Abstract
OBJECTIVE
To determine whether middle-aged and older individuals with impaired fasting glucose (IFG), but no clinical evidence of cardiovascular disease, exhibit abnormal changes in proximal thoracic aortic stiffness or left ventricular (LV) mass when compared with healthy counterparts.
RESEARCH DESIGN AND METHODS
From the Multi-Ethnic Study of Atherosclerosis, 2,240 subjects with normal fasting glucose (NFG), 845 with IFG, and 414 with diabetes, all aged 45 to 85 years and without preexisting coronary artery disease, underwent MRI determinations of total arterial and proximal thoracic aortic stiffness and LV mass. The presence or absence of other factors known to influence arterial stiffness was assessed.
RESULTS
After adjustment for clinical factors known to modify arterial stiffness, proximal thoracic aortic stiffness was not increased in those with IFG compared with those with NFG (1.90 ± 0.05 versus 1.91 ± 0.04 10−3 mmHg−1, respectively, P = 0.83). After accounting for clinical factors known to influence LV mass, LV mass was increased in those with diabetes relative to those with NFG (150.6 ± 1.4 versus 145.8 ± 0.81 g, P < 0.0009) but not in those with IFG in comparison with NFG (145.2 ± 1.03 versus 145.8 ± 0.81 g, P = 0.56).
CONCLUSIONS
Middle-aged and older individuals with the pre-diabetes state of IFG do not exhibit abnormal proximal thoracic distensibility or LV hypertrophy relative to individuals with NFG. For this reason, an opportunity may exist in those with IFG to prevent LV hypertrophy and abnormal aortic stiffness that is observed in middle-aged and older individuals with diabetes.
Individuals with diabetes exhibit increased arterial stiffness that is associated with future cardiovascular morbidity and mortality, peripheral arterial disease, impaired myocardial function, left ventricular (LV) hypertrophy, and congestive heart failure (1–6). For those with diabetes, different clinical sequelae occur depending on the location of abnormal stiffening within the arterial system (7–9). For example, abnormal proximal thoracic aortic stiffness contributes to abnormal ventricular vascular coupling, LV hypertrophy and dysfunction, and exercise intolerance (10). In peripheral arteries, abnormal arterial stiffness is associated with endothelial dysfunction and arteriosclerosis (11,12).
Individuals with impaired fasting glucose (IFG) exhibit a higher rate of cardiovascular events compared with those with normal fasting glucose (NFG) (9,13–15). Importantly, however, studies to date (7,8) have not clarified whether subjects with IFG exhibit abnormal arterial stiffness, especially within the proximal thoracic aorta, that in other populations is associated with cardiac dysfunction and exercise intolerance (10).
To determine if middle-aged and older individuals with IFG but no clinical cardiovascular disease exhibit abnormal changes in arterial stiffness, including the proximal thoracic aorta, compared with healthy counterparts, we analyzed MRI measures of arterial stiffness in participants from the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that total arterial and proximal thoracic aortic stiffness would be altered in participants with IFG as well as those with diabetes when compared with individuals with NFG. We also sought to determine if LV mass, which is directly influenced by proximal thoracic aortic stiffness, was abnormal in individuals with IFG and diabetes.
RESEARCH DESIGN AND METHODS
Study subjects.
The recruitment criteria of the individuals in MESA have been previously described (16). MESA is a large population-based sample of men and women from four ethnic groups (white, black, Hispanic, and Chinese) aged 45 to 84 years and without clinical cardiovascular disease. A total of 5,004 MESA participants received cardiovascular MRI studies, of whom 3,499 participants had examinations of proximal thoracic aortic stiffness. Institutional review committees in each participating site approved the study, and all participants provided written informed consent.
Participants in this study were characterized using criteria established by the American Diabetes Association into one of three groups using their fasting plasma glucose level (17). This included those with NFG (fasting glucose level of <100 mg/dl), IFG (fasting glucose level of 100–125 mg/dl), and diabetes (fasting glucose level of ≥126 mg/dl). Participants with a history of diabetes (with or without treatment) were classified into the diabetes group regardless of their fasting glucose level. Blood pressure was measured after 5 min of rest with sequential Dinamap measures, with a 5-min rest period between each sampling. Hypertension was defined by a systolic blood pressure (sBP) of 140 mmHg or greater or a diastolic blood pressure (dBP) of 90 mmHg or greater that occurred as the average of the second and third Dinamap measures on the initial clinic visit. Participants were also identified as hypertensive if they self-reported the presence of hypertension and they used any antihypertensive medication. Cigarette smoking was defined as ever smoked (>100 cigarettes in one's lifetime).
Magnetic resonance imaging technique.
Using MRI, two previously described noninvasive measures of cardiovascular stiffness were obtained and used as the primary stiffness outcomes for the study. The first was assessment of proximal thoracic aortic distensibility: a measure of cardiac cycle–dependent changes in aortic area after accounting for brachial pulse pressure and resting vessel area (10). The second was an assessment of total cardiovascular stiffness that involved determination of change in LV stroke volume after accounting for brachial pulse pressure. In addition to these measures, LV mass was measured (3).
