Abstract
OBJECTIVE
Hepatocyte nuclear factor 1β (HNF1β) is a transcription factor that is critical for pancreatic cell formation and glucose homeostasis. Previous studies have reported that common variants of HNF1β were associated with type 2 diabetes in Caucasians and West Africans. However, analysis in the subjects from the Botnia study and Malmö Preventive Project produced conflicting results, and the role for HNF1β in type 2 diabetes susceptibility remains unclear. We therefore investigated common variants across the HNF1β gene in a Chinese population.
RESEARCH DESIGN AND METHODS
Fifteen tagging single nucleotide polymorphisms (SNPs) were analyzed for association with type 2 diabetes in subjects with type 2 diabetes (n = 1,859) and normal glucose regulation (n = 1,785).
RESULTS
Consistent with the initial study, we observed evidence that the risk G allele of rs4430796 in intron 2 was significantly associated with type 2 diabetes (odds ratio 1.16 [95% CI 1.05–1.29], P = 0.0035, empirical P = 0.0475). Furthermore, the at-risk G allele was associated with earlier age at diagnosis in the type 2 diabetic subjects (P = 0.0228).
CONCLUSIONS
The result of this study provides evidence that variants in the HNF1β region contribute to susceptibility to type 2 diabetes in the Chinese population.
Hepatocyte nuclear factor 1β (HNF1β, also known as transcription factor [TCF]2), is a homeodomain-containing transcription factor that functions as a homodimer or heterodimer with its homologous partner HNF1α (1). HNF1β is widely expressed in a number of tissues where it takes a vital role in embryonic development and pancreatic cell formation and is involved in the β-cell transcription factor network (2,3). Heterozygous mutation of the HNF1β gene was identified in an autosomal dominant, early-onset subtype of diabetes known as maturity-onset diabetes of the young type 5 (MODY5) in Caucasian, Japanese, and Chinese populations (4–6). It is well recognized that common variants of genes that cause monogenic forms of diabetes may also have a role in common type 2 diabetes susceptibility, such as P12A polymorphism in PPARG, E23K polymorphism in KCNJ11, and common variants in the promoter region of HNF4A (7–9). Recently, single nucleotide polymorphisms (SNPs) on HNF1β were identified to be associated with type 2 diabetes in Finland, Sweden, and Canada populations (10,11). The association signals, which were separated by recombination hot spots, were located on introns 2 (rs757210) and 6–8 (rs1008284 and rs3110641). Moreover, a genome-wide association study on prostate cancer identified that the G allele of rs7501939 and A allele of rs4430796 (both in the intron 1–2 region) were associated with increased risk of prostate cancer in Caucasians and reduced risk of type 2 diabetes in Caucasians and Africans but not Hong Kong Chinese (12). However, the study by Holmkvist et al. (13) produced the conflicting results that common variants in HNF1β did not predict future risk of type 2 diabetes in two prospective studies. Thus, extensive study, especially in other ethnic groups, will improve our understanding of the role of HNF1β in type 2 diabetes predisposition. We therefore selected tagging SNPs spanning the HNF1β gene and performed a case-control study in a Chinese Han population.
RESEARCH DESIGN AND METHODS
All subjects were of eastern Chinese Han ancestry and resided in Shanghai. All case subjects were from the 2001–2005 inpatient database of the Shanghai Diabetes Institute and were diagnosed with type 2 diabetes by the 1999 World Health Organization criteria (14). Type 1 diabetes and mitochondrial diabetes were excluded (15). The control subjects were selected from the Shanghai Diabetes Studies, a community-based epidemiological survey for diabetes (16). The inclusion criteria for the control subjects were 1) age >40 years, 2) normal glucose regulation confirmed by a standard 75-g oral glucose tolerance test (fasting plasma glucose <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l), and 3) absence of family history of diabetes stated on a standard questionnaire. In the end, the study groups comprised 1,859 subjects with type 2 diabetes and 1,785 subjects with normal glucose regulation. The phenotypic characteristics of the study group are described in Table 1.
TABLE 1.
