Abstract
OBJECTIVE
Ciliary neurotrophic factor (CNTF) reverses muscle insulin resistance by increasing fatty acid oxidation through gp130-LIF receptor signaling to the AMP-activated protein kinase (AMPK). CNTF also increases Akt signaling in neurons and adipocytes. Because both Akt and AMPK regulate glucose uptake, we investigated muscle glucose uptake in response to CNTF signaling in lean and obese mice.
RESEARCH DESIGN AND METHODS
Mice were injected intraperitoneally with saline or CNTF, and blood glucose was monitored. The effects of CNTF on skeletal muscle glucose uptake and AMPK/Akt signaling were investigated in incubated soleus and extensor digitorum longus (EDL) muscles from muscle-specific AMPKα2 kinase-dead, gp130ΔSTAT, and lean and obese ob/ob and high-fat–fed mice. The effect of C2-ceramide on glucose uptake and gp130 signaling was also examined.
RESULTS
CNTF reduced blood glucose and increased glucose uptake in isolated muscles in a time- and dose-dependent manner with maximal effects after 30 min with 100 ng/ml. CNTF increased Akt-S473 phosphorylation in soleus and EDL; however, AMPK-T172 phosphorylation was only increased in soleus. Incubation of muscles from AMPK kinase dead (KD) and wild-type littermates with the PI3-kinase inhibitor LY-294002 demonstrated that PI3-kinase, but not AMPK, was essential for CNTF-stimulated glucose uptake. CNTF-stimulated glucose uptake and Akt phosphorylation were substantially reduced in obesity (high-fat diet and ob/ob) despite normal induction of gp130/AMPK signaling—effects also observed when treating myotubes with C2-ceramide.
CONCLUSIONS
CNTF acutely increases muscle glucose uptake by a mechanism involving the PI3-kinase/Akt pathway that does not require AMPK. CNTF-stimulated glucose uptake is impaired in obesity-induced insulin resistance and by ceramide.
Skeletal muscle glucose uptake is regulated by both intrinsic and circulating factors involving the phosphatidylinositol (PI3)-kinase/Akt and the AMP-activated protein kinase (AMPK) signaling pathways (1). The regulation of muscle glucose uptake by both pathways converges on AS160 (TBC1D4) and TBC1D1 where phosphorylation inhibits the negative regulation of GLUT4 vesicle translocation (2–4) to the plasma membrane (5,6). In insulin-resistant skeletal muscle, reduced insulin receptor substrate (IRS) and Akt activation results in reduced skeletal muscle glucose uptake (7,8). In contrast, activation of AMPK by endogenous circulating factors, including adiponectin (9,10) and interleuken (IL)-6 (11–13), and by pharmacological agents such as 5-aminoimidazole-4-carboxamide riboside (AICAR) (14,15) increases muscle glucose uptake, and this response is maintained in muscle from diabetic rodents and humans (16–19).
We have recently shown that the ciliary neurotrophic factor (CNTF), a member of the IL-6 family, also activates muscle AMPK and increases lipid oxidation (20). CNTF elicits intracellular signaling pathways by ligand binding to the CNTF receptor α (CNTFRα), which initiates heterodimerization and activation of gp130 and leukemia inhibitory factor (LIF) receptor, the two transmembrane β subunits of the receptor complex (21). This differs from the initial step in IL-6 signaling, which induces homodimerization of two gp130 subunits and does not involve LIF (22). Formation of the CNTFRα-gp130-LIF complex leads to association with isoforms of janus kinases, and these subsequently phosphorylate specific tyrosine residues on the intracellular domains, creating docking sites for Src homology–containing tyrosine phosphatase 2 (SH2)-containing proteins (23,24). Phosphorylation of Y757 of the intracellular domain of the β subunit leads to activation of the PI3-kinase/Akt pathway. Activation of the PI3-kinase by gp130-LIF relies on association of the p85 subunit with the regulatory SH2 domain–containing adapter molecule GAB1 coordinated by SHP2 (25), and the assembling of the complex does not involve IRS-1 (26). Phosphorylation of four more distal tyrosine residues leads to activation of the signal transducer and activator of transcription (STAT) 1 and 3 (23). This distal domain is responsible for AMPK activation by an adenosine nucleotide-dependent mechanism (27). Because both AMPK and PI3 kinase/Akt signaling are enhanced by activation of the gp130-LIF receptor, our first aim was to test whether CNTF increases glucose uptake in skeletal muscle and the signaling pathway(s) invoked.
The etiology of impaired muscle insulin signaling with obesity is multifactorial and appears to be related to at least two major events: chronic low-grade inflammation and intramyocellular lipid accumulation (7,28). Both events activate inhibitory IRS-1 kinases such as IκB kinase-β (29,30), c-jun terminal amino kinase (28), and protein kinase C θ (31), which impair insulin signaling by inhibiting IRS-1 association with PI3-kinase subunits. Although this is believed to be a significant element in muscle insulin resistance, the sphingolipid ceramide also impairs insulin signaling by promoting protein phosphatase 2A–dependent Akt dephosphorylation, thereby inhibiting Akt activation (32,33). Thus, because gp130-LIF signaling to glucose uptake would be expected to be independent of IRS-1, our second aim was to determine whether gp130-LIF signaling toward glucose uptake is maintained in mice with obesity-induced insulin resistance. Analysis of the molecular pathways of CNTF signaling to glucose uptake may provide important insights into the function of gp130-LIF signaling and reveal the potential of a novel therapeutic target for type 2 diabetes.
