Abstract
OBJECTIVES
To comprehensively inventory the proteins that control the G1/S cell cycle checkpoint in the human islet and compare them with those in the murine islet, to determine whether these might therapeutically enhance human β-cell replication, to determine whether human β-cell replication can be demonstrated in an in vivo model, and to enhance human β-cell function in vivo.
RESEARCH DESIGN AND METHODS
Thirty-four G1/S regulatory proteins were examined in human islets. Effects of adenoviruses expressing cdk-6, cdk-4, and cyclin D1 on proliferation in human β-cells were studied in both invitro and in vivo models.
RESULTS
Multiple differences between murine and human islets occur, most strikingly the presence of cdk-6 in human β-cells versus its low abundance in the murine islet. Cdk-6 and cyclin D1 in vitro led to marked activation of retinoblastoma protein phosphorylation and cell cycle progression with no induction of cell death. Human islets transduced with cdk-6 and cyclin D1 were transplanted into diabetic NOD-SCID mice and markedly outperformed native human islets in vivo, maintaining glucose control for the entire 6 weeks of the study.
CONCLUSIONS
The human G1/S proteome is described for the first time. Human islets are unlike their rodent counterparts in that they contain easily measurable cdk-6. Cdk-6 overexpression, alone or in combination with cyclin D1, strikingly stimulates human β-cell replication, both in vitro as well as in vivo, without inducing cell death or loss of function. Using this model, human β-cell replication can be induced and studied in vivo.
Recent reports demonstrate that human islet transplantation is technically feasible (1,2), that replication is a major means of maintaining β-cell numbers in rodents, and that all rodent adult β-cells are capable of replicating (3–5). In addition, advances in understanding the signaling pathways and growth factors that can induce β-cell replication continue to accrue (6–13). It is now clear that both type 1 and type 2 diabetes result from a decline in β-cell mass (14–16). Collectively, these observations underscore a need to understand, and to therapeutically exploit the molecular mechanisms underlying β-cell replication and regeneration. Abundant data in mice have demonstrated that the “G1/S checkpoint,” or the “G1/S pathway” (supplemental Fig. 1, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db08-0631/DC1), in the cell cycle is critical in regulating rodent β-cell replication and physiological function (rev. in 17–26).
In contrast to this richness in knowledge regarding β-cell cycle control in the mouse, relatively little is known regarding cell cycle control in the human islet (17,23,26). This is important because the fundamental reason for studying β-cell replication is to leverage such knowledge for expanding functional human β-cell mass for the treatment or prevention of diabetes. Significantly, whereas abundant examples exist for robust induction of rodent β-cell replication (3–6,9–13,17–19,27,28), multiple reports suggest that in contrast to rodents, human β-cell replication is a rare event; indeed, several authors have reported that they cannot demonstrate replication in human β-cells either under basal conditions or in response to growth factors (15,17,29–31).
We therefore sought to catalog in the human islet the molecules that control the G1/S checkpoint of the cell cycle. We report such a G1/S roadmap and compare it with that of the murine islet. We demonstrate that whereas cdk-6 is difficult to detect or absent in murine β-cells, it is present in human β-cells. Furthermore, we show that cdk-6 and a d-cyclin partner can be used to markedly accelerate replication in human β-cells in vitro. Most importantly, we show that combined overexpression of cdk-6 with cyclin D1 also leads to human β-cell replication in vivo and results in enhanced human islet engraftment and function in an in vivo transplant diabetes model.
RESEARCH DESIGN AND METHODS
Human islets.
Human islets were obtained through the National Institutes of Health– and Juvenile Diabetes Research Foundation–supported Islet Cell Resource Consortium (http://icr.coh.org). On arrival, human islets were washed with PBS and incubated in RPMI medium (Gibco, Grand Isle, NY) containing 5.5 mmol/l glucose, 1% penicillin, and streptomycin with 10% FBS until they were used for experiments. Receipt of, and work with, human islets was approved in advance by the University of Pittsburgh Institutional Review Board.
Immunoblotting and RT-PCR.
Human and animal islet extracts were prepared within 24–48 h of arrival using nuclear extract buffer resolved using 7.5–15% SDS-PAGE depending on the molecular weight of the protein in question and then transferred to Immobilon-P membranes (Millipore, Bradford, MA) as we have described previously (23,24). Primary antisera and dilutions used are shown in supplemental Table 1 (available in the online appendix). Each immunoblot was performed a minimum of three times on a minimum of three different islet preparations, each accompanied by an appropriate housekeeping protein control, either tubulin or actin. Representative immunoblots and actin/tubulin controls are shown in Figs. 1–8. RT-PCR for cdk-4 and cdk-6 were preformed as described previously (9,13,22,23) using primers described in the legend to Fig. 1.
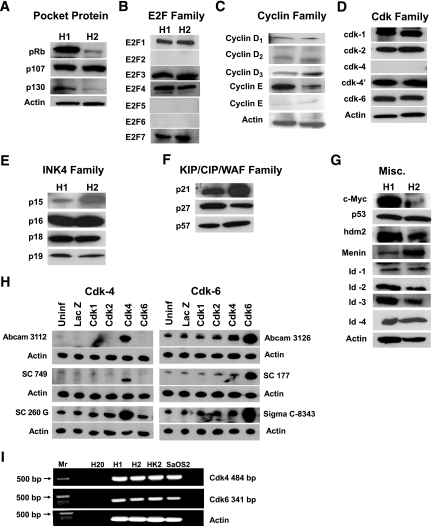
FIG. 1.