Clinical characteristics of the study subjects
| Case subjects | Control subjects | |
|---|---|---|
| n | 1,859 | 1,785 |
| Sex (male/female) | 973/886 | 739/1,046 |
| Ages (years) | 61.16 ± 12.63 | 57.37 ± 12.33 |
| BMI (kg/m2) | 24.08 ± 3.53 | 23.63 ± 4.23 |
| Fasting plasma glucose (mmol/l) | 13.04 ± 5.19 | 4.99 ± 0.50 |
| Age at diagnosis (years) | 54.10 ± 11.85 | — |
Data are n or means ± SD.
This study was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Written informed consent was obtained from each participant.
Anthropometric parameters such as height, weight, waist and hip circumference, and blood pressure were measured as previously described (16,17). For the case subjects, fasting and postprandial plasma glucose and serum insulin were measured. For the control subjects, blood samples were obtained at 0 and 120 min during the oral glucose tolerance test, and plasma glucose and serum insulin levels were examined. Insulin resistance and pancreatic β-cell function were assessed by homeostasis model assessment (18).
Genotyping.
We initially selected 20 SNPs spanning the HNF1β region, including both 1) tagging SNPs selected from the HapMap Phase II Chinese Han database that captured all common variants (minor allele frequency >0.1) from 3 kb upstream to 1 kb downstream of the gene based on an r2 of ≥0.7 and 2) SNPs previously reported to be associated with type 2 diabetes. SNPs were genotyped by matrix-assisted laser desorption ionization–time of flight mass spectroscopy (MassARRAY Compact Analyzer, Sequenom, San Diego, CA). Genotyping data underwent a series of quality-control checks, and cleaned data were used in the further association analyses. Fifteen SNPs had a call rate >95%, whereas five SNPs were excluded because of low genotyping call rate (rs1016990, rs7407025, rs1008284, rs3110640, and rs11263755). SNPs with Hardy-Weinberg equilibrium test P values <0.05 in the case or control subjects were excluded. No SNP was excluded because of inconsistency with Hardy-Weinberg expectations. The average reproducibility rate among 1,500 duplicate genotype pairs was 100%. The overall call rate for the remaining 15 SNPs was 99.1%.
Statistical methods.
The Hardy-Weinberg equilibrium test was performed in the case and control groups separately for each variant using χ2 test. Pairwise linkage disequilibrium was computed from the combined data of case and control subjects by calculating D′ and r2 in Haploview (version 4.1) (19). Sliding windows consisting of two or three adjacent SNPs were generated for haplotype analyses using PLINK (version 0.99) (20). Allele, genotype, and haplotype frequencies for case and control subjects were compared using the χ2 test or Fisher's exact test. Odds ratios (ORs) with 95% CIs were presented. Correction of multiple testing was performed in Haploview through 10,000 permutations. Empirical P values of logistic regression analysis were calculated through 500 permutations that generated simulation data by randomly shuffling case and control status. In the control group, continuous diabetes-related traits were analyzed, and quantitative traits were transformed to reduce skewness. All statistical analyses were performed by SAS (version 8.0; SAS Institute, Cary, NC), unless otherwise specified. A two-tailed P value <0.05 was considered significant. Considering a risk allele frequency of 20% and an additive model, we had ∼80% power to detect an OR of 1.15 at the 0.05 level.