RESEARCH DESIGN AND METHODS
Animals.
C57Bl6/J, ob/ob, AMPKα2 kinase-dead (KD) and gp130ΔSTAT mice aged 8 to 18 weeks with corresponding littermate controls were used in experiments. Transgenic mice overexpressing a KD form of the AMPKα2 protein under control of the muscle creatine kinase promoter have been described previously (34). Briefly, the KD mutation was generated by Lys45Arg mutagenesis to encode a KD α2 protein that displaces all detectable endogenous α2 and 50–70% of endogenous α1 protein from αβγ heterotrimer complexes (34,35). The knockin mutant gp130ΔSTAT mice express a COOH-terminal truncation mutation of gp130 that prevents activation of STAT1/3 as described previously (36). This mutation has a deletion of the distal intracellular domain responsible for AMPK and STAT activation. All mice were kept at a 12:12 h light:dark cycle at 20–21°C and provided ad libitum access to food and water. Mice were maintained on a standard rodent chow diet/low-fat diet (5% calories fat; Harlan Teklad) except in high-fat diet (HFD) experiments, in which the diet was composed of 45% calories from fat (SF04–027; Specialty Feeds). All procedures were approved by the St. Vincent's Hospital Animal Ethics Committee.
CNTF tolerance test.
C57Bl6/J mice were fasted for 6 h before being injected with either saline or 0.3 mg/kg CNTF, a concentration of CNTF that activates AMPK and induces weight loss without eliciting an inflammatory response (20). Blood glucose was monitored over 150 min.
Muscle incubations.
Soleus (oxidative and glycolytic fibers) and extensor digitorum longus (EDL; primarily glycolytic fibers) muscles were dissected from anesthetized mice (6 mg of pentobarbital per 100 g−1 body weight) and transferred to incubation flasks containing 2 ml of essential buffer (Krebs-Henseleit buffer, pH 7.4, with 2 mmol/l pyruvate, 8 mmol/l mannitol, and 0.1% BSA), gassed with 95% O2 + 5% CO2, and maintained at 30°C as previously described (15). For all experiments, muscles were preincubated for 15 min in this buffer before it was replaced with buffer containing AICAR (2 mmol/l for 40 min; Toronto Research Chemicals Inc, Ontario, Canada), CNTF (at concentrations and durations indicated above, Axokine; Regeneron Pharmaceuticals Inc, New York), or insulin (30 nmol/l [additivity study] or 2.8 nmol/l [HFD study] for 40 min, Actrapid; Novo Nordisk, Bagsvaerd, Denmark). In separate experiments, muscles were incubated in LY-294002 hydrochloride (37) (60 nmol/l for 30 min; Sigma-Aldrich Corp, St. Louis, MO) before treatment with CNTF or insulin as described.
2-deoxy-d-glucose (2DG) uptake was measured over 10 min by replacing existing incubation buffer with the buffer described above but with the addition of 0.5 μCi/ml−12-[2,6-3H]-deoxy-d-glucose, 1 mmol/l 2-deoxy-d-glucose, and 0.2 μCi [1-14C]-mannitol/ml. After preparing muscles as described below, radioactivity was measured in muscle lysates by liquid scintillation counting (Tri-Carb 2000; Packard Instrument Co).
Muscle lysate preparation.
Muscles were homogenized in ice-cold buffer (50 mmol/l Hepes, pH 7.4, 150 mmol/l NaCl, 10 mmol/l NaF, 1 mmol/l sodium pyrophosphate, 0.5 mmol/l EDTA, 250 mmol/l sucrose, 1 mmol/l dithiothreitol, 1% TritonX-100, 1 mmol/l Na3VO4, and one Roche protease inhibitor tablet per 50 ml buffer) using an electrical homogenizer. Lysates were prepared as previously described (38) and stored at −80°C until analysis. Protein content in lysates was measured by the bicinchoninic acid method (Pierce).
Immunoblotting.
Expression or phosphorylation of investigated proteins was determined in muscle lysates by SDS-PAGE and immunoblotting using the following primary antibodies: pan-αAMPK, phospho-AMPK T172, and phospho–acetyl CoA carboxylase (ACC) S222 (as previously described [(39)]), STAT3, phospho-STAT3 Y705, Akt, and phospho-Akt S473 (Cell Signaling Technology Inc). Secondary antibodies were horseradish-conjugated protein G (Bio-Rad Laboratories, Richmond, CA). Bands were visualized using an enhanced chemoluminescence system and quantified using ImageQuant TL 05 software (Amersham Biosciences, U.K.). Values obtained using phospho-specific antibodies are expressed as the ratio to the total content of the protein measured after stripping the membrane and reprobing.
AMPK activity.
AMPKα1 and -α2 activities were measured from 100 μg of muscle lysate protein using rabbit polyclonal AMPK antibodies for immunoprecipitation as previously described (40).
Muscle lipids.
Lipids were extracted from freeze-dried powdered gastrocnemius muscle tissues using chloroform:methanol:PBS (1:2:0.8) and 0.2% SDS. Triacylglycerol were saponified in an ethanol-KOH solution at 60°C, and glycerol content was measured fluorometrically. Diacylglycerols and ceramides were extracted and quantified according to the methods of Preiss et al. (41).
Muscle cell studies.
