Abstract
OBJECTIVE
To investigate how insulin sensitivity and glucose metabolism differ in adipocytes between different fat depots of male and female mice and how sex steroids contribute to these differences.
RESEARCH DESIGN AND METHODS
Adipocytes from intra-abdominal/perigonadal (PG) and subcutaneous (SC) adipose tissue from normal, castrated, or steroid-implanted animals were isolated and analyzed for differences in insulin sensitivity and glucose metabolism.
RESULTS
Adipocytes from both PG and SC depots of females have increased lipogenic rates compared with those from males. In females, intra-abdominal PG adipocytes are more insulin-sensitive than SC adipocytes and more insulin-sensitive than male adipocytes from either depot. When stimulated by low physiological concentrations of insulin, female PG adipocytes show a robust increase in Akt and extracellular signal–related kinase (ERK) phosphorylation and lipogenesis, whereas male adipocytes show activation only at higher insulin concentrations. Adipocytes from females have higher mRNA/protein levels of several genes involved in glucose and lipid metabolism. After castration, adipocytes of male mice showed increased insulin sensitivity and increased lipogenic rates, whereas adipocytes of females demonstrate decreased lipid production. Increasing estrogen above physiological levels, however, also reduced lipid synthesis in females, whereas increasing dihydrotestosterone in males had no effect.
CONCLUSIONS
There are major sex differences in insulin sensitivity in adipose tissue, particularly in the intra-abdominal depot, that are regulated by physiological levels of sex steroids. The increased sensitivity to insulin and lipogenesis observed in adipocytes from females may account for their lower level of insulin resistance and diabetes risk despite similar or higher fat content than in males.
Obesity increases dramatically the probability of developing type 2 diabetes, dyslipidemia, cardiovascular disease, fatty liver, and even some types of cancer (1). This occurs in large part as a result of decreased sensitivity of tissues to insulin, that is, insulin resistance. Adipocytes in different fat depots appear to have a distinct impact in insulin sensitivity. Accumulation of visceral fat is linked to the development of these metabolic complications, whereas accumulation of subcutaneous fat is not (2–4).
Fat accumulation in different depots is also sexually dimorphic. In humans, men accumulate more visceral fat, whereas women accumulate more subcutaneous fat and have a higher percentage of body fat compared with men. There is also evidence indicating that insulin sensitivity differs between males and females (5,6). Thus, despite having lower fat mass, the prevalence of diabetes and early abnormalities of glucose metabolism is higher in men than in women (7). Women also have decreased susceptibility to fatty acid–induced peripheral insulin resistance (8). In different rodent models of glucose intolerance, insulin resistance, and diabetes, males show a stronger phenotype than females (9–11). These sex-related differences in insulin sensitivity and adipose tissue development and function could be attributable in part to actions of estrogen and testosterone. For example, decreases in estrogen and increases in testosterone levels that occur during menopause are associated with loss of subcutaneous and gain of visceral fat and increase in insulin resistance (12). However, how insulin action differs between males and females and how these differences account for a sex-specific regulation of adipose tissue development and function are largely unknown.
In this study, we have investigated how insulin sensitivity and glucose metabolism differ in adipocytes from visceral and subcutaneous depots between male and female mice and how sex steroids could contribute to these differences. We find sex-specific differences in insulin action in adipocytes that may contribute to the sexual dimorphism of insulin resistance and suggest the possibility of more effective sex-specific therapies for obesity, diabetes, and metabolic syndrome.
RESEARCH DESIGN AND METHODS
Animals and physiological measurements.
C57BL/6 mice obtained from Taconic Laboratories were used between 9 and 15 weeks of age. Mice (9 weeks old) were castrated, sham operated, or implanted with 1.5 mg dihydrotestosterone (males) or 0.1 mg 17β-estradiol (females) pellets in a slow (21 days) release form. Glucose tolerance tests and insulin tolerance tests were performed on 9- and 12-week-old mice, respectively. Leptin, adiponectin, free fatty acid (FFA), estradiol, and dihydrotestosterone (DHT) serum levels were determined using ELISA. All protocols for animal use and euthanasia were approved by the Animal Care Use Committee of the Joslin Diabetes Center in accordance with National Institutes of Health guidelines.
Adipocyte isolation and lipogenesis.
Adipocytes from perigonadal (PG) and flank inguinal subcutaneous (SC) adipose depots from each individual mouse were isolated by collagenase digestion (13). Lipogenesis assays were performed as described in the online appendix (available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1054/DC1). To assess cell number, aliquots of 200 μl adipocytes were fixed with osmic acid and counted in a Coulter counter (14).
Gene expression.
Total RNA was extracted from 100 μl of isolated adipocytes (Rneasy; QIAGEN). Reverse transcription was performed using 0.5 μg of RNA, and quantitative real-time PCR was performed using a fluorescent temperature cycler (ABI 7900) with SYBR Green.
Western blot.