Immunoblots for the G1/S control molecules in the human islet. Each panel represents two examples of immunoblots of human islet extracts, and each is representative of at least three, and as many as eight, human islet preparations. Islets were extracted between 24 and 48 h of arrival. Antisera are shown in supplemental Table 1 of the online appendix. A: The pocket proteins. B: E2F family. C: Cyclin family. D: cdk family (cdk-4 and cdk-4′ represents immunoblots preformed with Abcam and sc-260G antisera as explained in H). E: INK4 family. F: KIP/CIP/WAF family. G: Miscellaneous other G1/S molecules (note that HDM2 is the human homolog of murine MDM2, the ubiquitin ligase for p53). H: Results obtained with multiple cdk-4 and cdk-6 antisera. To define the specificity of several available cdk-4 and cdk-6 antisera, human islets were transduced with adenoviruses (250 MOI) encoding cdk-1, cdk-2, cdk-4, and cdk-6 for 1 h, incubated for 48 h, and then extracted, resolved on SDS-PAGE, and immunoblotted for the six antisera shown. As can be seen, none of the cdk-4 or cdk-6 antisera cross-reacted with cdk-1, cdk-2, cdk-4, or cdk-6, and each was upregulated by the cognate cdk. All three cdk-6 antisera recognized cdk-6 in normal nontransduced islets, but only the sc-260 cdk-4 antiserum was able to detect cdk-4 in normal human islets. I: RT-PCR for cdk-4 and cdk-6 in human islets. MR indicates molecular weight markers, and H2O indicates a lane with no polymerase. Human osteosarcoma cells (SaOS2) and the human kidney cell line HK2 are positive controls. Sequencing of the amplified PCR products confirmed the presence of human cdk-4 and cdk-6. The primers used spanned intron–exon boundaries and generated products of the expected size: 484 bp for cdk-4 (vs. 754 for the genomic product) and 341 bp (vs. 8,457 for the genomic product). The primers used were: cdk-6 5′-GGCGCCTATGGGAAGGTGTTC-3′; 5′-AAAGTCCAGACCTCGGAGAAGC-3′; cdk-4 5′-GTGGCTGAAATTGGTGTCGG-3′; 5′-GCCATCTGGTAGCTGTAGATTC-3′.
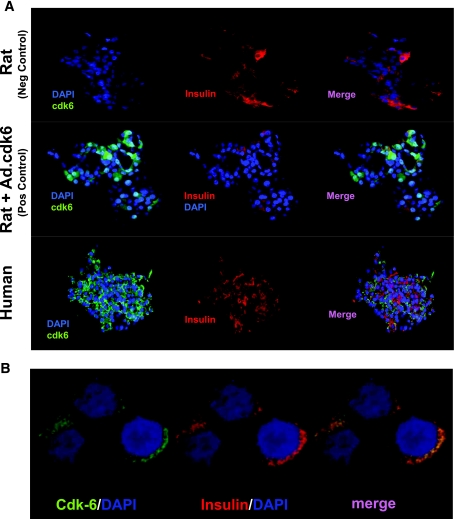
FIG. 2.
Confocal immunohistochemistry for cdk-6 in rat and human islets. A: In the upper panel, as a negative control, rat islets that do not contain cdk-6 were stained for insulin (red) or cdk-6 (green). Cdk-6 is not detectable in rat islets. In the middle panel, when rat islets are transduced with Ad.cdk-6 as a positive control, cdk-6 immunoreactivity is easily observed. In the lower panel, when human islets are stained for cdk-6, immunoreactivity is easily observed and colocalizes with insulin-positive cells. B: A higher-power magnification demonstrates that cdk-6 is present in the cytoplasm of human β-cells. (A high-quality digital representation of this figure is available in the online issue.)
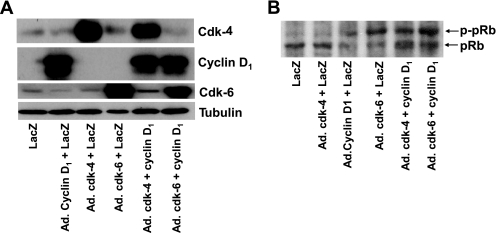
FIG. 3.
The effect of overexpression of cdk-4, cdk-6, and cyclin D1 on phosphorylation of the retinoblastoma protein, pRb. A: cdk-4, cdk-6, and cyclin D1 were overexpressed in human islets by their cognate virus but not in human islets transduced with Ad.lacZ or in normal human islets. As assessed using densitometry, cdk-4 was overexpressed 9.8-fold, cdk-6 5.5-fold, and cyclin D1 24.8-fold. B: Transduction of human islets with Ad.cdk-6, Ad.cyclin D1, or combinations of Ad.cdk-4 plus Ad.cyclin D1 or Ad.cdk-6 plus Ad.cyclin D1 leads to phosphorylation of endogenous pRb. This does not occur in the relevant controls or with Ad.cdk-4 alone. In all experiments, 500 MOI was used for 1 h with, for example, 250 MOI of Ad.lacZ plus 250 MOI of Ad.cdk-6 in lanes that contained a single cell cycle activator or 500 MOI of Ad.lacZ in which no cell cycle activator was included. Extracts were prepared and immunoblots performed 3 days after transduction.
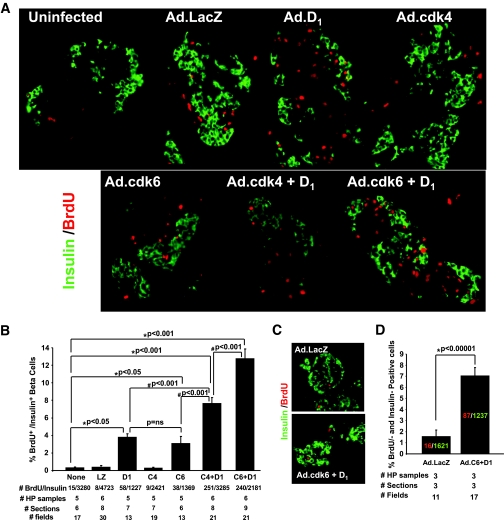
FIG. 4.
Induction of proliferation by cdk-4, cdk-6, and cyclin D1. A: Examples of isolated human islets stained for insulin (green) and BrdU (red) 3 days after transduction with adenoviruses encoding cdk-6 plus cyclin D1. B: Quantification of the BrdU incorporation into β-cells under each of these conditions. Bars represent SE. “# BrdU/insulin” refers to the number of cells that are positive for BrdU and insulin as a function of the total number of β-cells counted. “HP samples” refers to the numbers of human pancreatic islet samples examined. “# sections” refers to the number of tissue sections examined. “# fields” refers to the number of high-powered microscope fields examined. LZ, Ad.lacZ; D1, Ad.cyclin D1; C4, Ad.cdk-4; C6, cdk-6. The total MOI used for each experiment was 500, and where combinations were used, for example, “D1,” 250 MOI of each Ad.cyclin D1 and Ad.lacZ. C–D: Studies in isolated cultured human islets were similar to those in A and C but were studied 10 days after transduction with BrdU labeling for the final 24 h before harvesting the islets. The colored numbers within the bars indicate the absolute numbers of BrdU+ and insulin+ cells. (A high-quality digital representation of this figure is available in the online issue.)
