Abstract
OBJECTIVE
Glucagon-like peptide-1 receptor (GLP-1R) agonists are used to treat type 2 diabetes, and transient GLP-1 administration improved cardiac function in humans after acute myocardial infarction (MI) and percutaneous revascularization. However, the consequences of GLP-1R activation before ischemic myocardial injury remain unclear.
RESEARCH DESIGN AND METHODS
We assessed the pathophysiology and outcome of coronary artery occlusion in normal and diabetic mice pretreated with the GLP-1R agonist liraglutide.
RESULTS
Male C57BL/6 mice were treated twice daily for 7 days with liraglutide or saline followed by induction of MI. Survival was significantly higher in liraglutide-treated mice. Liraglutide reduced cardiac rupture (12 of 60 versus 46 of 60; P = 0.0001) and infarct size (21 ± 2% versus 29 ± 3%, P = 0.02) and improved cardiac output (12.4 ± 0.6 versus 9.7 ± 0.6 ml/min; P = 0.002). Liraglutide also modulated the expression and activity of cardioprotective genes in the mouse heart, including Akt, GSK3β, PPARβ-δ, Nrf-2, and HO-1. The effects of liraglutide on survival were independent of weight loss. Moreover, liraglutide conferred cardioprotection and survival advantages over metformin, despite equivalent glycemic control, in diabetic mice with experimental MI. The cardioprotective effects of liraglutide remained detectable 4 days after cessation of therapy and may be partly direct, because liraglutide increased cyclic AMP formation and reduced the extent of caspase-3 activation in cardiomyocytes in a GLP-1R–dependent manner in vitro.
CONCLUSIONS
These findings demonstrate that GLP-1R activation engages prosurvival pathways in the normal and diabetic mouse heart, leading to improved outcomes and enhanced survival after MI in vivo.
The protection against ischemic damage provided by cycling periods of ischemia and reperfusion, that is, ischemic preconditioning (IP), may last for hours or even days (1). Although the molecular basis of ischemic preconditioning is complex and incompletely understood, there is active interest in the development of therapeutic interventions that protect the myocardium against ischemic injury.
Glucagon-like peptide-1 (GLP-1), a member of the proglucagon-derived peptide family (2), exerts favorable actions on cardiovascular function in both preclinical and clinical studies. A functional GLP-1 receptor (GLP-1R) is expressed in the heart (3), and GLP-1R agonists directly activate cardiomyocyte signaling pathways (4). Because the GLP-1R is also expressed in the endocrine pancreas, GLP-1 may regulate cardiac function indirectly through metabolic control of glucose, insulin, glucagon, and free fatty acids. Furthermore, GLP-1R agonists activate the peripheral and central nervous system (CNS), including regions of the CNS important for control of cardiovascular function (5–7). The mechanisms through which GLP-1 can modulate cardiac function are complex and incompletely understood.
Transient GLP-1 administration improves outcomes in experimental models of cardiac injury such as pacing-induced heart failure (8) and experimental ischemia induced by coronary artery ligation (9–13). A pilot study of GLP-1 administration for 72 h in human subjects with left ventricular (LV) dysfunction after myocardial injury and angioplasty demonstrated reduced hospital stay and improved global and regional LV wall motion scores, benefits that remained detectable even several weeks after hospital discharge (14). Similarly, a 5-week course of GLP-1 infusion improved parameters of LV function, functional status, and quality of life in both diabetic and nondiabetic subjects with congestive heart failure (15).
Although the glucoregulatory actions of the first clinically approved GLP-1R agonist, exenatide, have been extensively studied in the clinic (2), there is limited information available about the effects of exenatide on the normal or ischemic heart (16). A second GLP-1R agonist, liraglutide, is a human dipeptidyl peptidase-4 (DPP-4)-resistant GLP-1 analog that exhibits a prolonged pharmacokinetic profile, relative to native GLP-1, resulting from noncovalent association with albumin (2,17,18), and has completed phase 3 clinical trials in human subjects with type 2 diabetes. Although liraglutide appears to be a promising antidiabetic agent (19), the effects of liraglutide on the cardiovascular system have not been examined.
We have now assessed whether liraglutide exerts cardioprotective actions in a preclinical murine model of experimental ischemia after coronary artery occlusion. We show that treatment with liraglutide before induction of ischemia leads to activation of prosurvival kinases and cytoprotective genes in the heart and limits infarct size, expansion, and cardiac rupture in the normal and diabetic heart. Moreover, liraglutide increases cAMP and reduces apoptosis in a GLP-1R–dependent manner in murine cardiomyocytes cultured in vitro. These findings extend our understanding of the cardioprotective actions of GLP-1R agonists and provide testable hypotheses for examining the cardiovascular effects of GLP-1R agonists in human subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Animals.
Protocols were approved by the Animal Care Committee of the Toronto General Hospital in accordance with guidelines of the Canadian Council for Animal Care. Male 10- to 12-week-old C57BL/6 mice were obtained from Charles River (Montreal, PQ, Canada) and housed for at least 2 weeks before experimentation.
Drug treatments.