Isolated adipocytes were treated with or without 0.1 or 10 nmol/l insulin for 5–30 min. Proteins were extracted from 200 μl of cell suspension homogenized in radioimmunoprecipitation assay buffer with 1% SDS. Thirty to 50 μg of protein was subjected to SDS-PAGE, transferred to polyvinylidine fluoride membranes, and blots were probed with the respective antibodies. The antigen-antibody complex was detected by using the HRP-coupled secondary antibodies and enhanced chemiluminescence.
Data are reported as means ± SEM. Comparisons for the glucose tolerance test, insulin tolerance test, and lipogenesis experiments were made using repeated-measures ANOVA and the rest of the comparisons were made using Student's t test; P < 0.05 was considered significant.
RESULTS
Female mice and female adipocytes are more insulin-sensitive than males.
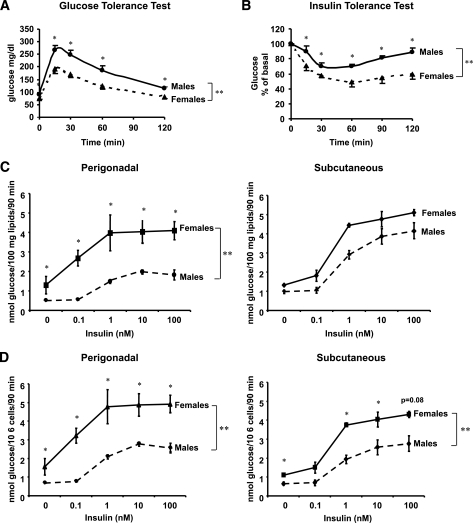
Previous studies have suggested that female humans and rodents are more insulin-sensitive than their male counterparts. When C57BL/6 mice were subjected to intraperitoneal glucose tolerance tests, female mice had lower glucose levels than male mice at every time point (P < 0.05) (Fig. 1A), suggesting increased insulin sensitivity. This was confirmed by insulin tolerance tests, which showed a greater fall in blood glucose in response to insulin in female as compared with male mice (Fig. 1B).
FIG. 1.
Female mice and female adipocytes have increased insulin sensitivity. Glucose tolerance test was performed after an overnight fast. Mice received an intraperitoneal injection of 2 g/kg body wt glucose. Blood glucose was measured from tail vein samples at the indicated times (A). Insulin tolerance test was performed on random-fed mice. Animals received injections of 1 unit/kg body wt insulin. Blood glucose was measured at the indicated time points (B). Data points are means ± SE with eight mice in each group. Adipocytes from PG and SC fat were isolated from male and female mice and stimulated with or without insulin in the presence of 14C-glucose for 90 min as described in research design and methods. Radioactive glucose incorporated into lipids was measured for each condition in the extracted lipids and normalized to total lipid content (C) or to cell number (D) as determined by counting of osmic acid fixed cells, n = 3. **P < 0.01 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons.
To analyze how glucose and lipid metabolism differ between male and female adipocytes, we measured the ability of isolated adipocytes to synthesize lipids through de novo lipogenesis. Under both basal and insulin-stimulated conditions, female adipocytes from PG and inguinal SC depots showed increased lipid synthesis as compared with male adipocytes whether expressed by lipid content (PG, P < 0.0001) (Fig. 1C) or by cell number (PG, P < 0.001; SC, P < 0.01) (Fig. 1D). The difference between sexes for PG fat was 263% (P < 0.005) in the basal state and 225% at maximal insulin concentration (P < 0.001). Female adipocytes from the PG depot also showed increased insulin sensitivity when compared with male adipocytes from the same depot. The insulin concentration causing a half maximal effect (EC50) on lipogenesis in female adipocytes from the PG depot was 0.19 ± 0.12 nmol/l, whereas in male adipocytes, it was 3.5 times higher, 0.67 ± 0.11 nmol/l (P = 0.001). Subcutaneous adipocytes from males and females showed a smaller difference in basal and insulin-stimulated effect than the PG fat that was statistically significant when normalized by cell number (P < 0.01); however, no difference in insulin sensitivity was observed between male and female adipocytes from the SC depot (Fig. 1C–D).
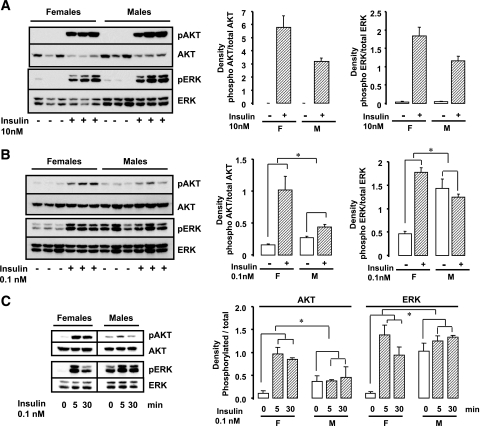
Adipocytes from female mice have increased insulin stimulation of Akt and extracellular signal–related kinase.