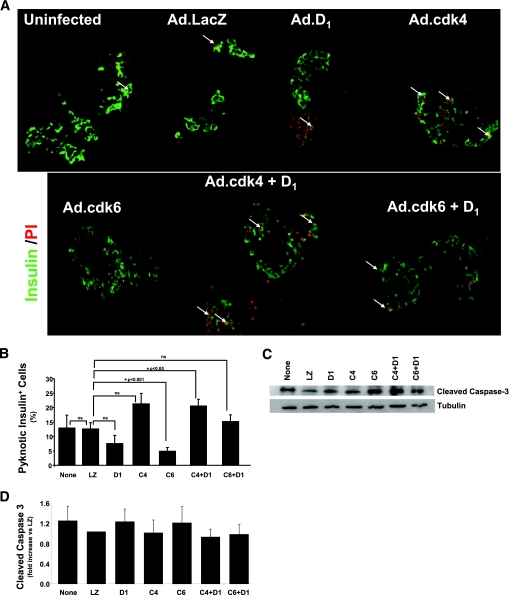
FIG. 5.
Cell death in human islets transduced with cdk-4, cdk6 and cyclin D1. Abbreviations are the same as in Fig. 4. A: Isolated human islets costained with propidium iodide (red) islets and anti-insulin antisera (green) 3 days after adenoviral transduction. Arrows illustrate pyknotic nuclei that are comparable among the several conditions. B: Quantification of these data. Cell death rates are high in cultured human islets but were not exacerbated by cdk-6, cyclin D1, or the combination. C: An immunoblot for cleaved caspace-3 in the several groups 3 days after adenoviral transduction. D: The densitometric quantification of six such experiments. Bars indicate means ± SEM. (A high-quality digital representation of this figure is available in the online issue.)
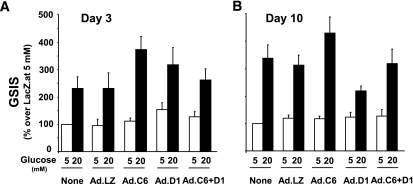
FIG. 6.
Glucose-stimulated insulin secretion (GSIS) in isolated human islets transduced with cdk-6 and cyclin D1. GSIS was studied at either 3 days (A) or 10 days (B) after transduction. Five different human islet preparations were used for each panel. Insulin secretion was determined using a human insulin ELISA kit (Alpco). Bars indicate mean ± SEM. Abbreviations are the same as in Fig. 4.
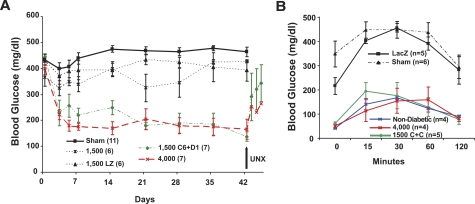
FIG. 7.
Cdk-6 and cyclin D1 enhance human islet function in vivo in streptozotocin-induced diabetic NOD-SCID mice. Bars indicate mean ± SEM. A: Sham-transplanted mice and mice transplanted with 1,500 IEQ alone or 1,500 IEQ transduced with Ad.lacZ are shown in the black lines as described in the key within the figure. Mice transplanted with 1,500 IEQ transduced with Ad.cdk-6 plus Ad.cyclinD1 are shown in green and compared with 4,000 normal, nontransduced IEQ. UNX, unilateral nephrectomy. The numbers in the key refer to the number of experimental animals in each group. The characteristics of the donor islets as provided by the ICRC procurement centers were as follows: average age ± SEM 42.8 ± 2.1 years (range 20–66), average viability 88.7% ± 1.4%, and average purity 77.9% ± 2.2%. B: Intraperitoneal glucose tolerance testing in normal (nondiabetic) NOD-SCID mice, sham-transplanted streptozotocin-induced diabetic NOD-SCID mice, diabetic NOD-SCID mice transplanted with 4,000 IEQ human islets, 1,500 human IEQ transduced with Ad.lacZ, or 1,500 IEQ transduced with 1,500 IEQ expressing cdk-6 plus cyclin D1. Studies were performed 14–21 days following transplantation. The numbers in parentheses indicate the numbers of animals studied.
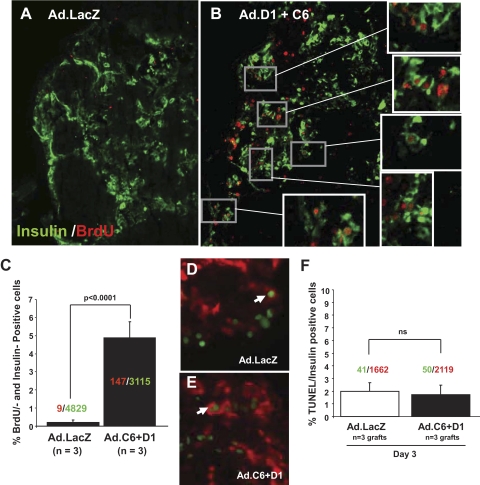
FIG. 8.
Effects of cdk-6 and cyclin D1 on β-cell proliferation and cell death in vivo. A–B: Proliferation in human β-cells in vivo 3 days after transduction and transplantation. BrdU is shown in red and β-cells are shown in green. Three grafts (three each from Ad.lacZ or Ad.cdk-6/cyclinD1) of human islets were removed by unilateral nephrectomy at day 3 after transduction (day 2 after transplantation) and examined for proliferation. The expanded boxes show examples of BrdU-positive β-cells. C: Quantification of BrdU-positive insulin-positive cells as a function of total insulin-positive cells. The numbers shown within the bars indicate the number of BrdU-positive (red) β-cells and of insulin-positive (green) cells. The numbers below the bars indicate the numbers of animals studied with two sections per animal. D and E: TUNEL staining in human islets at 3 days after transplantation. These are the same grafts as shown in A–C. F: Quantification of TUNEL staining in D and E in vivo 3 days after adenoviral transduction and transplantation. Labels are the same as in Fig. 4D. (A high-quality digital representation of this figure is available in the online issue.)