The volume of individual i.p. injections was 100 μl. The experimental protocols are summarized in supplemental Fig. 1 (available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-1193). One group of animals (n = 75) was injected with the GLP-1R agonist liraglutide (Novo Nordisk, Novo Alle, Bagsvaerd, Denmark) at a previously used dose of 200 μg/kg i.p. twice daily (18) for 7 days before permanent surgical ligation of the left anterior descending (LAD) artery as previously detailed (20). A parallel group of control animals (n = 75) was injected with an equivalent volume of the vehicle, PBS. After this treatment period, some animals (n = 10 per group) were killed just before planned LAD ligation with hearts dissected immediately, weighed, and frozen. A second group of mice received PBS or liraglutide (n = 10 each) for 7 days and were subjected to sham surgery without LAD occlusion. Because a regimen of liraglutide 200 μg/kg i.p twice daily (LIR 200) induced weight loss in mice, we also studied the effects of LAD ligation in separate groups of mice after administration of a lower dose of liraglutide (75 μg/kg i.p. twice daily: LIR 75) that did not produce significant weight loss or pair-feeding to induce weight loss in control animals comparable to that seen in mice treated with LIR 200. Separate groups of PBS- and liraglutide-treated mice (n = 13 per group) were killed on day 4 post–myocardial infarction (MI) for biochemical and histological analyses. LV tissues from infarct and peri-infarct zones and remote (noninfarcted) area were separated, snap-frozen, and stored at −80°C. The remaining mice were monitored for 28 days post-MI for survival analysis and histomorphometry for infarct size. In separate studies, mice were maintained on a high-fat diet (45% Kcal from fat, D-12451; Research Diets) for 1 month, following which diabetes was induced by treatment with streptozotocin (90 mg/kg i.p.). After stratification by degree of hyperglycemia at 3 weeks after streptozotocin injection, mice were randomized to receive treatment for 7 days with placebo (PBS, 100 μl i.p. twice daily), metformin in chow (6.76 g/kg of mouse diet), or liraglutide 75 μg twice daily (LIR 75) before sham or LAD-ligation surgery (n = 23/group: 5 sham, 18 LAD-ligation). Diabetic mice were maintained on the high-fat diet until euthanasia on day 28 postsurgery. To investigate the role of the known GLP-1R in the cardiac actions of liraglutide, male Glp1r-/- mice, 10 to 12 weeks of age, in the C57BL/6 background and their littermate (Glp1r+/+) controls (n = 6/group), were injected with either PBS or 75 μg liraglutide twice daily for 1 week, then killed and their hearts used for Western blot assessment of prosurvival kinases.
Necropsy.
Cardiac examinations were performed on deceased mice post-MI. The presence of a large amount of blood or clot around the heart and in the thoracic cavity as well as a perforation of the infarct or peri-infarct area was taken to indicate cardiac rupture.
Blood glucose.
Before anesthesia, nonfasting blood glucose measurements were obtained through a tail nick using a handheld glucometer and One-Touch glucometer strips (LifeScan Canada, Burnaby, BC, Canada).
Cardiac hypertrophy.
Heart-to-body weight ratios were calculated for each animal at the time of terminal euthanasia.
Infarct size.
Demarcation of the infarct area was performed in separate groups of PBS- and liraglutide-treated mice using either 2,3,5-triphenyl tetrazolium chloride (TTC) or hematoxylin and eosin (H-E) staining at 2 and 28 days post-MI, respectively, as described (20,21). Sections from three levels of each heart (5 μm: apical, midventricular, and basal) were stained with H-E and scanned, and the circumference of the fibrotic infarct area and entire left ventricle was measured with Image J software (NIH). The mean of measurements of percent infarct size (circumference) was then calculated (infarct circumference/total LV circumference × 100%).
For analysis after TTC staining, hearts were sectioned perpendicular to the long axis with a thickness of ∼2 mm from the apical, midventricular, and basal (immediately below the ligation point) regions and incubated in the prewarmed (30°C) fresh TTC solution for 15 min. They were then weighed and transferred to a 4% paraformaldehyde solution for 1 h before image acquisition with a digital camera. Infarct and total left ventricle areas were then measured by Image J for determination of percent infarct size (area) by calculation (infarct area/total LV area × 100%).
Zymography.
Matrix metalloproteinase-9 (MMP-9) was assessed in the infarct zone 4 days post-MI as described (22).
Cell culture, cAMP assay, and tumor necrosis factor-α–induced apoptosis.
Cardiomyocytes from newborn mice were prepared using a modified protocol (23). For cAMP assay, neonatal mouse cardiac myocytes were preincubated with IBMX (250 μmol/l; Sigma) for 30 min to inhibit cAMP degradation followed by subsequent treatment periods of 20 min for liraglutide (100 nmol/l), 30 min for the GLP-1R antagonist exendin (Ex)9–39 (1 μmol/l), and 15 min for forskolin (100 nmol/l). For the liraglutide + Ex9–39 treatment, cells were exposed to Ex9–39 for 30 min before coincubation with fresh Ex9–39 and liraglutide for an additional 20 min. All experiments were performed in quadruplicate. Samples were collected and analyzed using a cAMP radioimmunoassay kit (Amersham, Little Chalfont, U.K.). For tumor necrosis factor-α (TNF-α) experiments, cells were grown on six-well dishes, serum deprived for 24 h, incubated for 1 h with 10,100 or 1,000 nmol/l doses of liraglutide with or without 10 μmol/l Ex9–39 followed by coincubation with liraglutide and TNF-α (100 ng/ml; Sigma) for another 24 h to induce apoptosis (24). As a positive control for the induction of apoptosis, another group of cardiomyocytes was exposed to 0.5 μmol/l H2O2 as described (25). All experiments were performed in triplicate. Western blot analysis using whole cell extracts was used to quantify levels of cleaved caspase 3.
Western blot.