MRI studies were performed at six participating sites using 1.5-Tesla magnets (three from General Electric Medical Systems [Waukesha, WI] CV/i or LX platforms and three from Siemens Medical Solutions [Erlangen, Germany] Symphony or Sonata platforms). Participants were scanned in a supine position using a torso phased-array coil placed anteriorly and posteriorly and equipment approved for the MRI environment.
LV parameters.
The left ventricle was imaged according to previously published techniques in short axis slices starting from the base at the mitral valve plane to the apex (18). According to previously published methods (18), LV stroke volume was determined in each individual for use in the calculation of total vascular stiffness (19–21). For LV volume and mass determinations, the epi and endocardial border of each slice was planimetered manually at end diastole and end systole, and volumes were calculated by summation (Simpson's rule) as previously described. The LV ejection fraction was calculated from the ratio of the difference between LV end-diastolic and end-systolic volumes relative to end-diastolic volumes. The LV mass was calculated from summation of the LV myocardial volumes during end diastole in each short axis slice at end diastole and multiplied ×1.05. The variability of the MRI readings was determined from a set of 155 duplicate readings to be 4.77% (95% CI 3.63–6.02) for end-diastolic volume and 11.61% (9.55–13.71) for end-systolic volume (18).
Total and proximal thoracic aortic stiffness.
Images of the proximal thoracic aorta were obtained axially at the level of the main pulmonary artery identified on a sagittal scout image. Imaging parameters included a phase-contrast gradient-echo sequence with a 34-cm field of view, a 10-ms repetition time, a minimal full echo time, a 20° flip angle, an 8-mm-thick slice, a 256 × 224 matrix, 20 phases per cardiac cycle, two excitations, a 32-kHz bandwidth, and a velocity encoding of 150 cm/s. Brachial arterial pressure was measured noninvasively with a nonferromagnetic arm blood pressure cuff and recorded before and after at the time of the phase-contrast acquisition and then averaged to derive mean values. The pulse pressure was calculated from the difference between sBP and dBP.
Determination of stiffness in the ascending thoracic aorta was accomplished by measuring aortic distensibility according to previously published methods using the following formula whereby the area of the ascending aorta was identified from the phase-contrast, gradient-echo images throughout all phases of the cardiac cycle (10,22):
Aortic distensibility (10−3 mmHg − (1) + [maximal aortic area − minimal aortic area] [brachial pulse pressure × minimal aortic area].
To examine total vascular stiffness, the total arterial compliance was calculated according to the following previously published formula (Fig. 1) (19–21):
FIG. 1.
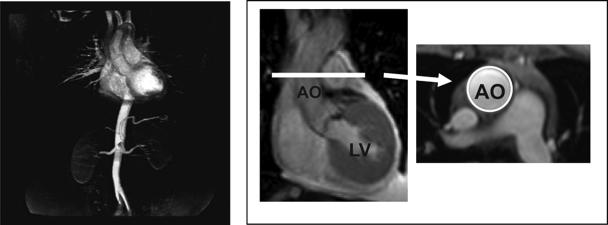
Formula for determining magnetic resonance imaging measures of total vascular stiffness (left panel) and proximal thoracic aortic stiffness (right panel). The left panel shows portions of the vascular system encompassed by the stiffness measure. In the right panel, coronal and transaxial views of the LV ascending thoracic aorta are provided. The circle demarcates the boundary of the ascending aorta (Ao), from which dimensions are determined by the calculation of proximal thoracic aortic stiffness. Total arterial stiffness = LV stroke volume/brachial artery pulse pressure. Proximal thoracic aortic stiffness = (area aorta end systole − area aorta end diastole)/ (brachial artery pulse pressure × area aorta end diastole).
Total arterial compliance (ml · mmHg−1) + LV stroke volume/pulse pressure.
Total arterial compliance (ml · mmHg−1) = LV stroke volume/pulse pressure.
Also, the inverse of total arterial compliance pulse pressure (PP)–to–stroke volume (SV) ratio was indexed for body surface area (PP/SVi) to account for potential differences in body size that may influence arterial stiffness. Total arterial elastance was determined using the change in LV SV/mean arterial pressure.
Results were expressed as means ± SE of the estimate unless stated otherwise. Descriptive statistics were first examined stratified by fasting glucose status (NFG, IFG, and diabetes). Next, comparisons across the three levels of fasting blood glucose were made with an ANCOVA approach using PROC GLM in SAS 9.1 (SAS Institute, Cary, NC). We fit a series of progressively more complex models to examine the relationship of glucose tolerance status (coded as a three-level class variable: NFG, IFG, and diabetes) and two outcomes of interest (total arterial and proximal thoracic aortic stiffness). There was a total of three models fit: model 1, adjusted for age (continuous variable), race/ethnicity, sex, and participating site; model 2, adjusted for variables from model 1 along with sex, race/ethnicity, age, site, weight, total cholesterol, high-density lipoproteins, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, and estimated glomerular filtration rate; model 3, adjusted for variables from model 2 and hypertension (defined as the use of blood pressure medicine or an sBP ≥140 mmHg or dBP ≥90 mmHg on the first clinic examination). For each of these models, we first examined the overall main effect for glucose tolerance status. When this was significant, two specific pairwise comparisons among the three groups (NFG versus IFG and NFG versus diabetes) were examined using two-sample t tests based on the adjusted mean values from the model (using the LSMEANS option in PROC GLM).