To determine whether the increased insulin sensitivity in female adipocytes observed in lipogenesis was a result of increased insulin signaling, we assessed phosphorylation/activation of two insulin downstream targets, Akt and extracellular signal–related kinase (ERK), with two different doses of insulin: a low physiological dose (0.1 nmol/l) and a pharmacological dose (10 nmol/l). This revealed that high insulin concentrations for 5 min induced a similar level of Akt and ERK phosphorylation in both female and male adipocytes (Fig. 2A). By contrast, stimulation with the low insulin concentration for 5 or 30 min (Fig. 2B and C) induced a robust increase in Akt and ERK phosphorylation only in female adipocytes (7.1- to 11.1-fold for Akt and 3.9- to 13.9-fold for ERK) (Fig. 2B and C), whereas the same physiological concentration of insulin induced only a very modest effect (1.2- to 1.62-fold for Akt and 0.9- to 1.3-fold for ERK) (Fig. 2B and C) or no activation in male adipocytes (P < 0.05 for both Akt and ERK).
FIG. 2.
Increased insulin-stimulated Akt and ERK activation in female adipocytes. Adipocytes from PG fat isolated from male and female mice were treated with or without 10 nmol/l (A) or 0.1 nmol/l (B and C) insulin for 5 (A–C) or 30 min (C). Cell lysates were run on SDS-PAGE and subjected to Western blot analysis using antibodies directed against phosphorylated (pAkt Ser473, pERK Thr202/Tyr204) or total Akt and ERK. Each lane represents a pool of adipocytes from three to four mice. The data were also quantified by densitometric scanning of the autoradiographs (n = 2). *P < 0.05-fold stimulation females versus males.
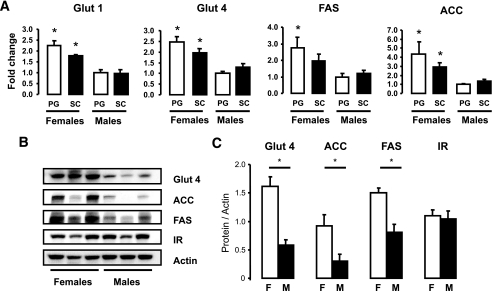
Female adipocytes show higher expression of glucose and lipid metabolism genes.
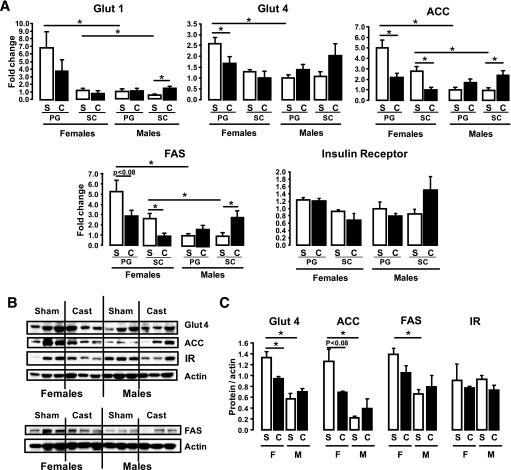
To determine whether differences in expression could explain the increased lipogenic activity and the increased insulin signaling in female adipocytes, we analyzed the mRNA expression patterns of genes involved in insulin signaling and in glucose and lipid metabolism using quantitative RT-PCR. We observed that adipocytes from both depots from female mice had higher mRNA levels of the glucose transporters (GLUT1, GLUT4) and the key lipogenic enzymes fatty acid synthase (FAS) and acetyl CoA carboxylase (ACC) than male adipocytes from the same depots (Fig. 3A). ACC mRNA showed a 4.3- and 2.1-fold difference between male and female cells for PG and SC fat (P < 0.05). Likewise, FAS mRNA was 2.7-fold higher in both depots in female versus male adipocytes (P < 0.05). GLUT4 was 2.4-fold (PG) and 1.5-fold (SC) higher in females, and GLUT1 was 2.4-fold (PG) and 1.8-fold (SC) higher in female adipocytes (P < 0.05). mRNA levels for each of these genes tended to be higher in female PG adipocytes than in SC adipocytes such that the female PG depot had the highest levels of expression of these genes among all depots compared. In males, the expression levels were similar between the two depots.
FIG. 3.
Female adipocytes have increased mRNA protein expression of lipid and glucose metabolism genes. Adipocytes from PG and SC fat isolated from male and female mice were lysed by homogenization using a denaturing guanidine-thiocyanate–containing buffer, mRNA was extracted, and quantitative real-time PCR was performed (A). Gene expression for GLUT1, GLUT4, FAS, and ACC mRNAs were normalized against the expression of TATA-binding protein. B: Proteins from similar adipocyte isolates were extracted and subjected to SDS-PAGE and Western blotted with antibodies to each of these proteins. Actin was used as a control for protein loading. Each lane represents a pool of adipocytes from three to four mice. C: The data were quantified by densitometry of immunoblots (n = 3) and normalized against actin. *P < 0.05.
These differences in mRNA were paralleled by differences in protein expression as determined by Western blot analysis. Thus, protein levels of GLUT4, ACC, and FAS were higher in female than in male adipocytes by two- to threefold (P < 0.05). By contrast, the insulin receptor and actin levels were not different (Fig. 3B and C). Thus, the greater level of lipid synthesis in female adipocytes compared with male adipocytes is attributable, at least in part, to increased glucose transporters and lipogenic enzyme levels.