Extracts from cells, whole hearts, and infarct regions were prepared as described (26). Rabbit polyclonal primary antibodies against GLP-1R (LS-A1205; MBL International), Nrf2 (C20; Santa Cruz Biotechnology), heme-oxygenase-1 (HO-1; Stressgen), peroxisome proliferator–activated receptor (PPAR)-β/δ (H-74; Santa Cruz), and monoclonal antibodies against Akt, phospho-Akt (Ser473), glycogen synthase kinase (GSK)-3β, phospho-GSK-3β (Ser9), and cleaved-caspase 3 (all from Cell Signaling) as well as goat-polyclonal anti–atrial natriuretic peptide (ANP) antibodies (Santa Cruz) were used per manufacturers' instructions. Mouse monoclonal anti–β-actin (Sigma) and anti–transcription factor II D (TFIID) (Santa Cruz) antibodies and a rabbit polyclonal anti-GAPDH (Santa Cruz) were used to evaluate the amount of protein loaded in each sample.
RT-PCR.
Total RNA was isolated from hearts using TRIZOL (Invitrogen), quantified (2 μg), and treated with DNase-I. cDNA synthesis was performed by Superscript III reverse transcriptase (Invitrogen) and specific primers for HO-1 (F: GGC-CTT-CTG-GTA-TGG-GCC-TCA-CTG-G; R: GCC-TCT-GAC-GAA-GTG-ACG-CCA-TCT-G) and Nrf2 (F: TCT-CCT-CGC-TGG-AAA-AAG-AA; R: AAT-GTG-CTG-GCT-GTG-CTT-TA). PCR products were visualized on 1.5% agarose gels with ethidium bromide. GAPDH reaction product served as a loading and RT efficiency control.
Ischemia-reperfusion.
Isolated hearts were prepared as previously described (27). Isolated hearts underwent a 20-min equilibration phase followed by a 40-min perfusion phase during which LV developed pressure (LVDP) was continuously recorded. Then, 30 min of sustained global ischemia was generated by clamping inflow to the heart followed by reperfusion for 40 min. In some experiments, liraglutide was added to the buffer during the final 20 min of the perfusion phase (i.e., preischemia); in others, it was added to the buffer only during reperfusion (i.e., postischemia). Recovery of LVDP was measured at the end of reperfusion. These experiments were also performed using hearts isolated from mice after 1 day (acute) or 7 days (chronic) of twice-daily liraglutide (200 μg/kg i.p.).
Ultrasound biomicroscopy.
Image acquisition and data analysis were carried out as described (28,29). Three groups of nondiabetic mice, including 8 control mice not subjected to LAD occlusion, and 15 liraglutide-treated and 15 saline-treated mice were studied on day 28 post-MI using high-frequency ultrasound imaging. Identical procedures were used to image diabetic mice (sham, n = 5/group; LAD-ligated, n = 10–11/group).
All data are expressed as mean ± SE. Survival analysis was done by the Kaplan-Meier method (Fig. 1A and B). Student's t test was used to compare two groups for data shown in Figs. 2–4. For analysis of echocardiographic data (Table 1) and specific time points in ischemia-reperfusion (I/R) experiments (Fig. 5) and cAMP and TNF-α studies (Fig. 6), a one-way ANOVA was used to evaluate the difference among groups. If the ANOVA was significant, the Student-Newman-Keuls (SNK) post hoc test was used for pairwise multiple comparisons. Significance was defined as P < 0.05.
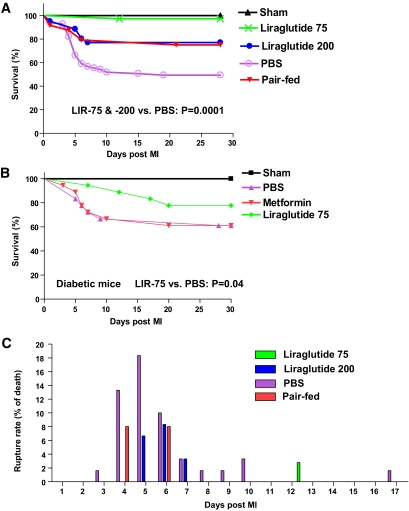
FIG. 1.
Liraglutide pretreatment improves outcomes after MI in mice. Kaplan-Meier survival curves show survival after MI in nondiabetic mice (A): sham (n = 20), liraglutide-MI (75 μg/kg, n = 35 or 200 μg/kg, n = 60), PBS-MI (n = 60), and pair-fed mice (n = 25), P = 0.0001 for LIR 75 and 200 versus PBS; and diabetic mice (B): sham (n = 15, 5/treatment group), PBS-MI (n = 18), metformin-MI (n = 18), liraglutide-MI (75 μg/kg, n = 18), P = 0.04 for LIR 75 versus PBS. C: Frequency and timing of cardiac rupture in nondiabetic mice is shown as a percentage of total group. One-week pretreatment with liraglutide (200 or 75 μg/kg i.p. twice daily) had no significant effects on random blood glucose levels in adult nondiabetic mice (PBS: 7.6 ± 4.0 versus LIR 200: 6.3 ± 0.6 versus LIR 75: 6.1 ± 0.4 mmol/l; P = 0.72). Blood glucose levels in diabetic mice are shown in supplemental Table 1 (available in the online appendix).
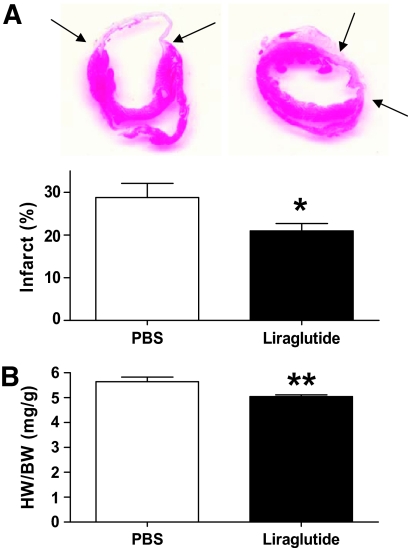
FIG. 2.