RESULTS
Of the 3,499 subjects (mean age 61 ± 10 years), 1,596 (46%) were men, 1,468 (42%) were white, 1,049 (30%) were black, 385 (11%) were Hispanic, and 597 (17%) were Chinese. As shown in Table 1, compared with those with NFG, those in the IFG group exhibited a higher BMI, waist-to-hip ratio, sBP, and triglyceride level as well as a lower HDL cholesterol level. As shown in Table 1, similar differences were also present in those with diabetes. When compared, subjects with NFG were younger and more frequently women and/or white.
TABLE 1.
Baseline characteristics
| NFG | IFG | Diabetes | |
|---|---|---|---|
| n | 2240 | 845 | 414 |
| Age (years) | 59.4 ± 9.9 | 62.2 ± 9.9* | 63.5 ± 9.4* |
| Male (%)† | 926 (41) | 459 (54) | 211 (51) |
| Race/ethnicity (%)† | |||
| White | 1,043 (47) | 324 (38) | 101 (24) |
| Black | 599 (27) | 260 (31) | 190 (46) |
| Chinese | 230 (10) | 109 (13) | 46 (11) |
| Hispanic | 368 (16) | 152 (18) | 77 (19) |
| Height (cm) | 166.6 ± 9.9 | 167.6 ± 9.9* | 166.7 ± 10.3 |
| Weight (kg) | 75.6 ± 15.7 | 81.3 ± 16.5* | 83.0 ± 16.4* |
| BMI (kg/m2) | 27.1 ± 4.7 | 28.8 ± 5.1* | 29.8 ± 5.3* |
| Waist circumference (cm) | 94 ± 13 | 100 ± 13* | 102 ± 13* |
| Waist-to-hip ratio | 0.90 ± 0.09 | 0.94 ± 0.07* | 0.96 ± 0.06* |
| Systolic blood pressure (mmHg) | 122 ± 20 | 128 ± 21* | 132 ± 22* |
| Diastolic blood pressure (mmHg) | 71 ± 10 | 73 ± 11* | 73 ± 10* |
| Hypertension (%) | 776 (35) | 413 (49) | 271 (65) |
| Antihypertension medications (%) | 535 (24) | 325 (38) | 219 (53) |
| Total cholesterol (mg/dl) | 194 ± 33 | 194 ± 34 | 190 ± 37* |
| Triglycerides (mg/dl) | 116 ± 63 | 131 ± 66* | 139 ± 74* |
| LDL cholesterol (mg/dl) | 117 ± 30 | 119 ± 30 | 113 ± 34* |
| HDL cholesterol (mg/dl) | 54 ± 15 | 49 ± 14* | 48 ± 14* |
| Fasting glucose (mg/dl) | 90 ± 6 | 107 ± 6* | 150 ± 50* |
| Creatinine (mg/dl) | 0.94 ± 0.20 | 0.97 ± 0.22 | 0.97 ± 0.57 |
| Calcium channel blockers | 197 (10) | 139 (16) | 80 (19) |
| Nitrates | 4 (0.2) | 1 (0.1) | 0 (0) |
Data are means ± SD.
*P < 0.05 compared with NFG.
†P < 0.05 among groups.
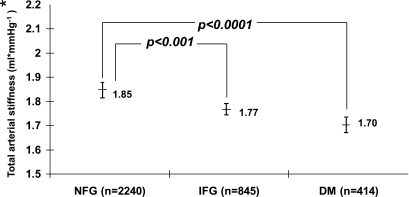
Across the study population, total and proximal thoracic aortic stiffness was not increased in those with NFG compared with those with IFG or diabetes (unadjusted data; Table 2). Also, arterial elastance (defined from the mean arterial pressure rather than pulse pressure/the LV stroke volume) was different in NFG (1.06 ± 0.01) relative to IFG (1.12 ± 0.01; P = 0.0005) or diabetic (1.14 ± 0.02; P = 0.007) participants. The differences in total vascular stiffness between subjects with NFG and IFG or diabetes persisted after adjustment for age, race/ethnicity, sex, and study site (model 1, Table 2). We examined sex by glucose status (NFG, IFG, and diabetes) interactions for all stiffness measures and found none to be significant (all with P ≥ 0.15).
TABLE 2.
Multivariate analysis of total arterial stiffness and proximal thoracic aortic stiffness among those with normal fasting glucose, impaired fasting glucose, and diabetes
| Total vascular stiffness |
Proximal thoracic aortic stiffness |
|||||
|---|---|---|---|---|---|---|
| (ml/mmHg) |
(10−10 mmHg−1) |
|||||
| NFG | IFG | Diabetes | NFG | IFG | Diabetes | |
| Unadjusted | 1.87 ± 0.01 | 1.73 ± 0.02† | 1.58 ± 0.03† | 1.94 ± 0.03 | 1.77 ± 0.05* | 1.45 ± 0.06† |
| Model 1 | 1.84 ± 0.01 | 1.74 ± 0.02† | 1.66 ± 0.03† | 1.90 ± 0.03 | 1.87 ± 0.04 | 1.64 ± 0.06† |
| Model 2 | 1.86 ± 0.01 | 1.78 ± 0.02† | 1.70 ± 0.03† | 1.92 ± 0.04 | 1.90 ± 0.05 | 1.66 ± 0.06† |
*P = 0.01 compared with NFG.