Muscle cells were differentiated and serum starved overnight. The next morning, 20 nmol/l C2-dihydroceramide (Toronto Research Chemicals Inc, Ontario, Canada) was added for 6 h before cells were treated for 20 min with either CNTF (10 ng/ml) or insulin (10 nmol/l) before collecting protein lysates or measuring 2DG uptake as previously described (20). C2C12 cell were used for signaling experiments and L6 muscle cells for 2DG uptake because of the limited capacity of C2C12 to increase glucose uptake in response to stimuli.
Data are expressed as means ± SE. Statistical evaluations were performed by either Student's t test or two-way ANOVA using the Student-Newman-Keuls method as a post hoc test when appropriate. Differences between groups were considered statistically significant if P < 0.05.
RESULTS
In vivo effects of ciliary neurotrophic factor on blood glucose.
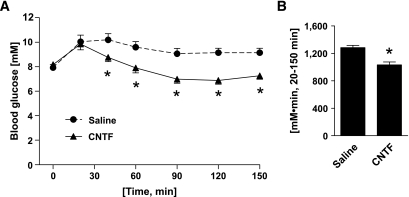
Injection of mice with CNTF was associated with a reduction in blood glucose after 40 min compared with saline-injected controls. Blood glucose remained reduced in the CNTF group throughout the remainder of the test (Fig. 1A). The AUC was 20% lower in CNTF-treated mice than in saline-injected controls (Fig. 1B).
FIG. 1.
In vivo effects of CNTF on blood glucose. A: CNTF tolerance test. Change in blood glucose over time. B: AUC. Animals were injected with either CNTF (0.3 mg/kg) or saline, and changes in tail blood glucose were monitored over 150 min. n = 10. Data are means ± SEM. *Significantly different from saline.
Muscle glucose uptake: ciliary neurotrophic factor dose response.
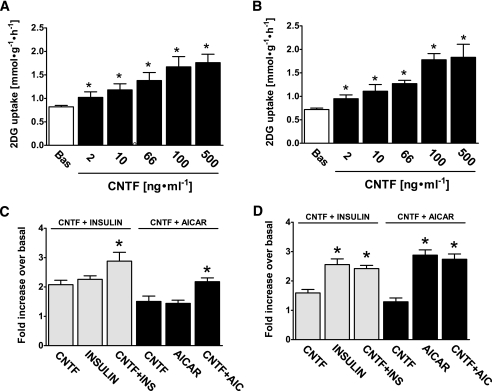
The effects of CNTF to reduce blood glucose in vivo may have been mediated by the suppression of hepatic glucose production or increased glucose uptake into adipose tissue and skeletal muscle. Therefore, to directly test the effects of CNTF on skeletal muscle glucose uptake, we incubated isolated muscles with CNTF at concentrations ranging from 2 to 500 ng/ml. CNTF increased muscle glucose uptake over 30 min in a dose-dependent manner with a significant increase at 2 ng/ml in both soleus and EDL muscles (Fig. 2A–B). Glucose uptake was elevated with increasing CNTF concentrations up to 100 ng/ml with no further increases observed at 500 ng/ml (Fig. 2A and B), consistent with dose-dependent effects on fatty acid oxidation observed previously (20). We next investigated if the effect of CNTF on glucose uptake was additive to the effects of insulin and AICAR. In soleus, CNTF, insulin, and AICAR independently increased glucose uptake to a similar degree, and combining AICAR and insulin with CNTF had an additive effect (Fig. 2C). In EDL, insulin and AICAR increased glucose uptake more strongly than CNTF and no further increase was observed when combining these treatments with CNTF (Fig. 2D).
FIG. 2.
Dose-dependent effect of CNTF on 2-deoxyglucose (2DG) glucose uptake and additive effects of CNTF on AICAR and insulin stimulated glucose uptake. A and B: Muscles were either incubated with vehicle (BAS) condition or stimulated with CNTF at indicated concentrations for 30 min; n = 8. *Significantly different from basal condition (P < 0.05). C and D: Muscles were incubated with CNTF (100 ng/ml), insulin (30 nmol/l), AICAR (2 mmol/l), or indicated combinations, and changes in 2DG uptake are expressed as fold increases over basal. n = 7–8. Data are means ± SEM. *Significantly different from CNTF in the same muscle type.
Time course of CNTF-stimulated muscle glucose uptake and signaling.
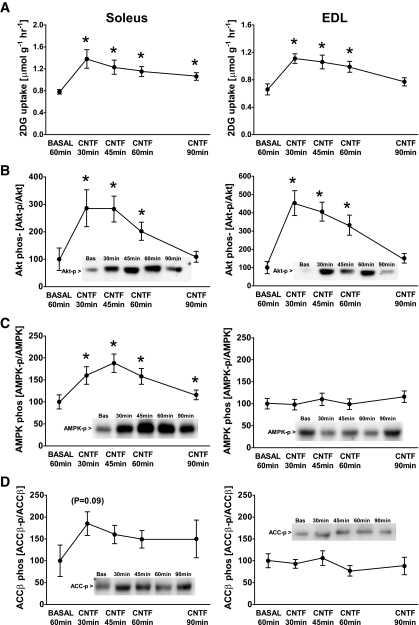
CNTF increased glucose uptake in soleus and EDL after 30 min—an effect that diminished thereafter in both muscle types (Fig. 3A). CNTF has been reported to increase the activity/phosphorylation of AMPK in muscle (20) and Akt in neurons (42), and because both kinases regulate muscle glucose uptake, we examined their activating phosphorylation. CNTF increased Akt S473-P nearly 200% in soleus and 450% in EDL muscle, and these effects diminished after 90 min (Fig. 3B). AMPK T172-P was increased 90% in the soleus with CNTF after 30 min, and this was maintained for 60 min (Fig. 3C). Although CNTF induced an apparent rise in ACCβ S218-P in soleus, this did not achieve statistical significance (P = 0.09) (Fig. 3D). CNTF had no detectable effect on AMPK- or ACCβ-P in EDL (Fig. 3C–D), which is consistent with previous observations in glycolytic muscle (20).