Effects of liraglutide pretreatment on infarct size and heart weight. A: Representative photomicrographs of H-E–stained hearts 28 days post-MI depict decreased infarct size (arrows) in liraglutide- (n = 36) versus PBS-treated mice (n = 21) as confirmed by morphometric quantification of percent total LV circumference (*P = 0.025). B: HW/BW ratio was reduced in liraglutide- versus PBS-treated mice 28 days post-MI (n = 36 and 21, respectively; **P = 0.001). Data shown are means ± SE. (A high-quality digital representation of this figure is available in the online issue.)
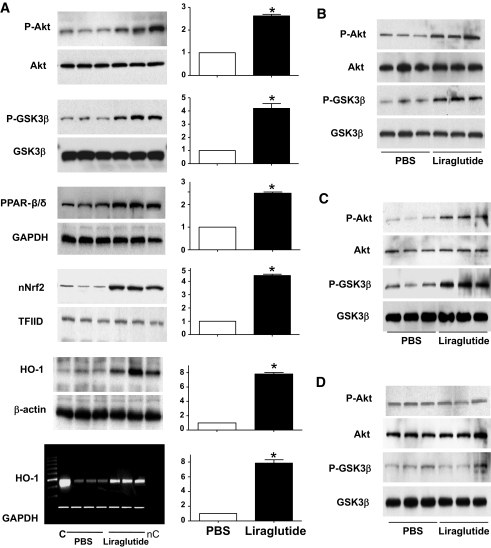
FIG. 3.
Effects of liraglutide pretreatment on levels of cardiac genes and proteins. The expression of genes/proteins before left anterior descending ligation is analyzed in liraglutide-treated hearts. A: Representative Western blots for wild-type mice treated with LIR 200 as outlined in supplemental Fig. 1 (available in the online appendix). Corresponding densitometric quantification (n = 6/group) of fold changes in phosphorylation of prosurvival kinases Akt and GSK3β and in expression of PPAR-β/δ, Nrf2, and HO-1 are shown in the top five panels. The bottom panel depicts a representative agarose gel (n = 6/group) showing HO-1–specific mRNA levels by RT-PCR. Data shown are means ± SE; *P < 0.05. B–D: Representative Western blot analysis of cardiac prosurvival kinases in LIR 75–treated wild-type (B), LIR 75–treated Glp1r+/+ littermate controls (C), and LIR 75–treated Glp1r-/- (D) mice, respectively.
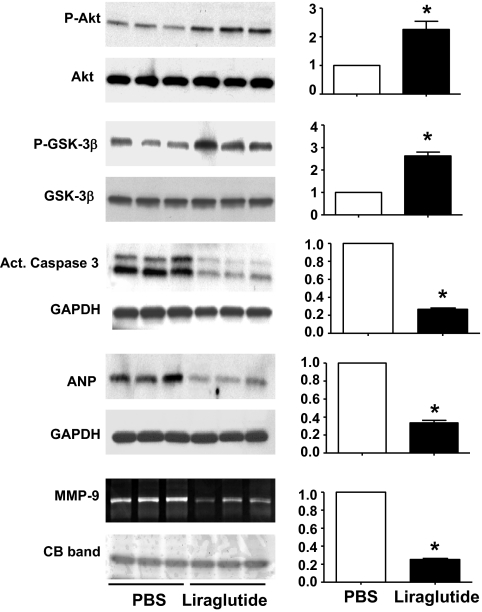
FIG. 4.
Effects of liraglutide pretreatment on cardioprotective signaling pathways after MI in mice. Liraglutide pretreatment for 7 days has persistent cardioprotective effects detectable 4 days post-MI as indicated by representative Western blots and corresponding densitometric quantification (n = 6/group) of fold changes in phosphorylation of Akt and GSK3β, cleavage of caspase-3, and expression of ANP and activity of MMP-9 by zymography in liraglutide- versus PBS-treated mice. Data shown are means ± SE; *P < 0.05.
TABLE 1.
Ultrasound biomicroscopy-defined cardiac dimensional, functional, and hemodynamic parameters in mice on day 28 postoperatively
| Parameter | Sham | MI-placebo | MI-liraglutide |
|---|---|---|---|
| n | 8 | 15 | 15 |
| Body weight (g) | 27.9 ± 0.3 | 29.5 ± 0.4 | 29.3 ± 0.5 |
| Aortic flow | |||
| Heart rate (bpm) | 393 ± 12 | 451 ± 18 | 435 ± 12 |
| Peak velocity (cm/s) | 86.3 ± 2.2 | 68.7 ± 2.7* | 80.3 ± 3.2† |
| VTI (cm) | 3.03 ± 0.08 | 2.09 ± 0.13* | 2.61 ± 0.12*† |
| AO diameter (mm) | 1.15 ± 0.02 | 1.15 ± 0.02 | 1.19 ± 0.01 |
| LV SV (μl) | 31.3 ± 0.9 | 22.1 ± 1.8* | 28.8 ± 1.4† |
| CO (ml/min) | 12.3 ± 0.5 | 9.7 ± 0.6* | 12.4 ± 0.6† |
| Mitral flow | |||
| Heart rate (bpm) | 413 ± 11 | 433 ± 14 | 424 ± 9 |
| Peak E velocity (cm/s) | 71.8 ± 1.9 | 59.0 ± 3.6* | 59.9 ± 2.2* |
| Peak A velocity (cm/s) | 47.0 ± 1.5 | 30.8 ± 3.5* | 43.6 ± 2.0† |
| Peak E/A ratio | 1.54 ± 0.04 | 2.54 ± 0.44* | 1.40 ± 0.06† |
| LV chamber dimensions by M mode | |||
| Heart rate (bpm) | 417 ± 10 | 442 ± 17 | 441 ± 15 |
| LV EDD (mm) | 4.33 ± 0.05 | 6.12 ± 0.23* | 5.52 ± 0.09*† |
| LV ESD (mm) | 3.33 ± 0.08 | 5.73 ± 0.30* | 5.02 ± 0.14*† |
| LV FS (%) | 23.0 ± 1.2 | 7.0 ± 1.5* | 9.2 ± 1.3* |
Data are means ± SE. In mitral inflow, the peak E velocity represents the maximal velocity of the early diastolic wave caused by active LV relaxation. The peak A velocity represents the maximal velocity caused by left atrial contraction in late diastole. *Difference (P < 0.05) with the corresponding value in the sham controls.