†P < 0.001 compared with NFG. Hypertension is defined by systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg that occurred as the average of the second and third Dinamap measures on the initial clinic visit. Model 1 adjusted for age, race/ethnicity, sex, and participating site. Model 2 adjusted for variables from model 1 and sex, race/ethnicity, age, site, weight, total cholesterol, HDL cholesterol, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, and estimated glomerular filtration rate.
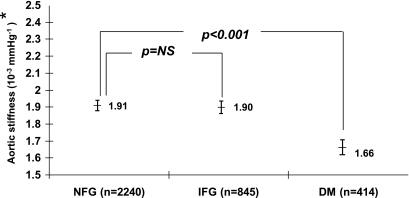
After adjusting for the variables included in model 1 of Table 2, the difference in proximal thoracic aortic stiffness remained present between NFG and diabetic subjects but not between NFG and IFG subjects (model 2, Table 2; Figs. 2 and 3). Age was the variable most associated with the difference in proximal thoracic aortic stiffness observed between subjects with NFG and IFG.
FIG. 2.
Comparison of adjusted total arterial stiffness of those with IFG or diabetes (DM) and those exhibiting NFG. *Adjusted for recruited age, race/ethnicity, sex, site, weight, total cholesterol, HDL cholesterol, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, estimated glomerular filtration rate, and hypertension.
FIG. 3.
Comparisons of adjusted proximal aortic stiffness of those with IFG or diabetes (DM) and those exhibiting NFG. *Adjusted for recruited age, race/ethnicity, sex, site, weight, total cholesterol, HDL cholesterol, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, estimated glomerular filtration rate, and hypertension.
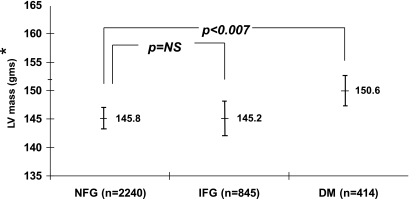
After the adjustments for model 3, total vascular stiffness remained increased in those with IFG compared with those with NFG (Fig. 2). However, the proximal thoracic aortic stiffness in those with IFG remained similar to that of those with NFG (Fig. 3). Across the study, the LV unadjusted mass in those with IFG or diabetes was increased when compared with those with NFG (150.8 ± 1.3 versus 140.4 ± 0.8 g, P < 0.0001, and 158.7 ± 1.9 versus 140.4 ± 0.8 g, P < 0.0001, respectively). When compared with individuals with NFG, and after accounting for the same covariates in Figs. 2 and 3, LV mass was increased in those with diabetes (151.1 ± 1.4 versus 145.2 ± 0.8 g, P < 0.007) but not in those with IFG (145.2 ± 0.9 versus 144.9 ± 1.0 g, P = not significant) (Fig. 4).
FIG. 4.
Comparison of adjusted left ventricular mass of those with IFG or diabetes (DM) and those exhibiting NFG. *Adjusted for recruited age, race/ethnicity, sex, site, weight, total cholesterol, HDL cholesterol, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, estimated glomerular filtration rate, and hypertension.
To determine if the variables in the numerators (LV SV for total and aortic area for proximal thoracic aorta) or denominators (PP for both) of our measures of stiffness were more influential in accounting for our results, we performed additional analyses adjusting the model 3 (results graphed in Figs. 2–4) stiffness measure for the variables in the numerator and denominator. After adjusting for LV SV, the total stiffness was 1.82 ± 0.01 ml/mmHg for NFG, 1.79 ± 0.02 ml/mmHg for IFG (P = 0.004 from NFG), and 1.74 ± 0.02 ml/mmHg for diabetes (P = 0.0005 from NFG); and after adjusting for PP, the total stiffness was 1.85 ± 0.01 ml/mmHg for NFG, 1.78 ± 0.01 ml/mmHg for IFG (P < 0.0001 from NFG), and 1.77 ± 0.02 ml/mmHg for diabetes (P < 0.0007 from NFG). After adjusting for the cardiac cycle–dependent change in aortic area, proximal thoracic aortic stiffness was 1.89 ± 0.03 10−3 mmHg−1 for NFG, 1.90 ± 0.04 10−3 mmHg−1 for IFG (P = 0.74 from NFG), and 1.81 ± 0.05 10−3 mmHg−1 for diabetes (P = 0.14 from NFG); and after adjusting for PP, proximal thoracic aortic stiffness was 1.91 ± 0.04 10−3 mmHg−1 for NFG, 1.90 ± 0.05 10−3 mmHg−1 for IFG, and 1.68 ± 0.06 10−3 mmHg−1 for diabetes (P = 0.0007 from NFG). Because many of the differences noted between the groups in model 3 remain (Figs. 2–4), the results of these adjustments suggest that both values in the numerators and denominators were important for influencing the differences or similarities in vascular stiffness noted between the groups assessed in this study.