Castration reverses lipogenic rates and gene expression.
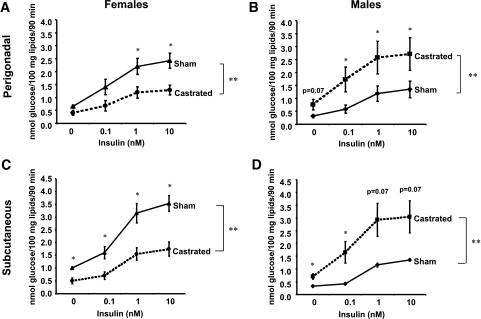
To determine the role of estrogen and testosterone on the sexual dimorphism of lipogenesis and insulin sensitivity, we analyzed the lipogenic capacity of fat cells taken from mice 6 weeks after castration or sham surgery. Adipocytes from castrated females had a decrease in the lipogenic rate (PG, P < 0.01; SC, P < 0.005) and in insulin sensitivity (only in PG depot) when compared with adipocytes from the sham controls, more closely resembling those of male adipocytes. PG adipocytes from castrated females had 47% lower rates of maximal lipogenesis (P < 0.05) compared with the control females. The half maximal response occurred at 0.4 nmol/l insulin compared with 0.2 nmol/l in the control females, although this difference was not statistically significant. In SC adipocytes from castrated females, both the basal and the maximal lipogenesis stimulated by insulin was reduced by 50% compared with control females (P < 0.05), whereas the half maximal response did not change and occurred at 0.5 nmol/l of insulin for both castrated and control animals (Fig. 4A and C).
FIG. 4.
Castration reverses lipogenic rates in male and female adipocytes. Male and female mice were castrated or sham operated. Six weeks after surgery, adipocytes from PG (A and B) and SC (C and D) fat were isolated and stimulated with or without insulin in the presence of 14C-glucose for 90 min. Radioactivity was measured in the extracted lipids and normalized by total lipid content. n = 6 animals per group. **P < 0.05 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons sham versus castrated.
Adipocytes from castrated male mice, on the other hand, showed increased lipogenic capacity and increased insulin sensitivity when compared with controls (Fig. 4B and D), similar to adipocytes from normal female mice. These effects were observed in both the PG and the SC depots. For male adipocytes, the half maximal lipogenesis from PG and SC depots occurred at 0.5 and 0.6 nmol/l insulin in controls, whereas in the cells from castrated males, the half maximal response occurred at 0.05 nmol/l (P < 0.02) and 0.3 nmol/l (not significant) insulin, respectively. Basal lipogenesis was increased 250% (P < 0.05) in PG cells and 210% (P < 0.05) in SC cells from castrated animals compared with controls, whereas the maximal lipogenesis was increased 200% in PG (P < 0.05) and 225% (not significant) in SC cells from castrated males compared with controls.
Quantitative RT-PCR analysis revealed that castration also caused a decreased gene expression of GLUT1, GLUT4, FAS, and ACC in female adipocytes from both depots when compared with adipocytes from sham-operated mice, whereas in male adipocytes, the expression of these genes increased in both depots after castration (Fig. 5A). Comparing adipocytes of the PG depot from castrated females with those from sham controls, GLUT4, ACC, FAS, and GLUT1 expression were reduced 35% (P < 0.05), 55% (P < 0.05), 45% (P < 0.05), and 44% (not significant). Likewise, in the SC depot from females, expression of the lipogenic genes ACC and FAS was dramatically reduced, whereas the expression of the glucose transporters 1 and 4 did not change after castration. In the case of the males, PG adipocytes showed a trend to increased expression of GLUT4, ACC, and FAS after castration; however, these changes were not statistically significant. On the other hand, castration in male mice resulted in a 250–300% increase in GLUT1, FAS, and ACC mRNA (P < 0.05) in SC adipocytes (Fig. 5A). Expression of the insulin receptor itself did not change in any of the depots in males nor females after castration (Fig. 5A). Western blot analysis of PG adipocytes from males and females after castration or sham surgery showed changes in agreement with the mRNA levels (Fig. 5B and C).
FIG. 5.
Castration reverses gene expression patterns in male and female adipocytes. Male and female mice were castrated (C) or sham operated (S). Six weeks after surgery, adipocytes from PG and SC fat were isolated, mRNA was extracted, and quantitative real-time PCR was performed (A). Gene expression for metabolic genes was assessed and normalized against the expression of TATA-binding protein. B: Proteins from similar cells were subjected to SDS-PAGE and Western blot with the respective antibodies. Actin was used as a control for protein loading. C: Quantitation of the proteins was achieved by densitometry normalized by the values for actin. *P < 0.05. IR, insulin receptor.
Effect of castration on body weight, adipose tissue weight, adipocyte size, and adipokine levels.