†Difference (P < 0.05) with the corresponding value in the placebo (PBS)-treated controls. AO, aortic orifice; CO, cardiac output; FS, fractional shortening; SV, stroke volume; EDD, end-diastolic diameter; ESD, end-systolic diameter; VTI, velocity–time integral of Doppler flow waveform.
FIG. 5.
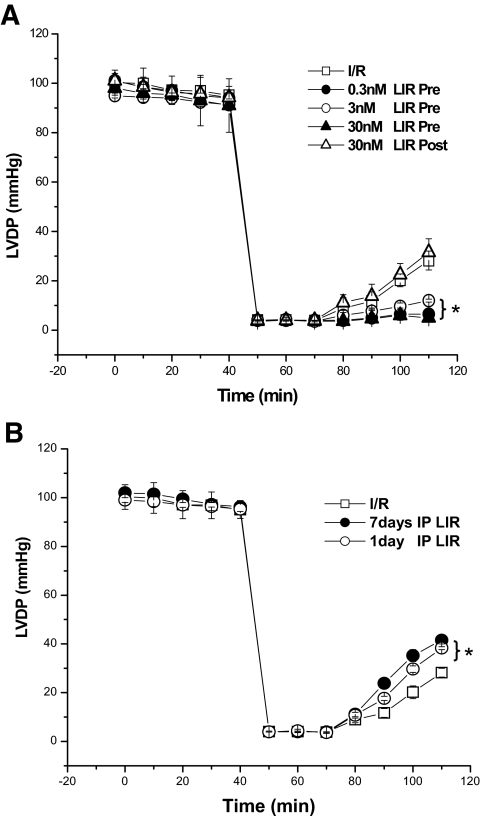
Effects of liraglutide on recovery of LV function after I/R injury in isolated hearts. A: Effect of direct infusions of liraglutide- (0.3, 3, and 30 nmol/l, n = 5/group) or no treatment (vehicle, n = 21) either pre- or postischemia in isolated murine hearts subjected to experimental I/R ex vivo. B: Two additional groups of mice (n = 5/group) received i.p. injections of liraglutide b.i.d. for 1 or 7 days before ex vivo experiments. Data shown are means ± SE; *P < 0.01 compared with untreated controls.
FIG. 6.
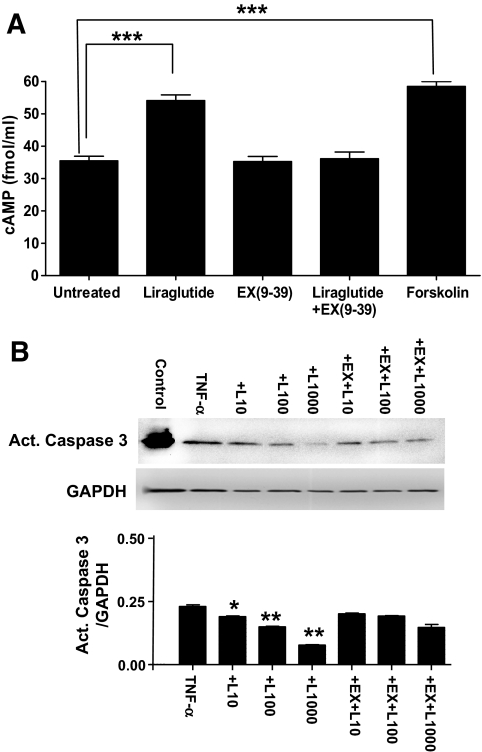
Liraglutide induces cAMP formation and reduces caspase-3 activation in murine cardiomyocytes in vitro. A: Liraglutide (100 nmol/l) increases cAMP formation in cultured neonatal cardiomyocytes. The actions of liraglutide were abolished by the GLP-1R antagonist exendin9–39. ***P < 0.001. B: Liraglutide (L: 10–1,000 nmol/l) reduced TNF-α–induced activation of caspase-3 in a dose-dependent manner in cultured neonatal mouse cardiomyocytes. Cotreatment with exendin9–39 (Ex: 10 μmol/l) abolished the protective effects of liraglutide. Positive control represents treatment of cells with the potent apoptosis-inducing agent H2O2. Data shown are means ± SE; *P < 0.01 and **P < 0.001 versus cultures only treated with TNFα.
RESULTS
Liraglutide increased survival in mice post-MI.