Human β-cell proliferation, cell death, and glucose-stimulated insulin secretion in vitro.
Human islet cell proliferation was assayed as reported previously (23) with the exception that the islets were labeled with bromodeoxyuridine (BrdU) for the final 24 h using a 1:1,000 dilution of BrdU (Sigma) and stained for BrdU and insulin as described subsequently for in vivo staining. Propidium iodide was used for nuclear staining and visualization of pyknotic nuclei. Glucose-stimulated insulin secretion was performed as described previously (23).
Quantification of transplanted human β-cell proliferation in vivo.
β-Cell proliferation was assessed in vivo in renal grafts as described previously (9,23–25,32) with the following modifications. Graft-containing kidneys were harvested on the third day after transplantation, 6 h after BrdU injection, fixed in 4% paraformaldehyde overnight at 4°C, paraffin-embedded, and sectioned. In the graft-containing kidneys, three serial sections separated by 25 μm were deparaffinized, rehydrated, and treated in prewarmed 1M HCl for 1 h at 37°C, blocked in 2% BSA/PBS for 1 h, then incubated overnight with anti-BrdU (Abcam, Cambridge, MA) and anti-insulin antibodies (Zymed, San Francisco, CA). After serial washing in PBST, slides were incubated for 1 h with secondary antibodies, gel-mounted, and coverslipped. Two sections per graft were analyzed. Three fields per section were quantified for BrdU- and insulin-positive cells.
Immunocytochemistry and laser confocal microscopy.
For cdk-6 immunohistochemistry, human islets were fixed in 2% paraformaldehyde for 12 min at room temperature followed by three 5-min washes in PBS. Islets were then blocked in 1× PBS, 0.2% Triton-X-100, 0.1 mmol/l CaCl, with 2% fetal bovine serum for 1 h at room temperature. Primary antibodies (supplemental Table 1, available in the online appendix) were used at 20°C overnight. Secondary antibodies were incubated for 1 h at room temperature. After three 5-min washes in PBS, islets were placed on microscope slides, coverslipped in antifade gel mount, and stored in the dark at 20°C. Islets were imaged using an Olympus Fluoview 1000 laser confocal microscope.
Adenoviruses.
Adenoviruses expressing human cyclin D1, human cdk-4 (Ad.hcdk-4), β galactosidase (Ad.lacZ), and green fluorescent protein driven by the CMV promoter were described previously (23). Adenovirus expressing human cdk-6 was prepared as described (23). Multiplicity of infection (MOI) were defined as in the past (23), and duration of exposure used is detailed in the figure legends.
Human islet marginal mass model in NOD-SCID mice.
Human islets were transplanted under the renal capsule of streptozotocin-induced diabetic NOD-SCID mice as described in detail previously (6,9,32,33). One islet equivalent (IEQ) was defined as 125 μm. All transplant studies with human islets in the streptozotocin-induced diabetes model were approved by, and performed in accordance with, the University of Pittsburgh Institutional Animal Care and Use Committee. Intraperitoneal glucose tolerance tests (IPGTTs) were performed as described previously (9,13,24,25).
For the BrdU and propidium iodide experiments, Student's unpaired two-tailed t-test was used. For the marginal mass NOD-SCID transplant model, one-way ANOVA for repeated measures was performed. P values <0.05 were considered significant.
RESULTS
The results of a screen for each of the G1/S regulatory molecules using protein extracts from human islets are shown in Fig. 1. In each of the panels, results are shown for two different, but representative, human islet extracts, labeled H1 and H2. As can be seen in Fig. 1A, each of the three members of the so-called “pocket protein family,” pRb, p107, and p130, are present in human islets. The pocket proteins regulate cell cycle progression by binding to the E2F family of transcription factors, which in turn regulate the expression of the myriad genes required for cell cycle progression. Figure 1B demonstrates that human islets contain E2Fs 1, 3, 4, and 7 but not E2Fs 2, 5, and 6.
The pocket proteins are phosphorylated by two kinase complexes, the cdks and their cyclin partners. Figures 1C and D demonstrate that human islets contain all three D cyclins, and easily measurable cyclin E, with less abundant, or at least less easily detectable, cyclin A. In addition, human islets contain easily demonstrable cdk1 (also called cdc2) and cdk2, the cognate partners of cyclins A and E.
We had preliminarily reported that human islets lack cdk-4 based on studies using antisera from Abcam and Santa Cruz and appropriate positive and negative controls (34). However, we were able to observe cdk-4 immunoreactivity using a third cdk-4 antiserum (cdk-4′ in Fig. 1D). To be certain that the cdk-4 immunoblots accurately reflected endogenous cdk-4 and not cross-reactivity with another cdk or other protein, we adenovirally overexpressed human cdk-1, cdk-2, cdk-4, and cdk-6 in human islets and performed immunoblotting using three different commercially available cdk-4 antisera. As can be seen in Fig. 1H, the Abcam 3112 and Santa Cruz 749 antisera failed to detect cdk-4 in human islets, although cdk-4 immunoreactivity was readily visible by both antisera when cdk-4 was overexpressed. In contrast, cdk-4 was easily observed in normal human islet preparations under basal conditions using the Santa Cruz 260G antiserum, and this was enhanced by overexpression of cdk-4 but not by overexpression of cdk-1, cdk-2, or cdk-6. These observations suggest that the three cdk-4 antisera do not cross-react with other cdks and that authentic cdk-4 is indeed present in human islets, but at sufficiently low levels that it is only observable using the third cdk-4 antiserum. RT-PCR and sequencing of the amplified cDNA products confirmed that human cdk-4 mRNA in present in human islets (Fig. 1I).