We tested for interactions between sex and measures of vascular stiffness by glucose group (NFG, IFG, and diabetes) and found them all to be nonsignificant. For vascular stiffness, the P value was 0.58; for LV end-diastolic mass, it was 0.17; and for aortic distensibility (stiffness), it was 0.95. These nonsignificant interactions suggest that there were no sex differences in the relation between glucose group and outcomes.
To determine if the findings relating to proximal aortic distensibility were influenced by the resting arterial diameter or the geometry of the aorta, we measured aortic compliance in our three patient groups. After adjusting for variables in model 3 (Figs. 2–4), aortic compliance was 1.48 ± 0.02, 1.44 ± 0.03, and 1.32 ± 0.04 in those with NFG, IFG, and diabetes, respectively. Like with aortic distensibility, compliance in participants with diabetes was reduced compared with that found in those with NFG (P = 0.0001) but not in those with IFG relative to those with NFG (P = 0.22).
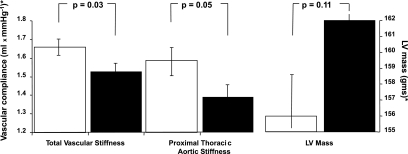
Of the 414 subjects with diabetes, 256 provided data on the duration of receipt of oral or insulin therapy. The duration of treatment ranged from 0 (current year) to 41 years, with a median of 6 years. As shown in Fig. 5, total and proximal thoracic aortic stiffness was worse for those treated for the median of 6 years or longer as opposed to less than the median of 6 years. Also, LV mass trended higher in those treated longer than 6 years for diabetes.
FIG. 5.
Mean ± SE of the estimates of total and proximal thoracic aortic stiffness and LV mass for participants with diabetes treated longer than 6 years (■) and 6 years or less (□). *Adjusted for recruited age, race/ethnicity, sex, site, weight, total cholesterol, HDL cholesterol, triglycerides, mean arterial pressure, waist-to-hip ratio, statin therapy, smoking, estimated glomerular filtration rate, and hypertension.
In addition to measuring total arterial elastance, a stiffness measure dependent on mean arterial pressure rather than PP, we also performed analysis adjusting our models for measures of mean arterial pressure rather than the prespecified diagnosis of hypertension. The differences and similarities between our participant groups persisted using mean arterial pressure as a covariate.
The use of statin therapy was 14.2% across the study population (11% of NFG, 16% of the IFG, and 26% of the diabetes participants). This may have influenced the lower LDL cholesterol level observed in our study population. After adjusting the total and proximal aortic stiffness for statin use (model 2, Table 2), differences between groups persisted.
Often, patients with diabetes or IFG exhibit features of the metabolic syndrome. We performed analyses to determine if the pressure of the metabolic syndrome would change the relations we observed in total arterial stiffness and proximal thoracic stiffness among our three groups. After accounting for the metabolic syndrome, all pairwise comparisons between NFG and diabetes or IFG remained unchanged.
DISCUSSION
Abnormally stiffened arteries are present in individuals with diabetes and are associated with an increase in cardiovascular events, LV afterload, and exercise intolerance (1,4,9). In this study, we sought to determine if individuals with IFG exhibited abnormal stiffening of the arterial tree (including the proximal thoracic aorta) that in studies of individuals with diabetes is associated independently with adverse cardiovascular events. There are two important findings in this study: Total vascular stiffness is worse in subjects with IFG compared with those with NFG. This finding is true regardless of age, sex, ethnicity, or other factors associated with abnormal vascular stiffening (Table 2; Fig. 2). After adjustment for factors known to influence vascular stiffness, proximal thoracic aortic stiffness and LV mass are similar in individuals with IFG and NFG (Figs. 3 and 4). In patients with diabetes, however, both proximal thoracic aortic stiffness and LV mass are elevated (Figs. 3 and 4).
In this cross-sectional analysis of the data from the MESA cohort study, subjects with IFG demonstrated higher BMI, blood pressure, total cholesterol, and serum triglyceride compared with those with NFG. These are all clinical features of the metabolic syndrome, which affects 47 million Americans and is associated with hyperinsulinemia and insulin resistance (23). After adjusting for these variables, the subjects with IFG demonstrated greater total arterial stiffness than those with NFG. The data in this study indicate that mild elevations of blood glucose adversely affect cardiovascular stif fening independent of other common cardiovascular risk factors associated with the metabolic syndrome.
There are several mechanisms by which total arterial stiffness becomes elevated in individuals with IFG or diabetes. Elevations in blood glucose lead to the formation and deposition of advanced glycation end products (24,25). These products promote the crosslinking of collagen that stiffens the structural components of the arterial wall (24,25). Diabetes also promotes increased lipid oxidation, vasoconstriction, tissue remodeling, low-grade inflammation, atherosclerosis, and sympathetic nervous system activation (26,27). Many of these processes inhibit endothelial nitric oxide synthase, which consequently impairs peripheral endothelial function and adversely effects vascular stiffness (24,25,28–30).