Immediate perioperative mortality (within 24 h of LAD ligation) was 8.3% in PBS- (n = 85) and 5% in LIR 200–treated (n = 60) mice (P = 0.43) with no deaths in the sham-operated animals (n = 20). By day 28 post-MI, LIR 200–treated mice exhibited reduced mortality compared with PBS-treated controls (12 of 60 versus 46 of 60; P = 0.0001) (Fig. 1A). Because mice treated with LIR 200 also exhibited weight loss compared with PBS-treated controls (−1.65 ± 0.05 versus +0.72 ± 0.02 g; P = 0.0001), we carried out additional experiments to determine whether the effects of LIR on survival post-MI were dependent on weight loss. A separate group of mice was pair-fed to achieve weight loss comparable to that of animals receiving LIR 200 for 7 days; no difference in survival was noted between mice treated with LIR 200 versus pair-fed controls (Fig. 1A). We next assessed a range of liraglutide doses on food intake and body weight and then determined the effect of pretreatment with a weight-neutral dose, LIR 75, on post-MI survival. Mice treated with LIR 75 for 7 days did not experience significant weight loss (0.30 ± 0.06 g; P = 0.85) but exhibited a marked improvement in survival after LAD ligation (Fig. 1A, P = 0.0001). Similarly, pretreatment of diabetic mice for 1 week with LIR 75 also reduced mortality after LAD occlusion (Fig. 1B, P = 0.04); in contrast, comparable treatment with metformin that produced equivalent reduction in glycemia (supplemental Table 1, available in the online appendix) did not improve survival after MI (P = 0.5, not significant). Together, these data suggest that pretreatment with liraglutide enhances survival after MI independent of effects on body weight or blood glucose.
Liraglutide prevented cardiac rupture post-MI.
To understand the mechanisms by which LIR improves outcomes after MI in mice, we performed postmortem examinations of all animals. Excluding day 1, postmortem analysis in nondiabetic mice revealed that all spontaneous death events within 10 days post-MI were associated with evidence of cardiac rupture. Timing and incidence of cardiac rupture differed among the groups, occurring as early as day 3 post-MI and peaking in incidence at day 5 post-MI in PBS-treated mice (Fig. 1C). By contrast, mice pretreated with LIR 200, and to an even greater extent LIR 75, exhibited fewer and later cardiac rupture events post-MI (Fig. 1C).
Liraglutide reduced infarct expansion post-MI.
Cardiac rupture is known to occur more frequently with larger infarcts (30) and is believed to manifest an imbalance between inflammatory and fibrotic responses as well hemodynamic forces acting on the infarct (31,32). To explore these possibilities in more detail, we first analyzed H-E–defined infarct size at day 28 post-MI in nondiabetic mice. Liraglutide (LIR 200) significantly reduced infarct size compared with PBS-treated mice (20.9 ± 1.7 versus 28.8 ± 3.3; percent total LV circumference, P = 0.02) (Fig. 2). Furthermore, cardiac hypertrophy, as manifested by heart weight/body weight (HW/BW) ratio, was significantly reduced in LIR 200–treated mice (Fig. 2). To determine if these results partly reflected survivor bias, we also examined TTC-defined infarct size at day 2 post-MI. This analysis revealed no significant difference in infarct size between LIR 200– (n = 14) and PBS-treated (n = 15) groups (40.2 ± 4.2 versus 39.3 ± 3.8; P = 0.86). Despite significantly higher survival in liraglutide-treated diabetic mice (Fig. 1B), no differences were observed in either infarct size or HW/BW ratios in diabetic animals at day 28 post-MI (data not shown).
Liraglutide activates cardioprotective signaling pathways in the heart.
To determine the mechanisms underlying liraglutide-induced improvements in survival and infarct remodeling post-MI, we studied the expression of selected genes and proteins known to modulate cardiomyocyte survival. LIR 200 administered twice daily for 7 days to normal healthy mice (without MI) increased phosphorylation of the prosurvival kinase Akt (pAkt/Akt: 2.10 ± 0.01-fold over control, P = 0.002, Fig. 3A). Similarly, liraglutide increased phosphorylation and thereby reduced the activity of GSK3β, a known Akt substrate, and increased levels of the nuclear receptor PPARβ/δ and the redox-sensitive basic leucine zipper transcription factor Nrf2. Liraglutide also induced mRNA and protein levels of HO-1 (Fig. 3A), a protein strongly implicated in cardioprotection in response to ischemic injury (33). To assess the importance of the known GLP-1R for the actions of liraglutide, we administered LIR 75 for an identical 7-day treatment period in mice with genetic absence of a functional GLP-1R and littermate controls. Liraglutide increased phosphorylation of Akt and GSK3β in both wild-type (Fig. 3B) and Glp1r+/+ littermate controls (Fig. 3C), but not in Glp1r-/- mice (Fig. 3D).
To assess whether the effects of liraglutide remained detectable after cessation of liraglutide therapy and before the peak incidence of mortality post-MI (which occurred on day 5; Fig. 1A), we examined the hearts of liraglutide- and PBS-treated controls 4 days post-MI (i.e., 4 days after the final dose of liraglutide or PBS). The HW/BW ratio was reduced in liraglutide-treated mice (5.53 ± 0.14 versus 5.83 ± 0.15 mg/g; P = 0.03) at day 4, and levels of phosphorylated Akt were significantly higher in the infarct area of hearts from liraglutide-treated mice (Fig. 4). Similarly, liraglutide pretreatment was associated with increased GSK phosphorylation at Ser 9 and a significant reduction in levels of cleaved caspase-3 and ANP (Fig. 4). Quantitative gelatin zymography showed a significant decrease in MMP-9 activity in hearts from liraglutide-treated mice (Fig. 4). These data demonstrate a persistent effect of liraglutide pretreatment on cardiac prosurvival and remodeling pathways detectable both before (Fig. 3) and after (Fig. 4) induction of ischemic injury.
Liraglutide-treated mice exhibit improved cardiac performance.
The results of cardiac ultrasound biomicroscopy performed 4 weeks after LAD ligation or sham surgery in nondiabetic mice are shown in Table 1. After experimental MI, measures of systolic function such as cardiac output and stroke volume were significantly increased in liraglutide-treated mice. Measures of diastolic function such as mitral inflow velocities (E/A ratio) were also improved in liraglutide-treated mice and LV dilatation in the liraglutide-treated group was less severe than in PBS-treated control mice (Table 1). In contrast, although liraglutide-treated diabetic mice exhibited a significant increase in cardiac output in the absence of experimental MI, no consistent differences in echocardiographic parameters were detected in diabetic mice treated with liraglutide versus metformin at day 28 post-MI (data not shown).