Surprisingly, in marked contrast to results in murine islets (17,23,35,36), human islets contain the cdk-4 homolog cdk-6 (Fig. 1D). To confirm that the cdk-6 immunoblot accurately reflected the presence of cdk-6 in human islets, we performed immunoblots using three different cdk-6 antisera, as shown in Fig. 1H. As can be seen in the figure, cdk-6 was observed in each of the human islet preparations with each of the three antisera used, and in each case, there was a specific increase in expression after transduction with Ad.cdk-6. Moreover, cdk-6 immunoreactivity was not enhanced by overexpression of cdk-1, cdk-2, nor cdk-4, indicating specificity of the antisera for cdk-6. RT-PCR and sequencing of the amplified cDNA products confirmed that human cdk-6 mRNA is present in human islets (Fig. 1I).
Upstream of the cdks and cyclins are two families of cell cycle inhibitors, the INK4 family and the CIP/KIP/WAF family. As can be seen in Figs. 1E and F, each of the four INK4s and each of the three CIP/KIP/WAF family members is easily detectable in human islets. Finally, Fig. 1G shows a number of additional G1/S regulatory molecules that are involved in cell cycle control.
To independently confirm the mentioned immunoblot studies, and to determine whether cdk-6 was present specifically in human β-cells, we performed immunohistochemistry for cdk-6. The upper row of Fig. 2A demonstrates that cdk-6 is absent or at least undetectable in rat islets. The middle row of this figure is a positive control and demonstrates that when human cdk-6 is expressed in rat islets using Ad.cdk-6, it is now easily observed in rat islets. These findings demonstrate that human cdk-6 can be reliably assessed using these immunohistochemistry techniques. The bottom row of Fig. 2A shows that native, nontransduced human islets contain easily measurable cdk-6 and that cdk-6 is present in the cytosol in β-cells. Interestingly, cdk-6 appears almost exclusively in the cytoplasmic compartment and is absent from the nucleus. These results are confirmed by higher-power magnification of human β-cells (Fig. 2B).
The preceding studies demonstrate that human islets contain cdk-6 but shed no functional light on whether cdk-6 drives cell cycle progression. To study cdk-6 function, we transduced human islets with adenoviruses expressing cdk-4 and cdk-6 alone or in combination with cyclin D1. As shown in Fig. 3A, the transductions were effective with each of the viruses leading to overexpression of the appropriate G1/S control protein. As can be seen in Fig. 3B, cyclin D1 and cdk-6 alone were able to phosphorylate pRb as were the combinations of cyclin D1 plus cdk-4 or cyclin D1 plus cdk-6.
These findings would suggest that the cdk-6/cyclin D1 combination might lead to proliferation of β-cells. To assess this, human islets were transduced with the combinations of viruses shown in Fig. 4A, and proliferation was assessed in human β-cells 3 days following transduction. As can be seen in the figure, BrdU-positive cells were rare to absent in nontransduced human islets and in human islets transduced with Ad.lacZ or cdk-4. In contrast, BrdU incorporation was regularly observed in human β-cells transduced with Ad.cdk-6 alone, Ad.cyclin D1 alone, or combinations of Ad.cdk-4 or Ad.cdk-6 plus Ad.cyclin D1. These results are quantified in Fig. 4B where it can be seen that overexpression of cdk-6 plus cyclin D1 induced a dramatic increase in proliferation with the combination leading to 13% of β-cells incorporating BrdU as compared with ∼0.3% under basal conditions or after Ad.lacz transduction, an approximate 40-fold increase in proliferation. BrdU incorporation occurs in cells other than β-cells (Fig. 4A). As shown in supplemental Fig. 2 (available in the online appendix), immunohistochemistry for insulin, glucagon, and somatostatin revealed few BrdU+ cells, suggesting that the remaining BrdU+ noninsulin+ cells in Fig. 4A are likely fibroblasts and endothelial, ductal, residual acinar, and other cell types transduced with Ad.cyclin D1-cdk-6 driven by the generalized CMV promoter.
To determine whether the β-cell proliferation was sustained for longer than 3 days, the experiments in Fig. 4A were repeated and proliferation assessed 10 days after transduction. As shown in Fig. 4C and D, BrdU incorporation in Ad.lacZ-transduced β-cells was 1.6% as compared with ∼7% in islets transduced with Ad.cdk-6 plus cyclin D1, indicating that cyclin-cdk–driven proliferation can be sustained for at least 10 days in vitro.
To determine whether the combinations of cell cycle activators also activated cell death in human β-cells, we performed propidium iodide staining and examined nuclear pyknosis as a measure of cell death. As can be seen in Fig. 5A and B, cell death represented by nuclear pyknosis was common among β-cells under basal conditions as well as following cell cycle activation by cdk-6 and cyclin D1, but there were no differences among the groups. These findings were confirmed by measuring cleaved caspace-3 in islets transduced with Ad.cdk-6 and cyclinD1 in the several combinations shown in Fig. 5C and D.
To determine whether transduction with cdk-6 plus cyclin D1 might result in dedifferentiation of β-cells, or loss of β-cell function, we examined glucose-stimulated insulin secretion studies at days 3 and 10 after transduction. As can be seen in Fig. 6, glucose-stimulated insulin secretion remained robust at both days 3 and 10.
Combined overexpression of cdk-6 and cyclin D1 enhances human islet engraftment, proliferation, and function in vivo.
To determine whether the proliferative effects of cdk-6 and cyclin D1 observed in vitro might translate to enhanced islet engraftment and function in the in vivo setting, we used the marginal mass model of human β-cell engraftment (6,9,32,33). As can be seen in Fig. 7A, sham-transplanted NOD-SCID mice induced to develop diabetes using streptozotocin remained hyperglycemic in the 450–500 mg/dl range for the 6-week duration of the experiment (negative control). In contrast, mice transplanted with 4,000 human IEQ (positive control) displayed random postprandial blood glucose values in the 150–160 mg/dl range. However, 1,500 nontransduced IEQ or 1,500 human islet IEQ transduced with Ad.LacZ caused only a mild attenuation in hyperglycemia in the 350–400 mg/dl range and therefore constituted an ideal marginal mass. Importantly, 1,500 IEQ transduced with Ad.cdk-6 plus cyclin D1 performed in a fashion far superior to the 1,500 IEQ controls and were indistinguishable from 4,000 human IEQ, indicating an approximate threefold enhancement of cdk-6/cyclinD1–transduced islets over controls. This improvement in blood glucose was sustained for the entire 6 weeks of the study but was reversed by unilateral nephrectomy.
