052, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Beunen G, Malina RM, Lefevre J, Claessens AL, Renson R, Simons J, Maes H, Vanreusel B, Lysens R: Size, fatness and relative fat distribution of males of contrasting maturity status during adolescence and as adults. Int J Obes Relat Metab Disord 18: 670– 678, 1994 [PubMed] [Google Scholar]
- 18.Must A, Naumova EN, Phillips SM, Blum M, Dawson-Hughes B, Rand WM: Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow-up. Pediatrics 116: 620– 627, 2005 [DOI] [PubMed] [Google Scholar]
- 19.van Lenthe FJ, Kemper CG, van Mechelen W: Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr 64: 18– 24, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Sandhu J, Ben-Shlomo Y, Cole TJ, Holly J, Davey Smith G: The impact of childhood body mass index on timing of puberty, adult stature and obesity: a follow-up study based on adolescent anthropometry recorded at Christ's Hospital (1936–1964). Int J Obes (Lond) 30: 14– 22, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Fox KR, Peters DM, Sharpe P, Bell M: Assessment of abdominal fat development in young adolescents using magnetic resonance imaging. Int J Obes Relat Metab Disord 24: 1653– 1659, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Brambilla P, Manzoni P, Agostini G, Beccaria L, Ruotolo G, Sironi S, Del Maschio A, Chiumello G: Persisting obesity starting before puberty is associated with stable intraabdominal fat during adolescence. Int J Obes Relat Metab Disord 23: 299– 303, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Lorentzon M, Swanson C, Andersson N, Mellstrom D, Ohlsson C: Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD Study. J Bone Miner Res 20: 1334– 1341, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury B, Sjostrom L, Alpsten M, Kostanty J, Kvist H, Lofgren R: A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord 18: 219– 234, 1994 [PubMed] [Google Scholar]
- 25.Starck G, Lonn L, Cederblad A, Forssell-Aronsson E, Sjostrom L, Alpsten M: A method to obtain the same levels of CT image noise for patients of various sizes, to minimize radiation dose. Br J Radiol 75: 140– 150, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Karlberg J: On the modelling of human growth. Stat Med 6: 185– 192, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Kindblom JM, Lorentzon M, Norjavaara E, Hellqvist A, Nilsson S, Mellstrom D, Ohlsson C: Pubertal timing predicts previous fractures and BMD in young adult men: the GOOD Study. J Bone Miner Res 21: 790– 795, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pepe MS, Heagerty P, Whitaker R: Prediction using partly conditional time-varying coefficients regression models. Biometrics 55: 944– 950, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Roemmich JN, Clark PA, Mai V, Berr SS, Weltman A, Veldhuis JD, Rogol AD: Alterations in growth and body composition during puberty: III. Influence of maturation, gender, body composition, fat distribution, aerobic fitness, and energy expenditure on nocturnal growth hormone release. J Clin Endocrinol Metab 83: 1440– 1447, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Guo SS, Huang C, Maynard LM, Demerath E, Towne B, Chumlea WC, Siervogel RM: Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. Int J Obes Relat Metab Disord 24: 1628– 1635, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH: Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337: 869– 873, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP: Body index measurements in 1996–7 compared with 1980. Arch Dis Child 82: 107– 112, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heude B, Lafay L, Borys JM, Thibult N, Lommez A, Romon M, Ducimetiere P, Charles MA: Time trend in height, weight, and obesity prevalence in school children from Northern France, 1992–2000. Diabetes Metab 29: 235– 240, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Karlberg J, Luo ZC, Albertsson-Wikland K: Body mass index reference values (mean and SD) for Swedish children. Acta Paediatr 90: 1427– 1434, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Sorensen TI, Sonne-Holm S: Risk in childhood of development of severe adult obesity: retrospective, population-based case–cohort study. Am J Epidemiol 127: 104– 113, 1998 [DOI] [PubMed] [Google Scholar]