Liraglutide treatment in vivo prevented ischemia–reperfusion injury of isolated hearts ex vivo.
To determine whether liraglutide exhibits rapid cardioprotective actions in the isolated mouse heart ex vivo, we examined the effects of acute liraglutide administration in the isolated perfused mouse heart. Direct infusions of liraglutide immediately before and postischemia did not improve functional recovery after ischemia-reperfusion (I/R) injury in the isolated mouse heart (Fig. 5A). In contrast, pretreatment of normal healthy mice with liraglutide (200 μg/kg b.i.d.) in vivo for 1 or 7 days before heart isolation enhanced recovery of LVDP after I/R relative to untreated controls (1 day: 41.54 ± 1.5, n = 4; 7 days: 38.31 ± 0.6 mmHg, n = 5; untreated: 28.24 ± 1.8 mmHg, n = 5; P < 0.01, Fig. 5B). These results imply that liraglutide-induced cardioprotection may require a minimum dose or pretreatment period for the activation of a cardiac gene and protein expression profile and/or may be indirect, reflecting the consequences of GLP-1R activation in noncardiac tissues.
Liraglutide induces cAMP and reduces apoptosis in neonatal mouse cardiomyocytes.
We next examined whether liraglutide exerts direct actions on mouse cardiomyocytes. Liraglutide (100 nmol/l) significantly increased cAMP formation in mouse cardiomyocytes (P < 0.0001) in a GLP-1R–dependent manner, because cAMP stimulation was abolished by the GLP-1R antagonist Ex9–39 (Fig. 6A). To determine whether liraglutide directly modulates cardiomyocyte survival, we examined the effects of liraglutide in TNF-α–treated neonatal cardiomyocytes. Liraglutide dose-dependently reduced TNF-α–induced activation of caspase 3 in cardiomyocytes in vitro. Furthermore, the protective effect of liraglutide was completely abolished after coincubation of cells with Ex9–39 (Fig. 6B).
DISCUSSION
In the present study, we demonstrate that liraglutide administration induced changes in the expression of cardioprotective proteins in the normal nonatherosclerotic murine heart characterized by phosphorylation of Akt and GSK3β and increased expression of Nrf2, PPAR-β/δ, and HO-1. These changes were associated with improved survival of mice after experimental ischemia despite cessation of liraglutide therapy. These findings, taken together with the results of echocardiography, demonstrate that a brief 7-day period of liraglutide pretreatment leads to improvements in cardiomyocyte survival and sustained improvement in cardiac function that remain detectable even 4 weeks after cessation of liraglutide and induction of experimental MI.
The beneficial effects of liraglutide were independent of weight loss, because a lower dose of liraglutide (75 μg/kg) that did not produce weight loss also protected the heart and increased survival to a greater extent than that observed with the higher 200 μg/kg liraglutide dose. The weight loss observed with higher pharmacological doses of GLP-1R agonists is not required for the beneficial effects of these agents on cardiovascular function (2).
Although the mechanisms underlying the reduced cardiac rupture and improved survival remain incompletely understood, notable findings from our studies include the modulation of putative mediators (Akt, GSK3β, HO-1, PPAR-β/δ, and Nrf2) known to be important for cardiomyocyte survival (34–38). We observed reduced levels of MMP-9 and cleaved caspase 3 in the infarct region of liraglutide-treated mice at day 4 post-MI. These findings are reminiscent of observations made in studies of the effects of GLP-1 on survival of β-cells and neurons, in which GLP-1R activation leads to inhibition of caspase activation and increased cytoprotection (39,40).
Functional assessment with echocardiography showed that LV systolic function remained improved even 4 weeks after the last dose of liraglutide. We observed significantly less dilatation of the left ventricle of liraglutide-treated mice. These findings are consistent with preclinical data demonstrating that GLP-1R agonists improve LV function in rodent and canine models of experimental cardiac dysfunction (8,9,11–13,41). The sustained benefits of transient liraglutide therapy that persisted for weeks after cessation of therapy is consistent with studies demonstrating that a 72-h infusion of GLP-1 in human subjects after MI produces sustained improvements in cardiovascular function that remained detectable even months after cessation of GLP-1 administration (14).
An unexpected finding was the observation that liraglutide, unlike native GLP-1 or GLP-19–36 (3), was not effective in directly improving LV pressure, or recovery of LV pressure after I/R injury, when administered either immediately before induction of ischemia or during the reperfusion phase to isolated hearts ex vivo. In contrast, administration of liraglutide in vivo for as little as 1 day (two injections) before ischemia was sufficient to confer a cardioprotective benefit in subsequent I/R experiments. Our findings raise the possibility that the cardioprotective actions of liraglutide are complex and may require a specific minimum dose or defined time period for induction of a protective gene/protein profile that promotes resistance to cardiomyocyte injury. Alternatively, the cardioprotective actions of liraglutide observed in vivo may be partly indirect, mediated perhaps through neural, hormonal, or metabolic factors. This latter possibility contrasts with findings demonstrating direct protection by native GLP-1 in I/R studies (9,10,42).