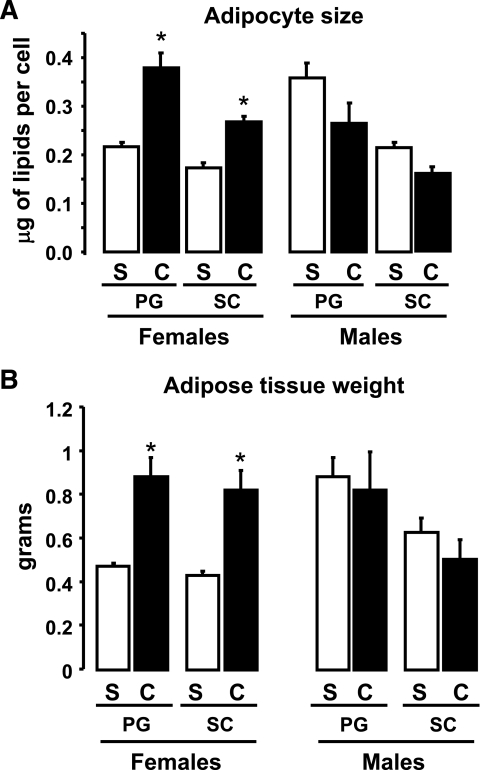
Castration is known to have effects on body weight (15,16). In these experiments, castration was performed at 9 weeks and the studies done 6 weeks later at 15 weeks of age. At this time, the average weight of the castrated males was 9.8% lower than the sham-operated control males (29.2 ± 2.0 g vs. 26.5 ± 2.9 g; not significant). On the other hand, the average weight of the castrated females was 14.5% higher than the control females (23.4 ± 1.0 g vs. 26.8 ± 2.5 g; P < 0.05). Regarding cell size, we observed that PG adipocytes from male mice were ∼60% larger than adipocytes from the analogous depot in females (P < 0.05). SC adipocytes from males were only 20% bigger than SC adipocytes from females. In females, PG adipocytes were 20% bigger than SC adipocytes, whereas in males, PG adipocytes were 60% bigger than SC adipocytes (P < 0.05) (Fig. 6A). After castration, adipocytes in female PG and SC depots increased in size by 70 and 50%, respectively (P < 0.05). In males, castration did not affect adipocyte size significantly, although there was a trend to decreased cell size in both depots (Fig. 6A).
FIG. 6.
Effect of castration on adipocyte size and adipose tissue weight. Male and female mice were castrated (C) or sham operated (S). Six weeks after surgery, total body weights and adipose tissue weights from PG and SC fat were assessed, and adipocytes were isolated as described in research design and methods. Mean cell size was calculated by dividing the total lipid content by the total number of cells on a sample. Graphs show the mean adipocyte size (A) or adipose tissue weight (B) from six animals per group. *P < 0.05.
Similar results were observed in adipose tissue weight. Male PG adipose tissue was double the size of female PG adipose tissue (0.88 ± 0.17 g vs. 0.47 ± 0.03 g, respectively; P < 0.05). In contrast, male SC adipose tissue was only 25% bigger than female SC adipose tissue (0.63 ± 0.13 g vs. 0.43 ± 0.04 g). In males, the PG fat pad was 33% heavier than the SC inguinal fat pad (P < 0.05), whereas in females, these two fat depots were of almost identical size (Fig. 6B). Castration resulted in a doubling of the size of both PG and SC fat pads in females (P < 0.05), whereas in males, there was no significant change in either depot weight after castration (Fig. 6B).
Factors that can modulate insulin sensitivity include the adipokines leptin and adiponectin. Serum adiponectin levels were increased in both males and females after castration compared with control mice (P < 0.05) with no significant difference between males and females (supplemental Fig. 1A, available in the online appendix). Leptin levels tended to be higher in females than in males and tended to increase after castration in both males and females, but these differences were not statistically significant (supplemental Fig. 1B of the online appendix).
Effect of different steroid levels on adipocyte lipogenesis in males and females.
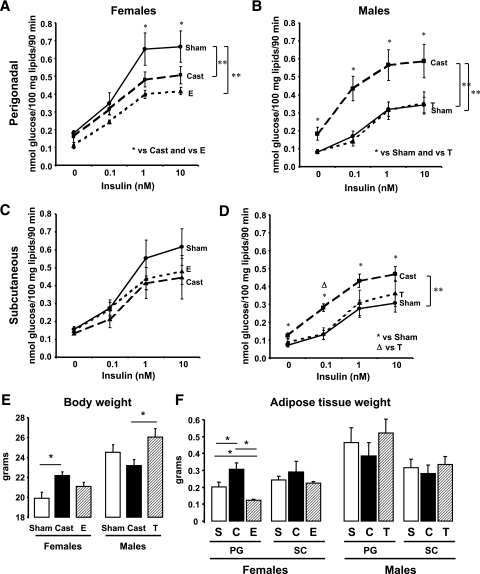
To assess more directly the effect of sex steroids on insulin sensitivity, we measured lipogenic rates on adipocytes from castrated female and male mice (with very low levels of estrogen or testosterone), control mice (with normal levels of sex steroids) and normal mice implanted for 3 weeks with estrogen or 5α-DHT (the most active metabolite of testosterone) pellets. As expected, estradiol and DHT levels were dramatically reduced in castrated animals and increased in implanted ones (supplemental Fig. 2 of the online appendix). To minimize the effects of body weight change after castration, we performed the lipogenesis assays only 3 weeks after the surgery.