FIG. 3.
Time-dependent effect of CNTF on 2-deoxyglucose (2DG) uptake, Akt S473, αAMPK T172, and ACCβ S218 phosphorylation in incubated mouse muscle. A: 2DG uptake in incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF at indicated periods of time. B: Akt S473 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF at indicated periods of time. C: αAMPK T172 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF. D: ACCβ S218 phosphorylation in lysates from incubated soleus and EDL at basal conditions and after incubation with 100 ng/ml CNTF. n = 8. Data are means ± SEM. *Significantly different from basal condition.
Effects of PI3-kinase inhibition and ablated AMPK activity on CNTF actions.
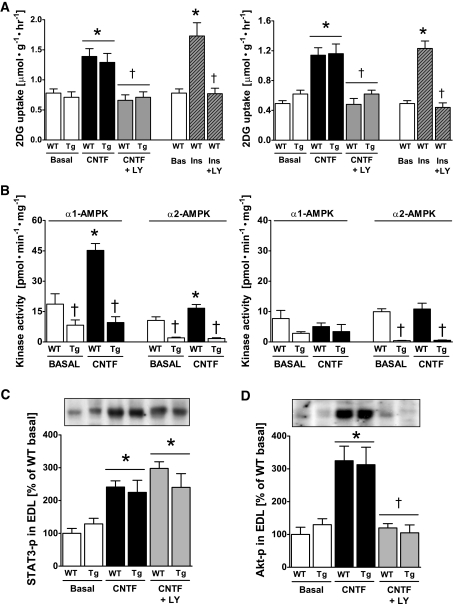
We next examined the relative importance of AMPK and PI3K/Akt signaling for CNTF-mediated glucose uptake. Muscles from wild-type and AMPK KD mice were preincubated with or without the PI3-kinase inhibitor, LY-294002, followed by stimulation with CNTF. Glucose uptake was increased to a similar degree with CNTF in wild-type and AMPK KD muscles, indicating that this response was AMPK independent. CNTF-stimulated glucose uptake was abolished by LY-294002 in both muscle types irrespective of mouse genotype (Fig. 4A), which is consistent with PI3-kinase/Akt signaling being essential for this response.
FIG. 4.
Effect of impaired AMPK signaling and the PI3-kinase inhibitor LY-294002 on CNTF-stimulated 2-deoxyglucose (2DG) uptake, AMPK activity, and Akt S473 and STAT3 Y705 phosphorylation in incubated mouse muscle from wild-type (WT) and AMPK KD (Tg) mice. A: 2DG uptake at basal and after incubation with 100 ng/ml CNTF with or without 60 nmol/l of LY-294002 in soleus (left panel) and EDL (right panel) muscle. In addition, incubation of wild-type muscle at basal with 2.8 nmol/l insulin (ins) with and without LY-294002. B: α-Isoform–specific AMPK activity in lysates from incubated soleus (left panel) and EDL (right panel) muscle at basal and after incubation with 100 ng/ml CNTF for 30 min. C: STAT3 Y705 phosphorylation in lysates from incubated EDL muscle at basal and after incubation with 100 ng/ml CNTF with and without LY-294002. D: Akt phosphorylation in lysates from incubated EDL at basal and after incubation with 100 ng/ml CNTF with and without LY-294002. n = 8. Data are means ± SEM. *Significantly different from basal condition. †Significantly different from CNTF or insulin stimulated. Significantly different from WT.
We next measured α-isoform–specific AMPK activity to verify that CNTF had activated AMPK signaling in wild-type mice and that the activation was impaired in AMPK KD muscles (Fig. 4B). As anticipated, overexpression of the AMPK KD construct lowered basal AMPK activity of both α-AMPK isoforms in both muscle types, with the most pronounced effect on α2-AMPK activity (34). In wild-type soleus, CNTF increased α1-AMPK activity by 180% and α2-AMPK activity by 35%, whereas CNTF had no effect on either AMPK isoform in AMPK KD muscles (Fig. 4B). CNTF did not increase AMPK activity in the EDL (Fig. 4B), which is consistent with CNTF not increasing AMPK phosphorylation in the EDL muscle (Fig. 3C).
Lastly, to establish whether inhibition of PI3-kinase or AMPK signaling altered proximal CNTF signaling through the gp130-LIF receptor complex, we examined STAT3 Y705-P in the EDL muscle of wild-type and AMPK KD mice in the absence or presence of LY-294002. Incubation with CNTF increased STAT3 Y705-P by 140% in wild-type muscle, and importantly, the increase was unaffected in AMPK-KD muscles and unaffected by coincubation with LY-294002 (Fig. 4C). We also verified that increases in Akt-P with CNTF were not affected by overexpression of the AMPK KD construct and that LY 294002 inhibited Akt activation (Fig. 4D). Similar findings were observed in the soleus muscle (data not shown).