Recent studies have implicated a role for the metabolite GLP-19–36 as a cardioactive peptide with inotropic effects in dogs with heart failure (41). The GLP-1 metabolite, GLP-19–36, also exerts cardioprotective actions when administered postischemia (3,16), further highlighting the complexity of different GLP-1–related peptides on the ischemic heart. Liraglutide is relatively resistant to cleavage by DPP-4 and contains two amino acid modifications, a substitution and an addition, together with a fatty acid side chain. Whether liraglutide is metabolized to a peptide with GLP-19–36–like activity remains unclear. The observation that liraglutide fails to induce the phosphorylation of Akt and GSK3β in the Glp1r-/- heart, together with the demonstration that liraglutide increased cAMP and reduced apoptosis in cardiomyocyte cultures in a GLP-1R–dependent manner, strongly implicates the known GLP-1R as a critical mediator of liraglutide action in the murine heart.
Drugs acting on the GLP-1R axis have been approved for the treatment of diabetes and have the added benefits of reducing appetite and body weight and, in some instances, blood pressure, leading to improvement of cardiovascular risk factors (43,44). Given the experimental data presented here and elsewhere (8,9,13,16), the possibility that these agents may have beneficial effects on cardiovascular outcomes of patients with diabetes, independent of improvements in blood pressure and blood glucose, requires further study.
Supplementary Material
Acknowledgments
These studies were supported in part by grants from the Heart and Stroke Foundation of Ontario (NA5926), CIHR Grant IRO 80668, and Novo Nordisk.
L.L.B. has served as a panel member and/or received a speaker's honorarium for symposia sponsored by Novo Nordisk, Merck Frosst, and GlaxoSmithKline. M.H. has served as a consultant to sanofi-aventis and Merck & Co. D.D. has served as an advisor or consultant to Amylin Pharmaceuticals, Arena, Arisaph Pharmaceuticals, ConjuChem, Eli Lilly, Emisphere Technologies, GlaxoSmithKline, Glenmark Pharmaceuticals, Hoffman LaRoche, Isis Pharmaceuticals, Mannkind, Merck Research Laboratories, Metabolex, Novartis Pharmaceuticals, Novo Nordisk, Phenomix, Takeda, and Transition Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Yellon DM, Dana A: The preconditioning phenomenon: a tool for the scientist or a clinical reality? Circ Res 87: 543– 550, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696– 1705, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M: Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117: 2340– 2350, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Vila Petroff MG, Egan JM, Wang X, Sollott SJ: Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res 89: 445– 452, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Barragan JM, Eng J, Rodriguez R, Blazquez E: Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol 277: E784– E791, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK: Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23: 2939– 2946, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK: Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110: 43– 52, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP: Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110: 955– 961, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM: Glucagon-like peptide-1 (GLP-1) can directly protect the heart against ischemia/reperfusion injury. Diabetes 54: 146– 151, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bose AK, Mocanu MM, Carr RD, Yellon DM: Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther 19: 9– 11, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bose AK, Mocanu MM, Carr RD, Yellon DM: Myocardial ischaemia–reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther 21: 253– 256, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP: Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther 312: 303– 308, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP: Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317: 1106– 1113, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP: Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109: 962– 965, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP: Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 12: 694– 699, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Sonne DP, Engstrom T, Treiman M: Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 146: 243– 249, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O: One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53: 1187– 1194, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M: Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50: 2530– 2539, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges JP, Verhoeven R, Buganova I, Madsbad S: Liraglutide, a long-acting human GLP-1 Analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes mellitus. Diabetes Care 6: 1608– 1610, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ohta K, Nakajima T, Cheah AY, Zaidi SH, Kaviani N, Dawood F, You XM, Liu P, Husain M, Rabinovitch M: Elafin-overexpressing mice have improved cardiac function after myocardial infarction. Am J Physiol Heart Circ Physiol 287: H286– H292, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Berr SS, Gilson WD, Toufektsian MC, French BA: Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast-enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation 109: 1161– 1167, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP: Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 115: 1398– 1407, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Li RK, Mickle DA, Weisel RD, Zhang J, Mohabeer MK: In vivo survival and function of transplanted rat cardiomyocytes. Circ Res 78: 283– 288, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Song W, Lu X, Feng Q: Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res 45: 595– 602, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Cook SA, Sugden PH, Clerk A: Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res 85: 940– 949, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Noyan-Ashraf MH, Wu L, Wang R, Juurlink BH: Dietary approaches to positively influence fetal determinants of adult health. FASEB J 20: 371– 373, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Tong H, Imahashi K, Steenbergen C, Murphy E: Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 90: 377– 379, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Zhou YQ, Foster FS, Nieman BJ, Davidson L, Chen XJ, Henkelman RM: Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics 18: 232– 244, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Zhou YQ, Zhu Y, Bishop J, Davidson L, Henkelman RM, Bruneau BG, Foster FS: Abnormal cardiac inflow patterns during postnatal development in a mouse model of Holt-Oram syndrome. Am J Physiol Heart Circ Physiol 289: H992– H1001, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su YD, Du XJ, Gao XM: Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physiol Heart Circ Physiol 294: H1815– 1822, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P: Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135– 1142, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS: Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med 197: 615– 624, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA: Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 89: 168– 173, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA: Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 94: 884– 891, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Heineke J, Molkentin JD: Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589– 600, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ: Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535– 1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG: Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J 20: 207– 216, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP: Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117: 3930– 3939, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN: Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9: 1173– 1179, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ: Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem 278: 471– 478, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nikolaidis LA, Elahi D, Shen YT, Shannon RP: Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289: H2401– 2408, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Kavianipour M, Ehlers MR, Malmberg K, Ronquist G, Ryden L, Wikstrom G, Gutniak M: Glucagon-like peptide-1 (7–36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides 24: 569– 578, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Inzucchi SE, McGuire DK: New drugs for the treatment of diabetes: part II: incretin-based therapy and beyond. Circulation 117: 574– 584, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Aulinger B, D'Alessio D: Glucagon-like peptide 1: continued advances, new targets and expanding promise as a model therapeutic. Curr Opin Endocrinol Diabetes Obes 14: 68– 73, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.