To more rigorously define graft function in vivo, we performed IPGTTs on mice 14–21 days after islet transplant. As shown in Fig. 7B, streptozotocin-induced diabetic NOD-SCID mice treated with sham or with 1,500 IEQ of Ad.lacZ-transduced grafts were markedly glucose intolerant. In contrast, normal NOD-SCID mice displayed excellent glucose tolerance as did streptozotocin-induced diabetic animals transplanted with 4,000 IEQ. Interestingly, diabetic animals transplanted with 1,500 IEQ transduced with Ad.cdk-6 plus cyclin D1 behaved similarly to normal NOD-SCID mice and to diabetic NOD-SCID mice transplanted with 4,000 IEQ. Furthermore, fasting glucose levels in the cdk-6 plus cyclin D1 group were ∼60 mg/dl and comparable with values in nondiabetic NOD-SCID mice and those transplanted with 4,000 IEQ.
To determine whether β-cell proliferation continued in vivo after transplantation of human islets, we repeated these experiments, this time sacrificing the transplant recipients at 3 days after transplantation after in vivo labeling with BrdU. As can be seen in Fig. 8A and B, BrdU incorporation into β-cells was abundant and easily observable in the cdk-6/cyclin D1 grafts in vivo. This was in marked contrast to sections from the control grafts in which little or no proliferation was observed in β-cells. These results are quantified in Fig. 8C, in which nine of 4,829 control graft β-cells (0.2%) were BrdU-positive as compared with 147 of 3,115 β-cells (4.7%) in the cdk-6/cyclin D1 group. This difference was highly significant.
To assess survival of human β-cells after transduction and engraftment, we examined transferase-mediated dUTP nick-end labeling (TUNEL) staining in these same human grafts in Fig. 8D and E obtained 3 days after engraftment. As can be seen in Fig. 8F, ∼2% of Ad.lacZ and Ad.cdk-6/cyclin D1–transduced β-cells were TUNEL-positive at day 3 after transplant.
DISCUSSION
Whereas many studies have demonstrated that rodent β-cells replicate (3–6,9–13,17–19,27,28), most investigators agree that human β-cell replication either in vitro or in vivo is a rare event (15,17,29–31). We had previously catalogued the molecules that control the G1/S transition in the murine islet (17,23,24). Because of the central role of the G1/S transition in β-cell cycle control, because of the reported differences in the rates of β-cell proliferation rates in human versus rodent β-cells, and in light of the need to develop a means of enhancing human β-cell regeneration, we analyzed the molecules that control the G1/S checkpoint in the human islet.
We find that in contrast to murine islets which contain little or no cdk-6 (17,23,35,36), each of the four G1/S cyclins and cdks is present in the human islet. In particular, cdk-6, an activator of cell cycle progression in many cell types, is abundant in human islets and specifically within the β-cell. The inability to detect cdk-6 in murine islets and the central role of cdk-4 in murine islets are supported by the observations that cdk-4 appears to be the downstream mediator of β-cell proliferation induced by the IRS-1–PI3 kinase pathway in murine β-cells (8) that knockout of cdk-4 leads to severe β-cell attrition and neonatal diabetic ketoacidosis in mice (21), whereas cdk-6 knockout mice display no β-cell phenotype but instead have severe hematologic abnormalities (36). Our observations suggest that although cdk-4 is essential to murine islet development and function, cdk-4 in human islets is likely redundant with cdk-6, which may play a similar and complimentary role in human β-cells.
In functional terms, overexpression of cdk-6 in combination with cyclin D1 leads to uniquely robust proliferation in human β-cells. We have previously demonstrated that cdk-4 overexpression in combination with cyclin D1 in human β-cells led to an approximate ninefold increase in proliferation (23). However, these studies were limited entirely to in vitro observations for 48–72 h. We report that a second combination of cell cycle activators (cdk-6 in combination with cyclin D1) can induce an even greater stimulation in human β-cell replication—for some, 40-fold. Furthermore, unlike cdk-4 overexpression, which alone is unable to either measurably phosphorylate pRb or activate β-cell replication (23) (Fig. 3B), cdk-6 overexpression alone is able to accomplish both of these outcomes, presumably by combining with endogenous β-cell d-type cyclins. Furthermore, as we previously reported for the cdk-4/cyclin D1 combination (23), cell death was not activated in human β-cells by the cdk-6/cyclin D1 combination. Future studies will be required to define whether cdk-6 alone will prove as effective in vivo as when supplied in combination with cyclin D1.
Perhaps the most critical observation in the current study is that, unlike prior in vitro studies with human islets and human autopsy specimens in which human β-cell replication does not occur (15,17,29–31), induction of human β-cell replication can be documented and quantified in vivo for the first time using the NOD-SCID human islet transplant model described here. In addition, the whole-animal glucose and insulin metabolic axis can simultaneously be monitored. Although the diabetic NOD-SCID mouse model is not novel, its use to document accelerated human β-cell replication is. This human islet transplant model is important, because it will permit investigators to ask for the first time, using human islets, whether interventions such as glucose infusion (37), diet-induced obesity (20), lipid infusion (38), pregnancy (27), or treatment with growth factors or drugs such as GLP-1, exenatide, gastrin, EGF, or others (9–13) can lead to activation of human β-cell replication in vivo.
In addition to the studies centered on cdk-6, the human G1/S proteome reveals a variety of additional novel observations. For example, human islets, like their murine counterparts, contain all seven INK4 and CIP/KIP/WAF family members, all three pocket proteins, menin, and p53. That is, every single cell cycle inhibitor that might be present in any cell type is present in the human islet; if one wanted to design a cell type that could not possibly replicate, this is how it would be accomplished. Their simple presence of course does not prove function, but this model will also allow further detailed in vivo study. For example, physiological and therapeutic activation of cell cycle progression in human β-cells, like their murine counterparts (19–26), might be accomplished by knockdown or silencing of individual or combinations of cell cycle inhibitors such as menin, p18 and p27, or pRb and p53.