RESULTS
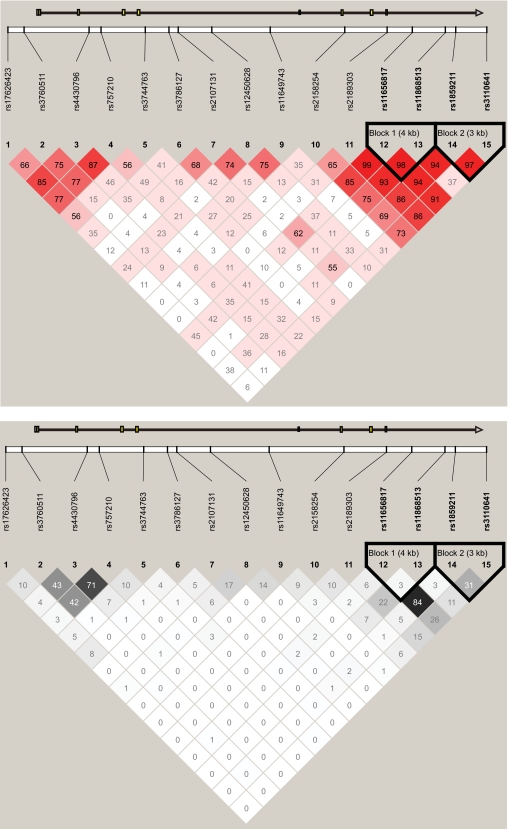
We examined 15 tagging SNPs spanning the HNF1β region. Figure 1 shows the pairwise linkage disequilibrium and haplotype block structure among these SNPs. The association of single SNPs with type 2 diabetes risk is shown in Table 2. Six of the 15 SNPs, including three previously reported SNPs, had a nominal P < 0.05. Three previously reported SNPs (rs4430796, rs757210 in intron 2, and rs3110641 in intron 8) were significantly associated with type 2 diabetes susceptibility (OR 1.13–1.16, P = 0.0035–0.0178). Three additional SNPs (two SNPs in intron 8 and one in the 5′ end of the gene region) also showed nominal significance (1.15–1.25, P = 0.0046–0.0178). Logistic regression analysis of type 2 diabetes with these six SNPs showed that the G allele of rs4430796 and A allele of rs11656817 tended to confer independent risks of type 2 diabetes (adjusted OR 1.135 and 1.211, P = 0.0162 and 0.0203, respectively). However, after adjustment for multiple testing by permutation, only the risk G allele of rs4430796 (in intron 2) showed significantly associated increased risk of type 2 diabetes (OR 1.16 [95% CI 1.05–1.29], P = 0.0035, empirical P = 0.0475). Logistic regression analysis adjusting for age, sex, and BMI also indicated that the association with SNP rs4430796 was statistically significant (1.18 [1.06–1.31], P = 0.0025, empirical P = 0.034). Sliding window haplotype analysis found that windows containing rs4430796 showed the strongest association with type 2 diabetes, which merely confirmed the results of single-SNP association analysis (Figure S1 available in an online appendix [http://diabetes.diabetesjournals.org/cgi/content/full/db08-1064/DC1]).
FIG. 1.
Linkage disequilibrium patterns of the 15 typed SNPs in the Chinese population.
TABLE 2.
Allele frequencies and association results for SNPs in the HNF1β gene
| Position (bp) | HNF1β region | Major/minor allele | Risk allele | Risk allele frequency |
OR (95% CI) | PSNP | Pgene | Adjusted PSNP* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Case subjects | Control subjects | |||||||||
| rs17626423 | −3,492 | 5′ | T/C | T | 0.886 | 0.885 | 1.01 (0.88–1.17) | 0.8804 | 1 | 0.4552 |
| rs3760511 | −1,438 | 5′ | C/A | A | 0.368 | 0.336 | 1.15 (1.04–1.27) | 0.0046 | 0.0624 | 0.0117 |
| rs4430796 | 6,836 | Intron 2 | A/G | G | 0.312 | 0.280 | 1.16 (1.05–1.29) | 0.0035 | 0.0475 | 0.0025 |
| rs757210 | 8,361 | Intron 2 | G/A | A | 0.289 | 0.264 | 1.13 (1.02–1.26) | 0.0178 | 0.2129 | 0.6318 |
| rs3744763 | 13,991 | Intron 4 | C/T | C | 0.557 | 0.545 | 1.05 (0.96–1.15) | 0.3065 | 0.9874 | 0.5032 |
| rs3786127 | 17,002 | Intron 4 | C/G | C | 0.837 | 0.834 | 1.02 (0.90–1.15) | 0.7708 | 1 | 0.7296 |
| rs2107131 | 18,187 | Intron 4 | C/T | T | 0.375 | 0.364 | 1.05 (0.95–1.15) | 0.3403 | 0.9934 | 0.3711 |
| rs12450628 | 22,455 | Intron 4 | C/T | C | 0.651 | 0.646 | 1.02 (0.93–1.12) | 0.6989 | 1 | 0.8704 |
| rs11649743 | 29,897 | Intron 4 | G/A | A | 0.328 | 0.323 | 1.02 (0.93–1.13) | 0.6373 | 1 | 0.8719 |
| rs2158254 | 39,381 | Intron 5 | G/A | A | 0.266 | 0.257 | 1.05 (0.94–1.16) | 0.4075 | 0.9981 | 0.2062 |
| rs2189303 | 44,771 | Intron 7 | C/T | T | 0.397 | 0.390 | 1.03 (0.94–1.13) | 0.5362 | 1 | 0.9497 |
| rs11656817 | 47,977 | Intron 8 | A/G | A | 0.916 | 0.898 | 1.25 (1.06–1.46) | 0.0067 | 0.0873 | 0.0210 |
| rs11868513 | 52,184 | Intron 8 | G/A | G | 0.721 | 0.719 | 1.01 (0.91–1.12) | 0.8353 | 1 | 0.8193 |
| rs1859211 | 53,504 | Intron 8 | A/G | A | 0.911 | 0.894 | 1.21 (1.03–1.41) | 0.0178 | 0.2138 | 0.0344 |
| rs3110641 | 57,459 | Intron 8 | C/T | C | 0.770 | 0.745 | 1.14 (1.03–1.27) | 0.0139 | 0.1742 | 0.0307 |
*Adjusted for age, sex, and BMI.