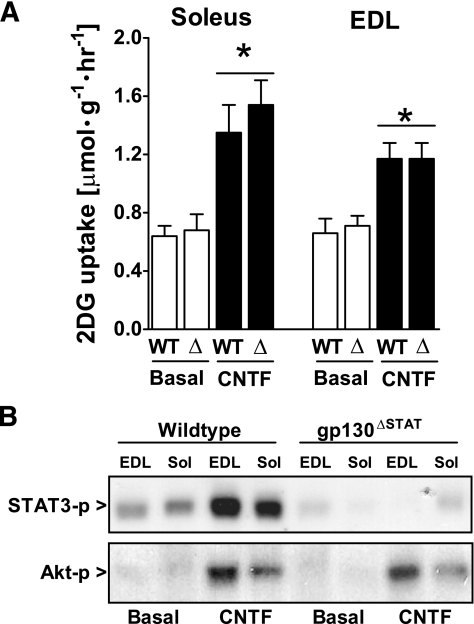
CNTF-stimulated glucose uptake in gp130ΔSTAT muscles.
Signaling from the gp130-LIF receptor complex activates two distinct intracellular signaling pathways. The first depends on phosphorylation of four tyrosine residues (Y765, Y812, Y904, and Y914) on the distal cytoplasmic tail of gp130, which is required and sufficient for activating STAT1/3 (23) and AMPK (20). The second arm requires phosphorylation of the more proximal Y757 residue in gp130, which induces ERK and PI3-kinase/Akt signaling (23). We incubated muscles from gp130ΔSTAT mice, which have a deletion of the cytosolic distal portion of the gp130 receptor required for induction of STAT1/3 and AMPK signaling but retain the Y757 residue that is required for activation of Erk and PI3-kinase/Akt signaling. Incubation with CNTF increased glucose uptake in soleus and EDL of wild-type mice, and this increase was maintained in gp130ΔSTAT knockin mutant mice (Fig. 5A). CNTF increased Akt S473-P in both genotypes, whereas STAT3 Y705-P was increased in wild-type but not in gp130ΔSTAT muscles as expected (Fig. 5B). Thus, CNTF increased glucose uptake normally despite impaired gp130 signaling and activation of AMPK.
FIG. 5.
CNTF-induced 2-deoxyglucose (2DG) uptake (A) and STAT3 Y705 and Akt S473 phosphorylation (B) in incubated soleus and EDL mouse muscle from wild-type (WT) and gp130ΔSTAT mice. (Muscles were either incubated at basal condition or with 100 ng/ml CNTF for 30 min [n = 8].) Data are means ± SEM. *Significantly different from basal condition.
Effects of obesity on CNTF-stimulated muscle glucose uptake and signaling.
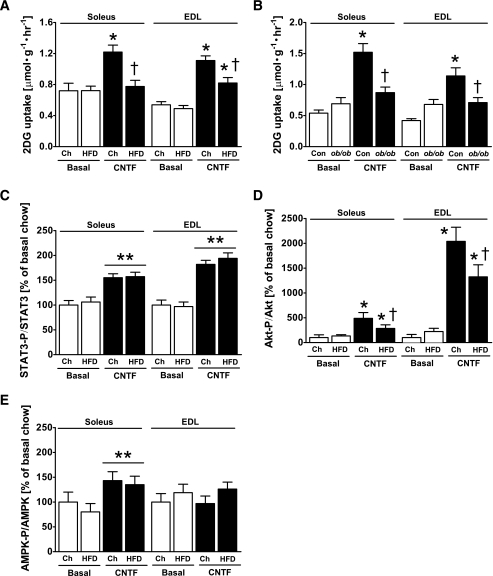
It is well established that obesity is associated with impaired insulin sensitivity in skeletal muscle, and impaired activation of IRS-1 has been attributed a primary role (28,43). Because CNTF stimulates glucose uptake downstream of IRS-1, we tested whether CNTF-stimulated glucose uptake is maintained in muscles from mice fed a HFD for 12 weeks and obese mice deficient in leptin (ob/ob). As dictated by design, the high-fat feeding increased body weight by ∼20% (chow, 28.4 ± 0.5 g; HFD, 34.0 ± 0.7 g), fasting insulin levels (836 ± 76 pg/ml to 1,670 ± 377 pg/ml), and the AUC during a GTT (chow, 539 ± 23; HFD, 870 ± 94). Similarly, ob/ob mice had dramatically increased body mass (wild-type, 29.6 ± 0.7 g; ob/ob, 57.3 ± 2.8 g) and AUC during a GTT (WT, 574 ± 26; ob/ob, 1,347 ± 98). CNTF increased glucose uptake by 80 to 100% in the soleus and EDL in chow-fed mice, whereas CNTF-stimulated glucose uptake was completely blunted in the soleus and reduced by ∼50% in EDL of mice fed a HFD (Fig. 6A). Similarly, CNTF increased muscle glucose uptake in both muscle types of lean littermates of ob/ob mice; however, this increase was abolished in muscles from ob/ob mice (Fig. 6B).
FIG. 6.
CNTF-stimulated 2-deoxyglucose (2DG) uptake in high-fat diet–fed (A) and ob/ob mice (B), STAT3 Y705 (C), Akt S473 (D), and AMPK T172 (E) phosphorylation in soleus and EDL mouse muscle from mice fed standard chow (Ch) or an HFD for 12 weeks; n = 8–10 for HFD experiment and 7–8 for ob/ob experiment. Data are means ± SEM. *Significantly different from basal condition (P < 0.05, P < 0.001). †Significantly different from CNTF-stimulated chow-fed/control mice.