In females, estrogen treatment caused a significant decrease in the lipogenic rates in adipocytes from the PG depot (P < 0.005) (Fig. 7A). This was manifest by a 38% decrease in insulin-stimulated lipogenesis in PG adipocytes from estrogen-treated females compared with those from controls (P < 0.05). However, the half maximal response occurred at ∼0.2 nmol/l insulin, similar to the controls, showing that estrogen treatment does not alter insulin sensitivity. Conversely, short-term castration decreased lipogenic rates in the PG adipocytes (P < 0.01) as seen in the previous experiments. Interestingly, short-term castration did not alter insulin sensitivity in PG adipocytes from females, because half maximal lipogenesis was similar to those from controls, that is, 0.2 nmol/l insulin (Fig. 7A). In female SC adipocytes, on the other hand, there was no significant change in the lipogenic rates at the high insulin concentrations after castration or estrogen treatment (Fig. 7C). Although castrated females increased their body weight by ∼12% (P < 0.05) (Fig. 7E), only the PG depot increased in weight (∼50%, P < 0.05), whereas the size of the SC depot did not change (Fig. 7F). Likewise, females implanted with estrogen pellets showed a significant decrease in weight in the PG fat pad (∼35%, P < 0.05) but had no change in weight in the SC depot compared with the controls or in total body weight.
FIG. 7.
Effect of short-term castration and steroid treatment on lipogenesis, body weight, and adipose tissue weight in males versus females. Male and female mice were castrated, implanted with 17β-estradiol or 5α-DHT pellets, or sham operated. Three weeks after surgery, adipocytes from PG (A and B) and SC (C and D) fat were isolated and stimulated with or without insulin in the presence of 14C-glucose for 90 min. Radioactivity was measured in the extracted lipids and normalized by total lipid content. E and F: Total body weight (E) and adipose tissue weights (F) from PG and SC fat from sham-operated (sham or S) castrated (cast or C) implanted with estrogen-containing pellets (E) or DHT-containing pellets (T) were assessed. The graphs show the mean from eight animals per group. **P < 0.05 by repeated-measures ANOVA. *P < 0.05 by Student's t test for point-to-point comparisons.
In males, short-term castration caused the same effect as the 6-week castration with increased lipogenic rates and increased insulin sensitivity in both depots. In the PG depot, basal and maximal lipogenesis were increased 230 and 171% (P < 0.05), and in the SC depot, these rates increased by 175 and 153% when compared with controls (P < 0.05). Insulin sensitivity also increased in adipocytes from castrated males compared with controls. Half maximal lipogenesis in both depots occurred ∼0.1 nmol/l of insulin in adipocytes from castrated males, similar to control female PG fat, whereas in noncastrated controls, half maximal stimulation was at 0.4 (PG; P < 0.001) and 0.5 (SC; P < 0.005) nM insulin (Fig. 7B and D). DHT treatment did not cause any change in lipogenic rates or insulin sensitivity in either adipose depot. DHT did cause a nonsignificant 6% increase in body weight in males with no changes in the adipose tissue weight (Fig. 7E and F).
Effect of sex steroid levels on adiponectin, leptin, and free fatty acids.
Changes in the level of sex steroids had variable effects on levels of circulating adipokines. Adiponectin levels tended to increase in castrated females and were decreased by 60% in females implanted with estradiol (P < 0.05). In castrated males, adiponectin levels were increased (P < 0.05), and there was no change in DHT-implanted males. Adiponectin levels were similar between males and females. Leptin levels were not changed by any of the treatments in males or females, and the levels tended to be higher in females, but this difference was not statistically significant. Free fatty acids were not different between males and females and did not change with any of the treatments (supplemental Fig. 3 of the online appendix).
DISCUSSION
Adipose tissue plays a major role in the regulation of glucose homeostasis and insulin sensitivity. Interestingly, fat accumulation is sexually dimorphic. Females have a higher percentage of body fat and tend to accumulate more SC fat than males, whereas males have a lower percentage of body fat and accumulate more visceral fat. Despite the higher level of body fat, female humans and rodents are more insulin sensitive than males. Thus, women have improved glucose tolerance and increased insulin sensitivity compared with men (17,18) and are more resistant to fatty acid–induced insulin resistance (8,19,20). Prevalence of early abnormalities of glucose metabolism is three times higher in men compared with women (7). Likewise, female mice are less prone to diet-induced insulin resistance (10,21,22), and many genetically induced forms of insulin resistance have a milder phenotype in females compared with males (11,21,22). Indeed, we find that the increased insulin sensitivity of female versus male mice can be detected on simple intraperitoneal glucose and insulin tolerance tests.