Because rs4430796 exhibited strong association with diabetes in our population, further analyses focused on this SNP for diabetes-related traits. We first treated age at diagnosis as a quantitative trait and analyzed its association with rs4430796 in the type 2 diabetic subjects. There was evidence that the G allele of rs4430796 was associated with younger age at diagnosis (52.40 ± 11.18 years for G/G, 53.94 ± 11.85 years for G/A, and 54.61 ± 11.97 years for A/A, P = 0.0228). When treating age at diagnosis as a dichotomous trait to stratify the type 2 diabetic patients and comparing the allele frequencies of rs4430796 with control subjects, we also found that the association signal was slightly stronger in the early-onset (age of diagnosis <45 years) subgroups (OR 1.27 [95% CI 1.07–1.49], P = 0.0051, Table 3).
TABLE 3.
Allelic association analysis of rs4430796 among type 2 diabetic and control subjects stratified according to age at diagnosis of type 2 diabetes
| n | Allele frequencies |
OR (95% CI) | P vs. control subjects | ||
|---|---|---|---|---|---|
| A | G | ||||
| Type 2 diabetic patients | 1,856 | 0.688 | 0.312 | 1.16 (1.05–1.29) | 0.0035 |
| Early onset | 395 | 0.670 | 0.330 | 1.27 (1.07–1.49) | 0.0051 |
| Late onset | 1,461 | 0.693 | 0.307 | 1.14 (1.02–1.26) | 0.0211 |
| Control subjects | 1,781 | 0.720 | 0.280 | 1.0 | — |
Early-onset type 2 diabetes age of diagnosis <45 years; late-onset type 2 diabetes age of diagnosis ≥45 years.
In subjects with normal glucose regulation, this SNP was not associated with plasma glucose levels; adiposity-related anthropometrics such as BMI, waist circumference, and waist-to-hip ratio; lipid profile; insulin sensitivity; or insulin secretion (supplementary Table S1).
DISCUSSION
We replicated previous findings that a SNP (rs4430796) in intron 2 of HNF1β was significantly associated with type 2 diabetes in our Chinese population. Our results are consistent with those reported by Gudmundsson et al. (12), and the effect size of the at-risk G allele for rs4430796 in our Chinese subjects (OR 1.16) is quite similar to that determined by the DECODE findings (for the G allele, overall OR 1.10). Notably, the allele frequency for the SNP rs4430796 is quite different between different ethnic groups. The at-risk G allele for rs4430796 had a frequency of 0.25–0.30 in the Chinese population but a frequency of 0.47–0.54 and 0.70–0.73 in Caucasians and West Africans, respectively (11,12). Although the minor G allele identified in the Chinese and Caucasian populations is the major allele in West Africans, this allele showed the same direction of its effect for type 2 diabetes. rs4430796 was not significantly associated with type 2 diabetes in Hong Kong Chinese, although the effect sizes between the Shanghai and Hong Kong samples were similar (for the risk G allele, OR 1.16 and 1.12, respectively) and in the same direction. One potential explanation for the discrepancy between the current findings and the results in the Hong Kong population is the difference in the control ascertainment. The Shanghai control subjects were >40 years of age and had measurements for both fasting plasma glucose and 2-h plasma glucose. In contrast to the individuals of our study, the Hong Kong control subjects were only tested for fasting plasma glucose. In addition, more than one-third of these control subjects were adolescents. All of these considerations raise the possibility of misclassification in the Hong Kong control samples; consequently, the effect size may be underestimated.