We then examined the mechanism by which obesity inhibited CNTF-stimulated glucose uptake. CNTF increased STAT3 Y705-P in skeletal muscle of chow- and HFD-fed animals (Fig. 6C), indicating no impairment of CNTF action at the receptor level. CNTF-induced Akt S473-P was increased by 4- and 19-fold in soleus and EDL of chow-fed mice, respectively, and this increase was markedly impaired with obesity in both muscle types (Fig. 6D). In contrast, AMPK T172-P in soleus was also unaffected by diet (Fig. 6E).
Muscle lipids and CNTF signaling.
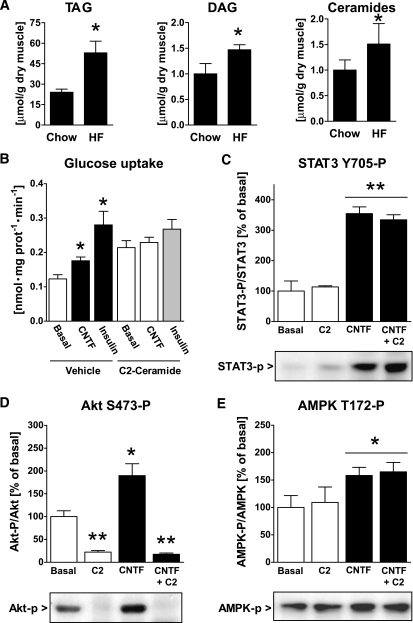
High-fat feeding increased muscle contents of triacylglycerol, diacylglycerol, and ceramide by ∼100%, ∼50%, and ∼50%, respectively, compared with chow-fed controls (Fig. 7A). Because ceramides have been shown to directly inhibit insulin-stimulated Akt phosphorylation (33), we tested whether exposure of cultured myotubes to a short-chain ceramide analog also impaired CNTF-stimulated glucose uptake and Akt phosphorylation. Consistent with our findings in obese skeletal muscle, myotubes treated with C2-ceramide had impaired CNTF-stimulated glucose uptake (Fig. 7B). Reductions in glucose uptake were not the result of inhibition of the gp130-LIF receptor complex because CNTF increased STAT3-P by ∼350% in both vehicle and C2-ceramide–treated cells (Fig. 7C). However, C2-ceramide reduced basal and CNTF-stimulated Akt S473-P (Fig. 7D). AMPK T172-P was increased (P < 0.05) by 60% with CNTF, and this increase was unaffected by C2-ceramide treatment (Fig. 7E).
FIG. 7.
Muscle lipids in gastrocnemius muscle from mice fed standard chow or an HFD and STAT3 Y705, Akt S473, and AMPK T172 phosphorylation in C2C12 myocytes. A: Muscle content of triacylglycerol (TAG), diacylglycerol (DAG), and ceramides. B: CNTF and insulin-stimulated 2-deoxy-d-glucose uptake in L6 myotubes treated with either C2-ceramide or vehicle for 6 h. C–E: Phosphorylation of CNTF-activated signaling molecules in C2C12 myotubes treated with C2-ceramide for 6 h or CNTF for 30 min or in combination. *Significantly different from basal condition (P < 0.05, P < 0.001).
DISCUSSION
CNTF can regulate metabolic and growth signaling pathways in several tissue types. The present study shows that CNTF regulates glucose uptake and delineates the proximal signaling events mediating this response. We show that acute exposure of CNTF reduces blood glucose in vivo and increases glucose uptake in soleus and EDL muscle in vitro through the PI3-kinase/Akt signaling pathway. It was expected that AMPK would also exert a role in this process, but we found that AMPK was not required for CNTF to stimulate glucose uptake. Skeletal muscle is the most important tissue for insulin-stimulated glucose disposal (44), and skeletal muscle insulin resistance is a major defect in most obese phenotypes. Accordingly, we investigated whether CNTF-stimulated glucose uptake was maintained in muscle from obese, insulin-resistant mice. The rationale for these studies was to establish whether gp130-LIF signaling could constitute an alternative pathway to substitute for insufficient insulin signaling to skeletal muscle glucose uptake. However, obesity was associated with impaired CNTF-stimulated glucose uptake, which, at least in part, may have been attributed to increased muscle lipids and impaired PI3-kinase/Akt signaling with CNTF.
CNTF is a peptide hormone of the IL-6 family, which is highly expressed in peripheral nerves and other tissues (24,45,46). CNTF levels are low in serum of healthy individual as a result of the absence of an exocytosis targeting sequence (47,48). Recent reports have shown significant promise for the use of CNTF as an antiobesity therapeutic because it suppresses food intake acutely (49) and induces hypothalamic neurogenesis (50), leading to a new set point in body mass. CNTF also has direct antidiabetic effects in peripheral tissues. Chronic treatment of obese diabetic mice with CNTF increases metabolic rate (51) and reduces liver steatosis by enhancing fat oxidation and reducing synthesis of complex lipids (52). We have recently shown that chronic CNTF treatment of obese diabetic mice reverses obesity-induced insulin resistance by activating AMPK and reducing muscle lipid accumulation (20). In line with this study, CNTF acutely prevents muscle insulin resistance in response to a 2-h lipid infusion by preventing lipid accumulation (27). The clinical efficacy and safety of CNTF as a possible therapeutic for obesity is supported by findings in humans demonstrating weight loss and improved glycemic control (53).