In the present study, we have further analyzed sex differences in adipocyte glucose and lipid metabolism as they relate to differences in insulin sensitivity at the cellular level. We find that adipocytes of female mice have increased lipogenic capacity compared with adipocytes from male mice and are also more insulin sensitive, especially those from the intra-abdominal (PG) depot, which are more insulin sensitive than female adipocytes from the SC depot and male adipocytes from both depots. This increased lipogenic capacity in female adipocytes is the result of at least two factors. One is enhanced insulin sensitivity at intermediate steps in the insulin signaling network such as stimulation of Akt and ERK. Thus, PG adipocytes from female mice show increased phosphorylation/activation of Akt at lower doses of insulin than male adipocytes, and Akt has been shown to be a key intermediate in insulin stimulation of glucose transport (23). The other factor is increased expression of glucose and lipid metabolism genes such as GLUT1, GLUT4, FAS, and ACC. Interestingly, despite the increased lipogenic rates, female adipocytes are smaller than male adipocytes, especially those from the PG depot, consistent with studies showing that females adipocytes have increased lipolytic rates compared with those from males (24,25). As a result, in humans, females have higher FFA serum levels than males (20) but appear to be protected against insulin resistance induced by elevated FFA (8). However, in mice, we did not detect any significant differences in the FFA serum levels between males and females.
Our results, as well as previous studies (26), demonstrate that female adipocytes have increased insulin sensitivity compared with male adipocytes. This is particularly true for female PG adipocytes, which have a lower EC50 (0.1–0.2 nmol/l insulin) for insulin stimulation of lipogenesis than female SC adipocytes (0.5–0.6 nmol/l) and male adipocytes from either depot (0.4–0.7 nmol/l). Female PG adipocytes have a higher lipogenic rate than male adipocytes. Because PG fat in females also has a higher lipolytic capacity than in males (25), this would suggest a higher metabolic turnover of PG fat in females leading to decreased fat accumulation in visceral depots in females compared with males.
Sex steroids are known to play a role in the regulation of adipose tissue development and function as well as whole-body insulin sensitivity. Ovariectomy reduced lipogenic capacity in female adipocytes from both PG and SC depots and reduced insulin sensitivity in female PG adipocytes, indicating a positive role of estrogen in insulin sensitivity and lipogenesis in females. The exact mechanism of this effect is confounded by the fact that castration increases food intake and fat mass in females, and this could contribute indirectly to a reduction in insulin sensitivity.
To differentiate the direct hormonal effects from the indirect effects on body weight, we shortened the period after castration before analysis to 3 weeks. Under these conditions, weight gain was reduced, and there was no effect of ovariectomy on insulin sensitivity and a reduced effect on lipogenesis, suggesting that body weight is a factor affecting adipocyte insulin sensitivity, although estrogen deficiency may also play a role. Indeed, short-term lack of estrogen after ovariectomy increased PG depot weight and estrogen pellet implantation in normal mice decreased PG depot weight as compared with controls. Interestingly, both of these manipulations affected the SC adipocytes in a similar fashion, whereas fat pad weight, lipogenic rate, and insulin sensitivity remained the same. Thus, an increase in circulating estrogen above the physiological levels preferentially alters PG adipose tissue metabolism, reducing lipogenic rates and depot size. These findings are in agreement with previous reports (27) and consistent with evidence that white adipocytes in different fat depots have fundamentally different properties and may have different developmental lineage (28). Which estrogen receptor (ER) is mediating these effects is unclear. In females, ERα expression is similar between depots, whereas we and others found that the levels of ERβ are higher in the SC depot (data not shown) (29). Studies with knockout mice of ERα or ERβ suggest that the effects of estrogen in the adipose tissue are mediated mainly through ERα (30–32). The preferential effect of estrogen on adipocyte metabolism in the PG depot in females might also involve differences in coactivators such as the steroid receptor coactivator p160 family of proteins (33).
In male mice, androgen receptor expression is higher in PG than SC fat (data not shown). Both short-term and long-term castration in males resulted in increased insulin sensitivity and increased lipogenesis in both PG and SC depots. This was independent of changes in the adipose tissue weight. Thus, in contrast to the preferential effect of estrogen in females on the PG depot, testosterone exerts an inhibitory effect on lipogenesis and insulin sensitivity in both PG and SC adipocytes. These results are the opposite of what happens in the androgen receptor knockout mice, which develop late-onset obesity with reduced energy expenditure (34). This difference in phenotypes might be explained by the fact that obesity is observed in the ARKO mice only in old mice (30 weeks old), suggesting that factors related to aging are necessary for the development of obesity in this context.
Adiponectin levels cannot explain the changes in insulin sensitivity in females. Adiponectin levels were increased in castrated females, but insulin sensitivity was decreased in the adipocytes. In females implanted with estradiol, adiponectin levels were decreased, but this was not reflected in lower insulin sensitivity. In males, adiponectin levels did increase after castration, which does correlate with increased insulin sensitivity.
In summary, our results demonstrate an important role for sex steroids and sex in modulating adipose mass and insulin sensitivity. Thus, female adipocytes have increased lipid synthesis compared with male adipocytes in both the PG and SC depots, and in females, PG adipocytes also have improved insulin sensitivity to lipogenesis and to insulin signaling. These contribute to whole-body insulin sensitivity, allowing female mice to remain insulin sensitive despite similar or higher fat mass.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health (DK31036, DK33201, and D55545 to C.R.K.). Y.M. was supported by the Pew Latin American Fellows Program in the Biomedical Sciences and by the mentor-based American Diabetes Association award.