Another novel observation is that human islets contain all three pocket protein members, pRb, p107, and p130; previously, only pRb has been identified in human islets (23). As another example, human islets lack E2F2, a protein that is present in murine islets (17,23,24) and the loss of which in knockout mice leads to severe pancreatic exocrine and endocrine dysfunction (39,40). Similarly, human islets contain E2F3 and E2F7, whereas their murine counterparts lack both E2F3 and E2F7 (17,23,24). The functional and therapeutic implications of these observations are currently unknown but clearly merit further exploration.
A number of additional important questions remain for future studies. One important question relates to the quantification of β-cell mass loss or accrual in this model. It would be ideal to be able to accurately assess β-cell mass as well as β-cell differentiation. However, this will be challenging given the complex mixture of cell types contained in islet grafts (6,32,41). Potential approaches to quantitate increments in β-cell mass might include intraocular anterior chamber transplant (42) or combining insulin promoter-driven viral delivery of luciferase with external imaging coupled with graft histomorphometry.
It is also important to emphasize that the immunoblots describing the human G1/S proteome were performed on whole human islets, which are comprised predominantly of non-β-cells. Thus, for the cell cycle proteins described (other than cdk-4 and cdk-6, which were confirmed to be present in the β-cell using immunohistochemistry), future studies will need to unequivocally document the presence or absence of these molecules in human β-cells.
Another important question relates to the duration of the adenovirus expression and β-cell replication induced by cdk-6/cyclin D1. We have examined only a single early time point, but it is important to know whether adenoviral expression is sustained for the entire 6 weeks of the experiment or even longer. In this regard, we have recently demonstrated, using a similar model, that adenoviral expression of hepatocyte growth factor in transplanted primate islets persists for at least 6 weeks (43).
Although we show that cdk-6 overexpression is able to activate the β-cell cycle, this does not address the physiological role of cdk-6 in the human β-cell nor mechanisms through which it acts. Further studies will be required to define the consequences of cdk-6 loss on human β-cells and to identify the cyclin INK/KIP/CIP partners of cdk-6. This is particularly interesting in that the large majority of cdk-6 is present in the cytosolic compartment, not the nuclear compartment where it might phosphorylate pocket proteins. Is cdk-6 a cytosolic protein only in resting β-cells that translocates to the nucleus in proliferating β-cells? Or does cdk-6 always remain in the cytoplasm? Can it drive cell cycle progression by sequestering CIPs/KIPs in the cytoplasm away from cdk-1/cdk-2 cyclin A/E complexes? Answering these questions and others will require purification of human β-cells, coimmunoprecipitaton, colocalization, live-cell imaging, and other studies. In addition, whether cdk-6 expression or activity is regulated by growth factors and signaling pathways in human islets, as is cdk-4 in the murine islet (8), provides fertile ground for future study.
Finally, cdks and cyclins are oncogenes. This raises the question as to whether sustained therapeutic cdk or cyclin activation might have oncogenic consequences. On the “high oncogenic risk” side of the equation, cdk-4 is associated with melanoma and other malignancies and cyclin D1 with insulinomas, breast cancer, and lymphomas (44–46). On the “low oncogenic risk” side of the equation, these cell cycle molecules of course are also normal regulators of cell cycle replication in all cells, including β-cells. Under normal physiological circumstances, their level of expression is tightly controlled and does not lead to tumor development but suffices to expand β-cell mass in embryology and childhood. Thus, if it were possible to control the duration and degree of expression of these molecules through the use of β-cell–specific and regulatable promoters, their degree and duration of activation might be engineered to mimic physiological β-cell replication and expansion. Because the islet grafts can be left in place for months or even 1 or 2 years, the model described here will permit accurate and meaningful study of their oncogenicity.
In summary, we have provided a human β-cell cycle “G1/S proteome,” which can serve as a road map or model to more deeply understand and exploit human β-cell replication. We have also developed an in vivo model in which to explore the function of these molecules as well as a broad variety of physiological maneuvers on human β-cell replication. We have also demonstrated a variety of other unanticipated differences between rodent and human G1/S regulatory machinery. Collectively, these studies make three fundamental points. First, rodent studies aimed at augmenting human β-cell replication need to be confirmed in human islets; if one wants to understand human β-cell replication, one will need also to study human β-cells. Second, although progress is being made in understanding the molecular details of cell cycle control in the pancreatic β-cell, current understanding is still rudimentary. Third, it is clearly possible to drive human β-cell replication in ways that retain differentiation and function in vivo, at least over a period of days to weeks. Approaches designed to exploit fundamental cell cycle control in human β-cells may provide insight into how best to expand human β-cells for therapeutic purposes in the treatment of diabetes.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants R-01 DK5502 and T-32 DK07052, American Diabetes Association Grant 1-06-134, and Juvenile Diabetes Research Foundation (JDRF) Grant 1-2008-39.
No potential conflicts of interest relevant to this article were reported.