In the study by Winckler et al. (11), the authors observed that other SNPs in intron 2 (rs757210) and intron 8 (rs3110641) were associated with type 2 diabetes in a large Caucasian sample (combined OR 1.12 and 1.10, P = 5 × 10−6 and 0.0006, respectively). SNP rs757210 is correlated with rs4430796 ( D′ = 0.87 and r2 = 0.71 in our population). In the present study, although neither of the two SNPs reached significance after a correction for multiple testing, these two SNPs showed a similar trend in the same direction in our samples. In the study by Holmkvist et al. (13), the three previously reported SNPs (including rs1008284, rs3110641, and rs757210) did not predict future risk. However, our study suggests that the strongest effect for type 2 diabetes susceptibility was at rs4430796, and further study to follow up and replicate the association is still necessary.
Our study is the first to demonstrate that the at-risk G allele for rs4430796 is also associated with earlier onset of type 2 diabetes. Interestingly, previous studies have provided evidence that common variants in the MODY1 gene (HNF4A) also associated with type 2 diabetes and age at diagnosis (21,22). Nevertheless, rs4430796 was in the intron region of HNF1β, with no obvious functional effect reported; thus, we cannot exclude the possibility that other, as yet unknown or untyped, SNPs in linkage disequilibrium with rs4430796 may be the causal variants.
There are several limitations of this study. First, although the age of diagnosis of diabetes was determined by physician-documented medical records, we cannot exclude the possibility that the case subjects had an earlier age of onset. Second, the SNPs that we successfully genotyped can only capture 60% of common SNPs in the HNF1β gene region under the threshold of r2 ≥ 0.7 based on phase 3 HapMap Chinese data. It may be inevitable that we miss some common variants in this region that contribute to diabetes.
In summary, our data replicated the previous finding by Gudmundsson et al. (12) and support the notion that genetic variation in the HNF1β is associated with type 2 diabetes. Further studies in other populations and of more SNPs at this gene will be required to further elucidate the role of HNF1β variation in the pathogenesis of type 2 diabetes.
Supplementary Material
Acknowledgments
This work was supported by research grants from the Shanghai Rising-Star Program (07QA14036), the Major Program of the National Natural Science Foundation of China (NSFC) (30630061 and 30600361), the National 863 project of China (2006AA02A409), the National 973 project of China (2006CB503901), and the project of Shanghai Key Laboratory of Diabetes Mellitus (08DZ2230200) and an EFSD grant.
No potential conflicts of interest relevant to this article were reported.
We thank all nursing and medical staff at the Shanghai Clinical Center for Diabetes for their dedication and professionalism.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mendel DB, Hansen LP, Graves MK, Conley PB, Crabtree GR: HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev 5: 1042– 1056, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Cereghini S: Liver-enriched transcription factors and hepatocyte differentiation. FASEB J 10: 267– 282, 1996 [PubMed] [Google Scholar]
- 3.Poll AV, Pierreux CE, Lokmane L, Haumaitre C, Achouri Y, Jacquemin P, Rousseau GG, Cereghini S, Lemaigre FP: A vHNF1/TCF2-HNF6 cascade regulates the transcription factor network that controls generation of pancreatic precursor cells. Diabetes 55: 61– 69, 2006 [PubMed] [Google Scholar]
- 4.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI: Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet 17: 384– 385, 1997. 9398836 [Google Scholar]