It is important to note that some of the mechanisms by which arteries stiffen after exposure to elevations in blood glucose occur rapidly, whereas others require more time to develop. For example, peripheral arterial endothelial function is known to deteriorate 1 h after high glucose oral intake (31). There is also emerging evidence that postprandial hyperglycemia is associated with the development of atherosclerosis, which in turn heightens peripheral arterial stiffness (32). It is not surprising that those with IFG or diabetes exhibit abnormal total vascular stiffness of the entire vascular tree because both IFG and diabetes adversely impact endothelial function, atherosclerosis, and vasomotor tone, each of which influences peripheral artery stiffness after a short duration of exposure.
The use of MRI in this study provided the opportunity to assess proximal thoracic aorta stiffness. The proximal thoracic aorta stiffens abnormally in those with diabetes (27), and several studies have identified the adverse impact of proximal thoracic aortic stiffness on LV performance and exercise capacity in those with diabetes and those with heart failure (10,22).
Our group and others have demonstrated an independent relationship between proximal thoracic aortic stiffness and increased LV mass and exercise intolerance in individuals with heart failure (10,22).
The results of this study indicate that proximal thoracic aortic stiffness, a stimulus for increased LV hypertrophy and diminished LV performance, is not dissimilar in those with IFG and NFG. In addition, those subjects with IFG had no increase in LV mass after accounting for other conditions known to influence LV mass. Our results parallel those of others indicating absence of a relationship between IFG and LV dysfunction or hypertrophy (33). These data indicate that regardless of sex, ethnicity, or age, those with IFG do not exhibit increased proximal thoracic aortic stiffness, and they suggest that prolonged exposure to high serum levels of glucose are necessary to enhance stiffening of the proximal thoracic aorta. This stands to reason given that prolonged exposure to higher levels of glucose increases the opportunity for advanced glycation end products to facilitate a higher number of crosslinks within the collagen of the wall of the central aorta.
Another possible mechanism by which stiffness in the ascending aorta may not be increased in those with IFG relates to the effects of atherosclerotic plaque burden. Diabetes is one of the most important risk factors for the presence of aortic atherosclerotic plaque, and a positive association has been shown between the presence of atherosclerotic plaque and arterial stiffness (34). Underlying inflammation may be less operative in patients with IFG. Scuteri et al. reported that increased large arterial stiffness and impaired endothelial function were found in the normotensive normoglycemic first-degree relatives of patients with diabetes independent of the presence of the metabolic syndrome (35). These data suggest that mechanisms other than the level of serum glucose such as endothelial dysfunction or low-grade inflammation may affect the stiffness of proximal thoracic aorta (36,37). Sengstock and associates (38) also demonstrated that insulin resistance was an important factor in the stiffening of arteries. Potential mechanisms mediating this factor include endothelial dysfunction and/or vascular smooth muscle proliferation.
To address the relation between prolonged exposure to high serum levels of glucose and arterial (both total and proximal thoracic aortic) stiffness, we reviewed the results of MESA participants who responded to the question of duration of diabetes treatment. We were able to assess the relation among treatment duration, central aortic stiffness, and LV mass. As shown in Fig. 5, after accounting for variables known to influence proximal thoracic aortic stiffness, total and aortic stiffness were worse for individuals treated for diabetes longer than 6 years. There was also a strong trend toward an increase in LV mass for those treated for diabetes longer than a median of 6 years.
What are some of the clinical applications of our study results? Those with IFG do not exhibit abnormalities of proximal thoracic aortic stiffness to the magnitude observed by those with diabetes. Data to date indicate that stiffening in the central aorta has hemodynamically detrimental effects on the cardiovascular system and a strong association with increased cardiovascular mortality. This may be one of the possible explanations for why those with IFG have no increase in LV mass and perhaps lower rates of cardiovascular events compared with those with diabetes. Preventing pre-diabetes states from developing into diabetes theoretically could prevent stiffening of central aorta and associated adverse clinical sequela.
Therapies designed to reduce stiffness in the proximal thoracic aorta may differ for middle-aged and older individuals with IFG versus diabetes. Studies involving the collagen crosslink breaker thiazolidinedione for the purpose of attenuating aortic stiffness and regressing LV hypertrophy are underway in patients with heart failure (39,40). In the absence of other clinical conditions known to stiffen the aorta, older individuals with IFG do not exhibit stiff aortas, so suitability for participation in these studies may differ.
Previously, in primary prevention strategies, statin therapy has been shown to reduce cardiovascular events (41), improve endothelial function, and regress atherosclerotic plaques in the aorta (42,43) in patients with diabetes. In patients with diabetes or IFG and concomitant hypercholesterolemia, guidelines exist for prescribing statin therapy to reduce LDL cholesterol levels (44). At present, however, guidelines do not exist for using vascular stiffness measures as surrogate end points for treatment in primary or secondary prevention strategies that involve patients with IFG or diabetes. Further study is needed to explore this issue.
Because individuals with IFG and diabetes often exhibit reduced exercise capacity (45,46), our results indicate that mechanisms other than abnormalities of aortic stiffness such as higher myocardial oxygen consumption at rest, an inability to adapt coronary flow adequately to higher metabolic demands during peak exercise, or abnormal peripheral arterial endothelial function may be more likely responsible for exercise intolerance in middle-aged and older individuals with IFG (45,46).