Because we previously showed that CNTF activates AMPK in muscle (20) and AMPK is well known to increase glucose uptake in skeletal muscle (15,54,55), we expected AMPK rather than PI3-kinase/Akt signaling to be required for CNTF-stimulated glucose uptake. However, multiple lines of evidence indicate that CNTF signaling to glucose uptake may be mediated by a PI3-kinase/Akt–dependent signaling pathway: 1) the kinetics in Akt S473 phosphorylation correlated with increases in glucose uptake in both soleus and EDL muscles; 2) incubation with the PI3-kinase inhibitor, LY-294002, completely blocked CNTF-stimulated Akt phosphorylation and glucose uptake; 3) CNTF increased glucose uptake was not impaired in muscles overexpressing a KD AMPKα2; 4) AMPK was not activated by CNTF in the EDL muscle despite a 100% increase in glucose uptake; 5) a knockin mutation of a truncated gp130 receptor, which impairs gp130 signaling toward STAT3 and AMPK, had no effect on CNTF-stimulated glucose uptake; and 6) obesity induced by an HFD or treatment of cells with C2-ceramide impaired CNTF stimulated glucose uptake and was associated with blunted Akt but not AMPK phosphorylation. However, our findings demonstrating that CNTF-stimulated glucose uptake was additive to a maximal dose of insulin in Soleus, but not EDL muscle suggest that under some conditions, AMPK activation may also play a role in the stimulation of glucose uptake in Soleus, albeit to a lesser extent than originally anticipated. A possible explanation for the limited role of AMPK in CNTF-stimulated glucose uptake is that CNTF predominantly activated the α1-AMPK isoform. Studies in AMPKα2-null mice have shown that AMPKα2, but not α1 is required for the regulation of muscle glucose uptake in response to stimuli such as AICAR (15), possibly explaining why CNTF activation of AMPK is not required to increase glucose transport.
Because we found CNTF to increase muscle glucose uptake, it was of interest to test the effects of CNTF in insulin-resistant muscle from obese mice. We found that the efficacy of CNTF to regulate glucose uptake was reduced by ∼50% or more in muscles from obese HFD-fed and ob/ob mice. CNTF resistance with obesity seems not to be the result of impaired activation of the gp130-LIF receptor complex because CNTF-induced phosphorylation of AMPK and STAT3 were normal in obese muscle. In contrast, CNTF-stimulated phosphorylation of Akt was suppressed in obese muscles and was associated with muscle DAG and ceramide accumulation. Ceramides downregulate Akt phosphorylation and membrane translocation by activating protein phosphatase 2A (56). Ceramides also increase the activity of protein kinase C ζ, leading to inhibitory Akt S34 phosphorylation (57). In addition to the finding that ceramides were elevated in muscle from obese mice, our studies in myotubes showed that the C2-ceramide not only reduced basal Akt phosphorylation but also completely prevented CNTF-induced Akt phosphorylation and CNTF-stimulated glucose uptake. These results agree with previous studies in myotubes (33) and isolated rodent (32) and human (58) skeletal muscle demonstrating impaired insulin-stimulated Akt activation and glucose uptake when ceramides are elevated. In the present studies, DAG and a reduction in PI-3 kinase activity may have also contributed to the reduced CNTF activation of Akt in muscles from obese animals. Importantly, the finding that neither the C2-ceramide nor obesity altered CNTF-induced STAT3 and AMPK phosphorylation is consistent with our previous findings that CNTF activation of AMPK and lipid metabolism is maintained in skeletal muscles from obese, insulin-resistant mouse models (20). Thus, although the signaling arm of CNTF to glucose uptake is compromised with obesity, the activation of AMPK and lipid oxidation is preserved.
In summary, our data show that CNTF-stimulated glucose uptake is associated with activation of the PI3-kinase/Akt signaling pathway. Furthermore, CNTF-stimulated Akt S473 phosphorylation and glucose uptake is impaired in muscles from obese insulin-resistant mice despite the maintenance of STAT3 and AMPK signaling. This mechanism may, in part, be attributable to ceramide accumulation with obesity, which in turn impairs CNTF signaling to the PI3-kinase/Akt signaling arm of the gp130 receptor. Because CNTF-induced activation of AMPK was maintained in obesity, CNTF or CNTF analogs provide the bases for a viable therapeutic to prevent muscle lipid accumulation and to restore insulin sensitivity in obesity.