- 5.Edghill EL, Bingham C, Ellard S, Hattersley AT: Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84– 90, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Fang Q, Zhang R, Lin X, Xiang K: Scanning for MODY5 gene mutations in Chinese early onset or multiple affected diabetes pedigrees. Acta Diabetol 41: 137– 145, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES: The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26: 76– 80, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, McCarthy MI, Hattersley AT, Frayling TM: Large-scale association studies of variants in genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52: 568– 572, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Silander K, Mohlke KL, Scott LJ, Peck EC, Hollstein P, Skol AD, Jackson AU, Deloukas P, Hunt S, Stavrides G, Chines PS, Erdos MR, Narisu N, Conneely KN, Li C, Fingerlin TE, Dhanjal SK, Valle TT, Bergman RN, Tuomilehto J, Watanabe RM, Boehnke M, Collins FS: Genetic variation near the hepatocyte nuclear factor-4α gene predicts susceptibility to type 2 diabetes. Diabetes 53: 1141– 1149, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Bonnycastle LL, Willer CJ, Conneely KN, Jackson AU, Burrill CP, Watanabe RM, Chines PS, Narisu N, Scott LJ, Enloe ST, Swift AJ, Duren WL, Stringham HM, Erdos MR, Riebow NL, Buchanan TA, Valle TT, Tuomilehto J, Bergman RN, Mohlke KL, Boehnke M, Collins FS: Common variants in maturity-onset diabetes of the young genes contribute to risk of type 2 diabetes in Finns. Diabetes 55: 2534– 2540, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Winckler W, Weedon MN, Graham RR, McCarroll SA, Purcell S, Almgren P, Tuomi T, Gaudet D, Boström KB, Walker M, Hitman G, Hattersley AT, McCarthy MI, Ardlie KG, Hirschhorn JN, Daly MJ, Frayling TM, Groop L, Altshuler D: Evaluation of common variants in the six known maturity-onset diabetes of the young (MODY) genes for association with type 2 diabetes. Diabetes 56: 685– 693, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gudmundsson J, Sulem P, Steinthorsdottir V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T, Gudbjartsson D, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Blondal T, Stacey SN, Helgason A, Gunnarsdottir S, Olafsdottir A, Kristinsson KT, Birgisdottir B, Ghosh S, Thorlacius S, Magnusdottir D, Stefansdottir G, Kristjansson K, Bagger Y, Wilensky RL, Reilly MP, Morris AD, Kimber CH, Adeyemo A, Chen Y, Zhou J, So WY, Tong PC, Ng MC, Hansen T, Andersen G, Borch-Johnsen K, Jorgensen T, Tres A, Fuertes F, Ruiz-Echarri M, Asin L, Saez B, van Boven E, Klaver S, Swinkels DW, Aben KK, Graif T, Cashy J, Suarez BK, van Vierssen Trip O, Frigge ML, Ober C, Hofker MH, Wijmenga C, Christiansen C, Rader DJ, Palmer CN, Rotimi C, Chan JC, Pedersen O, Sigurdsson G, Benediktsson R, Jonsson E, Einarsson GV, Mayordomo JI, Catalona WJ, Kiemeney LA, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K: Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39: 977– 983, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Holmkvist J, Almgren P, Lyssenko V, Lindgren CM, Eriksson KF, Isomaa B, Tuomi T, Nilsson P, Groop L: Common variants in maturity-onset diabetes of the young genes and future risk of type 2 diabetes. Diabetes 57: 1738– 1744, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications: part 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med 15: 539– 553, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Xiang K, Wang Y, Zheng T, Jia W, Li J, Chen L, Shen K, Wu S, Lin X, Zhang G, Wang C, Wang S, Lu H, Fang Q, Shi Y, Zhang R, Xu J, Weng Q: Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes 53: 228– 234, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jia WP, Pang C, Chen L, Bao YQ, Lu JX, Lu HJ, Tang JL, Wu YM, Zuo YH, Jiang SY, Xiang KS: Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia 50: 286– 292, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hu C, Jia W, Zhang R, Wang C, Lu J, Wu H, Fang Q, Ma X, Xiang K: Effect of RBP4 gene variants on circulating RBP4 concentration and type 2 diabetes in a Chinese population. Diabet Med 25: 11– 18, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263– 265, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira M, Bender D, Maller J, de Bakker P, Daly M, Sham P: PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet 81: 559– 575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damcott CM, Hoppman N, Ott SH, Reinhart LJ, Wang J, Pollin TI, O'Connell JR, Mitchell BD, Shuldiner AR: Polymorphisms in both promoters of hepatocyte nuclear factor 4-α are associated with type 2 diabetes in the Amish. Diabetes 53: 3337– 3341, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lehman DM, Richardson DK, Jenkinson CP, Hunt KJ, Dyer TD, Leach RJ, Arya R, Abboud HE, Blangero J, Duggirala R, Stern MP: P2 promoter variants of the hepatocyte nuclear factor 4α gene are associated with type 2 diabetes in Mexican Americans. Diabetes 56: 513– 517, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.