The investigators from the Hoorn Study (8) have reported that subjects with impaired glucose tolerance and diabetes had arterial stiffening in both the central and peripheral arterial system and that central arterial stiffness of those with impaired glucose tolerance was intermediate in severity between the group with normal glucose tolerance and diabetes. Our study had a similar finding on peripheral arterial stiffening, but our measure of aortic distensibility arterial stiffness was not increased in those with IFG. There are possible explanations on the difference of this finding. The criteria for classification of subjects with impaired glucose metabolism were different in our study and the Hoorn Study. Our study used fasting glucose according to the American Diabetes Association criteria, but the Hoorn Study defined glucose tolerance according to the WHO criteria. Subjects with IFG (American Diabetes Association criteria) and impaired glucose tolerance (WHO criteria) are not identical, and the pathophysiology of the disorders are different. When comparing subjects with IFG with those with impaired glucose tolerance, those with IFG are more strongly correlated with insulin resistance, whereas impaired glucose tolerance is more strongly correlated to impaired insulin secretion (47,48).
Aortic stiffness was measured differently in the two studies. Our study used aortic distensibility, whereas the Hoorn Study used aortic augmentation index. Aortic distensibility measures stiffness in the proximal ascending thoracic aorta, which has direct implications for stimulating LV hypertrophy; aortic augmentation index measures stiffness within the entire aorta. This latter value seems more similar to the assessment of total vascular stiffness that we report currently. We noted differences in total arterial stiffness among NFG, IFG, and diabetes (similar to the Hoorn results). Third, participants in the Hoorn Study were on average older than ours. Age is an important influence on aortic stiffening; stiffening within the cardiovascular system increases with age.
Our study has the following limitations. Up to 5% of subjects with IFG exhibit diabetes if they undergo 2-h glucose tolerance testing. Because those with diabetes exhibit stiffer arteries than those with IFG, it stands to reason that moving 5% of the IFG participants who are truly diabetic into the diabetes group would further reduce differences between our NFG and IFG groups. Brachial cuff pressure, rather than an invasive measure of central aortic pressure, was used in our calculations of total and proximal thoracic aortic stiffness. Our noninvasive determinations of arterial stiffness (ratios involving pulse pressure) are based on the principle that in a steady-state condition, the arterial tree can be modeled as an elastic chamber with a constant compliance (49). Although these measures have been validated with standard invasive assessment of arterial compliance in animals (50) and humans (21) and have been shown to be an independent predictor for cardiovascular death (21), younger, healthier individuals may have more amplification of PP than older individuals. To provide for this possibility, we accounted for age in our statistical models. The difference we observe between our IFG and NFG groups is present in our total arterial stiffness measure, but not in our aortic distensibility measure. This observation suggests that the lack of difference between NFG and IFG in aortic distensibility is in fact true. Also, LV mass, which parallels proximal thoracic stiffness, is not different in the NFG and IFG groups. Our LV mass determination does not involve PP.
It is important to recognize that our results reflect those generated from participants aged 45 to 85 years of age. We are uncertain of the effects of IFG and diabetes on stiffness parameters in individuals including children, teenagers, and younger adults.
In conclusion, in middle-aged and older adults, the pre-diabetes state of IFG is associated with an increase in arterial stiffening that primarily impacts regions exclusive of the ascending thoracic aorta. Perhaps prevention of diabetes in those with IFG would be associated with preservation of more favorable heart weight and proximal thoracic aortic distensibility, which are known risk factors for adverse cardiac events.