No potential conflicts of interest relevant to this article were reported.
We thank S. Gesta and B. Emanuelli for technical assistance and critical discussion of the work, O. Peroni for her assistance with the Coulter Counter, M. Rourk for his expertise in animal care, and 5P30 DK 36836 Specialized Assay Core at the Joslin Diabetes Center for leptin, adiponectin, and FFA measurements.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Lean ME: Pathophysiology of obesity. Proc Nutr Soc 59: 331– 336, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Mauriege P, Despres JP, Moorjani S, Prud'Homme D, Lamarche B, Bouchard C, Nadeau A, Tremblay A, Lupien PJ: Abdominal and femoral adipose tissue lipolysis and cardiovascular disease risk factors in men. Eur J Clin Invest 23: 729– 740, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF: The association of body fat distribution with hypertension, hypertensive heart disease, coronary heart disease, diabetes and cardiovascular risk factors in men and women aged 18–79 years. J Chronic Dis 40: 421– 428, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Kissebah AH, Krakower GR: Regional adiposity and morbidity. PMID 74: 761– 811, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Nuutila P, Knuuti MJ, Maki M, Laine H, Ruotsalainen U, Teras M, Haaparanta M, Solin O, Yki-Jarvinen H: Gender and insulin sensitivity in the heart and in skeletal muscles: studies using positron emission tomography. Diabetes 44: 31– 36, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Mittendorfer B: Insulin resistance: sex matters. Curr Opin Clin Nutr Metab Care 8: 367– 372, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kuhl J, Hilding A, Ostenson CG, Grill V, Efendic S, Bavenholm P: Characterisation of subjects with early abnormalities of glucose tolerance in the Stockholm Diabetes Prevention Programme: the impact of sex and type 2 diabetes heredity. Diabetologia 48: 35– 40, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT: Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes 50: 1344– 1350, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Clark JB, Palmer CJ, Shaw WN: The diabetic Zucker fatty rat. Proc Soc Exp Biol Med 173: 68– 75, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Zierath JR, Houseknecht KL, Gnudi L, Kahn BB: High fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 46: 215– 223, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK: Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest 106: 523– 531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM: Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev 27: 575– 605, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR: Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 103: 6676– 6681, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman SW, Salans LB: Determinations of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res 19: 269– 273, 1978 [PubMed] [Google Scholar]
- 15.Pallier E, Aubert R, Lemonnier D: Effect of diet and ovariectomy on adipose tissue cellularity in mice. Reprod Nutr Dev 20: 631– 636, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Wade GN, Gray JM: Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav 22: 583– 593, 1979 [DOI] [PubMed] [Google Scholar]
- 17.Boyns DR, Crossley JN, Abrams ME, Jarrett RJ, Keen H: Oral glucose tolerance and related factors in a normal population sample. I. Blood sugar, plasma insulin, glyceride, and cholesterol measurements and the effects of age and sex. BMJ 1: 595– 598, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yki-Jarvinen H: Sex and insulin sensitivity. Metabolism 33: 1011– 1015, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Hevener A, Reichart D, Janez A, Olefsky J: Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes 51: 1907– 1912, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Soeters MR, Sauerwein HP, Groener JE, Aerts JM, Ackermans MT, Glatz JF, Fliers E, Serlie MJ: Gender-related differences in the metabolic response to fasting. J Clin Endocrinol Metab 92: 3646– 3652, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE: Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 148: 231– 241, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Trevaskis JL, Meyer EA, Galgani JE, Butler AA: Counterintuitive effects of double-heterozygous null melanocortin-4 receptor and leptin genes on diet-induced obesity and insulin resistance in C57BL/6J mice. Endocrinology 149: 174– 184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saltiel AR, Kahn CR: Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799– 806, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Mittendorfer B, Horowitz JF, Klein S: Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab 281: E1333– E1339, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pujol E, Rodriguez-Cuenca S, Frontera M, Justo R, Llado I, Kraemer FB, Gianotti M, Roca P: Gender- and site-related effects on lipolytic capacity of rat white adipose tissue. Cell Mol Life Sci 60: 1982– 1989, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerre-Millo M, Leturque A, Girard J, Lavau M: Increased insulin sensitivity and responsiveness of glucose metabolism in adipocytes from female versus male rats. J Clin Invest 76: 109– 116, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS: Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983– 35991, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Gesta S, Tseng YH, Kahn CR: Developmental origin of fat: tracking obesity to its source. Cell 131: 242– 256, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mayes JS, Watson GH: Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5: 197– 216, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS: Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A 97: 12729– 12734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME: Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ Res 89: 534– 539, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Jaubert AM, Pecquery R, Dieudonne MN, Giudicelli Y: Estrogen binding sites in hamster white adipose tissue: sex- and site-related variations; modulation by testosterone. Gen Comp Endocrinol 100: 179– 187, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Louet JF, Coste A, Amazit L, Tannour-Louet M, Wu RC, Tsai SY, Tsai MJ, Auwerx J, O'Malley BW: Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci U S A 103: 17868– 17873, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H: Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 54: 1000– 1008, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.