Acknowledgments
This research was supported by grants from the National Health and Medical Research Council, Australia (G.R.S., M.J.W., and B.E.K.) and the National Institutes of Health (NIH), U.S. (M.J.B.; R01-DK56886). S.B.J. was supported by a Danish Research Council of Health and Diseases postdoctoral fellowship. M.J.W. is an R. Douglas Wright Fellow, and G.R.S. is a National Health and Medical Research Council of Australia Research Fellow and a Canadian Research Chair in Metabolism, Obesity and Type 2 Diabetes. B.E.K. is an Australian Research Council Federation Fellow.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Rose AJ, Richter EA: Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20: 260– 270, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bruss MD, Arias EB, Lienhard GE, Cartee GD: Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41– 50, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF: AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051– 2058, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ: Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787– 9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JE, Dudek RW, Whitehead DS, Downes DL, Frisell WR, Caro JF, Dohm GL: Immunolocalization of glucose transporter GLUT4 within human skeletal muscle. Diabetes 40: 150– 154, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE: Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599– 14602, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230– 50236, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ: Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728– 1731, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T: Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288– 1295, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB: Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A 99: 16309– 16313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA: Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55: 2688– 2697, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Geiger PC, Hancock C, Wright DC, Han DH, Holloszy JO: IL-6 increases muscle insulin sensitivity only at superphysiological levels. Am J Physiol Endocrinol Metab 292: E1842– 1846, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A: Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56: 1630– 1637, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ: Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47: 1369– 1373, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF: Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070– 1079, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H: 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes 52: 1066– 1072, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fiedler M, Zierath JR, Selen G, Wallberg-Henriksson H, Liang Y, Sakariassen KS: 5-aminoimidazole-4-carboxy-amide-1-beta-D-ribofuranoside treatment ameliorates hyperglycaemia and hyperinsulinaemia but not dyslipidaemia in KKAy-CETP mice. Diabetologia 44: 2180– 2186, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Steinberg GR, Smith AC, Van Denderen BJ, Chen Z, Murthy S, Campbell DJ, Heigenhauser GJ, Dyck DJ, Kemp BE: AMP-activated protein kinase is not down-regulated in human skeletal muscle of obese females. J Clin Endocrinol Metab 89: 4575– 4580, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Iglesias MA, Furler SM, Cooney GJ, Kraegen EW, Ye JM: AMP-Activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes 53: 1649– 1654, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR: CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12: 541– 548, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD: LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science 260: 1805– 1808, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, Taga T, Kishimoto T: IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science 260: 1808– 1810, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Ernst M, Jenkins BJ: Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 20: 23– 32, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Febbraio MA: gp130 receptor ligands as potential therapeutic targets for obesity. J Clin Invest 117: 841– 849, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T: Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol 18: 4109– 4117, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertola A, Bonnafous S, Cormont M, Anty R, Tanti JF, Tran A, Le Marchand-Brustel Y, Gual P: Hepatocyte growth factor induces glucose uptake in 3T3–L1 adipocytes through A Gab1/phosphatidylinositol 3-kinase/Glut4 pathway. J Biol Chem 282: 10325– 10332, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Watt MJ, Hevener A, Lancaster GI, Febbraio MA: Ciliary neurotrophic factor prevents acute lipid-induced insulin resistance by attenuating ceramide accumulation and phosphorylation of c-Jun N-terminal kinase in peripheral tissues. Endocrinology 147: 2077– 2085, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM: IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665– 668, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE: Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673– 1677, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M: IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191– 198, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI: PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823– 827, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167– 179, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schmitz-Peiffer C, Craig DL, Biden TJ: Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202– 24210, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ: A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport I skeletal muscle. Mol Cell 7: 1085– 1094, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Jensen TE, Rose AJ, Hellsten Y, Wojtaszewski JF, Richter EA: Caffeine-induced Ca2+ release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am J Physiol Endocrinol Metab 293: E286– 293, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U, Heath JK, Dunn AR: Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med 194: 189– 203, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtaszewski JF, Lynge J, Jakobsen AB, Goodyear LJ, Richter EA: Differential regulation of MAP kinase by contraction and insulin in skeletal muscle: metabolic implications. Am J Physiol 277: E724– E732, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF: The alpha2–5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 53: 3074– 3081, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE: AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett 443: 285– 289, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H: Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J 19: 1146– 1148, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM: Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem 261: 8597– 8600, 1986 [PubMed] [Google Scholar]
- 42.Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM: Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 18: 270– 282, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Petersen KF, Shulman GI: Etiology of insulin resistance. Am J Med 119: S10– 16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron AD, Brechtel G, Wallace P, Edelman SV: Rates and tissue sites of non-ins. Am J Physiol 255: E769– E774, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Gupta SK, Altares M, Benoit R, Riopelle RJ, Dunn RJ, Richardson PM: Preparation and biological properties of native and recombinant ciliary neurotrophic factor. J Neurobiol 23: 481– 490, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B, et al. : The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science 255: 1434– 1437, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Wenisch C, Linnau KF, Looaresuwan S, Rumpold H: Plasma levels of the interleukin-6 cytokine family in persons with severe Plasmodium falciparum malaria. J Infect Dis 179: 747– 750, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Stockli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, Lindholm D, Thoenen H: Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature 342: 920– 923, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Steinberg GR, Watt MJ, Fam BC, Proietto J, Andrikopoulos S, Allen AM, Febbraio MA, Kemp BE: Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology 147: 3906– 3914, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Kokoeva MV, Yin H, Flier JS: Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310: 679– 683, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Bluher S, Moschos S, Bullen J, Jr, Kokkotou E, Maratos-Flier E, Wiegand SJ, Sleeman MW, Mantzoros CS: Ciliary neurotrophic factorAx15 alters energy homeostasis, decreases body weight, and improves metabolic control in diet-induced obese and UCP1-DTA mice. Diabetes 53: 2787– 2796, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Sleeman MW, Garcia K, Liu R, Murray JD, Malinova L, Moncrieffe M, Yancopoulos GD, Wiegand SJ: Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc Natl Acad Sci U S A 100: 14297– 14302, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ettinger MP, Littlejohn TW, Schwartz SL, Weiss SR, McIlwain HH, Heymsfield SB, Bray GA, Roberts WG, Heyman ER, Stambler N, Heshka S, Vicary C, Guler HP: Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA 289: 1826– 1832, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Merrill GF, Kurth EJ, Hardie DG, Winder WW: AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273: E1107– E1112, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA: Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes 51: 284– 292, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Stratford S, Hoehn KL, Liu F, Summers SA: Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 279: 36608– 36615, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M: Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem 282: 12450– 12457, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005– 2011, 2002 [DOI] [PubMed] [Google Scholar]