Acknowledgments
This research was supported in part by NIH R01HL076438 and the Claude D. Pepper Older Americans (Independence Center of Wake Forest University) NIH Grant P60AG10484.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Boutouyrie P, Tropeano AI, Asmar R, et al. : Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10– 15, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Haider AW, Larson MG, Franklin SS, et al. : Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 138: 10– 16, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Hay I, Fetics B, et al. : Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107: 714– 720, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Shoji T, Emoto M, Shinohara K, et al. : Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12: 2117– 2124, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Schram MT, Kostense PJ, van Dijk RA, et al. : Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 20: 1743– 1751, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Salomaa V, Riley W, Kark JD, et al. : Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study Atherosclerosis Risk in Communities Study. Circulation 91: 1432– 1443, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Henry RM, Kostense PJ, Spijkerman AM, et al. : Hoorn Study. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 107: 2089– 2095, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Schram MT, Henry RM, van Dijk RA, et al. : Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension 43: 176– 181, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cruickshank K, Riste L, Anderson SG, et al. : Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 106: 2085– 2090, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hundley WG, Kitzman DW, Morgan TM, et al. : Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 38: 796– 802, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kinlay S, Creager MA, Fukumoto M, et al. : Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 38: 1049– 1053, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kizu A, Koyama H, Tanaka S, et al. : Arterial wall stiffness is associated with peripheral circulation in patients with type-2 diabetes. Atherosclerosis 170: 87– 91, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fisman EZ, Motro M, Tenenbaum A, et al. : Impaired fasting glucose concentrations in nondiabetic patients with ischemic heart disease: a marker for a worse prognosis. Am Heart J 141: 485– 490, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Henry P, Thomas F, Benetos A, et al. : Impaired fasting glucose, blood pressure and cardiovascular disease mortality. Hypertension 40: 458– 463, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Barr EL, Zimmet PZ, Welborn TA, et al. : Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 116: 151– 157, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. : Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 156: 871– 881, 2002 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 27: S5– S10, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Natori S, Lai S, Finn JP, et al. : Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 186: S357– S365, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Alfie J, Waisman GD, Galarza CR, et al. : Contribution of stroke volume in the change in pulse pressure pattern with age. Hypertension 34: 808– 812, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Chemla D, Hebert JL, Coirault C, et al. : Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 274: H500– H505, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Fagard RH, Pardaens K, Staessen JA, et al. : The pulse pressure-to-stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol 38: 227– 231, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Rerkpattanapipat P, Hundley WG, Link KM, et al. : Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol 90: 1221– 1225, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. : Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433– 438, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Wautier JL, Guillausseau PJ: Diabetes, advanced glycation endproducts and vascular disease. Vasc Med 3: 131– 137, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Barden A, Mori T, et al. : Advanced glycation end-products: a review. Diabetologia 44: 129– 146, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Ferreira I, Boreham CA, Twisk JW, et al. : Clustering of metabolic syndrome risk factors and arterial stiffness in young adults: the Northern Ireland Young Hearts Project. J Hypertens 25: 1009– 1020, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kimoto E, Shoji T, Shinohara K, et al. : Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 52: 448– 452, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Laakso M: Hyperglycemia as a risk factor for cardiovascular disease in type 2 diabetes. Prim Care 26: 829– 839, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Ishii H, Koya D, King GL: Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med 76: 21– 31, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Airaksinen KE, Salmela PI, Linnaluoto MK, et al. : Diminished arterial elasticity in diabetes: association with fluorescent advanced glycosylation end products in collagen. Cardiovasc Res 27: 942– 945, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Lee IK, Kim HS, Bae JH: Endothelial dysfunction: its relationship with acute hyperglycaemia and hyperlipidemia. Int J Clin Pract Suppl 129: 59– 64, 2002 [PubMed] [Google Scholar]
- 32.Shige H, Ishikawa T, Suzukawa M, et al. : Endothelium-dependent flow-mediated vasodilation in the postprandial state in type 2 diabetes mellitus. Am J Cardiol 84: 1272– 1274, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Bertoni AG, Goff DC, Jr, D'Agostino RB, Jr, et al. : Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 29: 588– 594, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Qureshi G, Brown R, Salciccioli L, et al. : Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness. Atherosclerosis 195: e190– e194, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Scuteri A, Tesauro M, Rizza S, et al. : Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis 18: 349– 356, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Nagano M, Nakamura M, Sato K, et al. : Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis 180: 189– 195, 2005 [DOI] [PubMed] [Google Scholar]
- 37.McEniery CM, Wallace S, Mackenzie IS, et al. : Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 48: 602– 608, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sengstock DM, Vaitkevicius PV, Supiano MA: Arterial stiffness is related to insulin resistance in nondiabetic hypertensive older adults. J Clin Endocrinol Metab 90: 2823– 2827, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Little WC, Zile MR, Kitzman DW, et al. : The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail 11: 191– 195, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kass DA, Shapiro EP, Kawaguchi M, et al. : Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 104: 1464– 1470, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Colhoun HM, Betteridge DJ, Durrington PN, et al. : Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet 364: 685– 696, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Lee JM, Wiesmann F, Shirodaria C, et al. : Early changes in arterial structure and function following statin initiation: quantification by magnetic resonance imaging. Atherosclerosis 197: 951– 958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koutopoulos AG, Athyros VG, Pehlivanidis AN, et al. : Long-term treatment effect of atorvastatin on aortic stiffness in hypercholesterolaemic patients. Curr Med Res Opin 19: 22– 27, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM, Cleeman JI, Merz CN, et al. : Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110: 227– 239, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Van d V, De WO, Gir M, et al. : Fasting blood glucose levels are related to exercise capacity in patients with coronary artery disease. Am Heart J 152: 486– 492, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Gravholt CH, Nyholm B, Saltin B, et al. : Muscle fiber composition and capillary density in Turner syndrome: evidence of increased muscle fiber size related to insulin resistance. Diabetes Care 24: 1668– 1673, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Petersen JL, McGuire DK: Impaired glucose tolerance and impaired fasting glucose—a review of diagnosis, clinical implications and management. Diab Vasc Dis Res 2: 9– 15, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Barzilay JI, Spiekerman CF, Wahl PW, et al. : Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet 354: 622– 625, 1999 [DOI] [PubMed] [Google Scholar]
- 49.de Simone G, Roman MJ, Koren MJ, et al. : Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 33: 800– 805, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Randall OS, Westerhof N, van den Bos GC, et al. : Reliability of stroke volume to pulse pressure ratio for estimating and detecting changes in arterial compliance. J Hypertens Suppl 4: S293– S296, 1986 [PubMed] [Google Scholar]