We thank the NIDDK- and JDRF-supported Islet Cell Resource Center for providing the majority of the islets used in these studies. We thank the Don and Arleen Wagner and the Kroh Family Foundations for supporting these studies. We thank Dr. Simon Watkins at the University of Pittsburgh Center for Biological Imaging for help with confocal microscopy and Bennet Smith for technical help. We also thank Dr. Alan Attie and Jeremy Lavine at the University of Wisconsin for helpful advice and comments.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343: 230– 238, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Rother KI, Harlan D: Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 114: 877– 883, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic ß-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41– 46, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Brennand K, Huangfu D, Melton D: All beta cells contribute equally to islet growth and maintenance. PLOS Biology 5: e163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553– 2561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasavada RC, Wang L, Fujinaka Y, Takane KK, Rosa TC, Mellado-Gil, Friedman PA, Garcia-Ocaña A: Protein kinase C-zeta activation markedly enhances beta cell proliferation. Diabetes 56: 2732– 2743, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Heit JJ, Apelqvist AA, Gu X, Winslow M, Neilson JR, Crabtree GR, Kim SK: Calcineurin/NFAT signaling regulates pancreatic ß-cell growth and function. Nature 443: 345– 349, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E: Akt induces β-cell proliferation by regulating cyclin d1, cyclin d2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 55: 318– 325, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Ocaña A, Takane KK, Reddy VT, Lopez-Talavera J-C, Vasavada RC, Stewart AF: Adenovirus-mediated hepatocyte growth factor transfer to murine islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem 278: 343– 351, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A: Combination therapy with epidermal growth factor and gastrin increases beta cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes 54: 2596– 2601, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696– 1705, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L: NeuroD-betacellulin therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 9: 596– 603, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fujinaka Y, Sipula D, Garcia-Ocaña A, Vasavada RC: Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic beta cell survival. Diabetes 53: 3120– 3130, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Meier JJ, Bushan A, Butler AE, Rizza RA, Butler PC: Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48: 2221– 2228, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta cell deficit and increased beta cell apoptosis in humans with diabetes. Diabetes 52: 102– 110, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Yoon KH, Ko H, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S: Selective ß cell loss and α cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88: 2300– 2308, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada RC, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta cell. Endocr Rev 27: 356– 370, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Heit JJ, Karnik SK, Kim SK: Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol 22: 311– 338, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Karnick SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McClean GW, Xiong Y, Meyerson M, Kim SK: Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27kip1 and p18Ink4c. Proc Natl Acad Sci U S A 102: 14659– 14664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M: Deletion of cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11: 175– 182, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Rane S, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M: Loss of expression of cdk4 causes insulin-deficient diabetes and cdk4 activation results in beta cell hyperplasia. Nat Genet 22: 44– 52, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Zhong L, Georgia S, Tschen S, Nakayama K, Nakayama K, Bhushan A: Essential role of skp-2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest 117: 2869– 2876, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF: Induction of beta cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenoviral delivery of cyclin-dependent kinase-4 and cyclin D1. Diabetes 53: 149– 159, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Cozar-Castellano I, Haught M, Stewart AF: The cell cycle inhibitory protein, p21cip, is not essential for maintaining beta cell cycle arrest or beta cell function in vivo. Diabetes 55: 3271– 3278, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Vasavada RC, Cozar-Castellano I, Sipula D, Stewart AF: Tissue-specific deletion of the retinoblastoma protein in the pancreatic beta cell has limited effects on beta cell replication, mass and function. Diabetes 56: 57– 64, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE: p16ink4a induces an age-dependent decline in islet regenerative potential. Nature 443: 453– 457, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sorenson RL, Brelje TC: Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29: 301– 307, 1997 [DOI] [PubMed] [Google Scholar]
- 28.De Leon DD, Deng S, Madani R, Ahima RS, Drucker DJ, Stoffers DA: Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes 52: 365– 371, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Tyrberg B, Eizirik DL, Hellerstrom C, Pipeleers DG, Anderssen A: Human pancreatic beta cell deoxyribonucleic acid synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology 137: 5694– 5699, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Brunn C, Mandrup-Poulsen T, Billlestrup N, Halban P: Proliferation of sorted human and rat beta cells. Diabetologia 51: 91– 100, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC: A selective decrease in beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 59: 817– 820, 1995 [PubMed] [Google Scholar]
- 32.Rao P, Roccisana J, Takane KK, Bottino R, Zhao A, Trucco M, Garcia-Ocana M: Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic severe combined immunodeficient mice. Diabetes 54: 1664– 1675, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Talavera J-C, Garcia-Ocaña A, Sipula I, Takane KK, Cozar I, Stewart AF: Hepatocyte growth factor gene therapy for pancreatic islets: reducing the minimal requisite islet transplant mass in a glucocorticoid-free rat model of allogeneic portal vein islet transplantation. Endocrinology 145: 467– 474, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Bigatel TA, Cozar-Castellano I, Velasquez S, Harb G, Fiaschi-Taesch N, Selk K, Takane KK, Stewart AF: Comprehensive comparative cell cycle analysis reveals critical differences between human and murine beta cell cycle regulation: human islets contain Cdk-6 but lack Cdk-4. In Proceedings of the 67th ADA Annual Meeting, Chicago, IL, June 2007 [Google Scholar]
- 35.Martin J, Hunt SL, Dubus P, Sotillo R, Nehme-Pelluard F, Magnuson MA, Parlow AF, Malumbres M, Ortega S, Barbacid M: Genetic rescue of cdk-4-null mice restores pancreatic beta cell proliferation but not homeostatic cell number. Oncogene 22: 5261– 5269, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M: Mammalian cell cycle without D-type cyclin-dependent kinases cdk-4 and cdk-6. Cell 118: 493– 504, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocaña A: Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56: 1792– 1801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, Semache M, Lausier JA, Roskens V, Mirmira RG, Jetton TL, Poitout V: Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and pdx-1 binding in islets. Diabetes 57: 424– 431, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li FX, Zhu JW, Tessem JS, Beilke J, Varella-Garcia M, Jensen J, Hogan CJ, DeGregori J: The development of diabetes in E2F1/E2F2 mutant mice reveals important roles for bone marrow derived cells in preventing islet loss. Proc Nat Acad Sci U S A 100: 12935– 12940, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglesias A, Murga M, Laresgoiti U, Skoudy A, Bernales I, Fullaondo A, Moreno B, Lloreta J, Field SJ, Real FX, Zubiaga AM: Diabetes and exocrine pancreatic insufficiency in E2F1/E2F2 double mutant mice. J Clin Invest 113: 1398– 1407, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayek A, Beattie GM: Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab 82: 2471– 2475, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Speier S, Nyqvist D, Cabrera O, Yu J, Damaris Molano R, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, Ricordi C, Leibiger IB, Caceido A, Bergstron P-O: Non-invasic imaging of pancreatic islet cell biology. Nat Med 14: 574– 578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiaschi-Taesch NM, Berman-Weinberg D, Sicari BM, Takane KK, Garcia-Ocaña A, Ricordi C, Kenyon NS, Stewart AF: Hepatocyte growth factor (HGF) enhances engraftment and function of non-human primate islets. Diabetes 57: 2745– 2754, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung DC, Brown SB, Grahme-Cooke F, Seto M, Warshaw AL, Jensen RT, Arnold A: Overexpression of cyclin D1 occurs frequently in human pancreatic endocrine tumors. J Clin Endocrinol Metab 85: 4373– 4378, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Sotillo R, Dubus P, Martin J, de la Cueva E, Ortega S, Malumbres M, Barbacid M: Wide spectrum of tumors in knock-in mice carrying a cdk4 protein insensitive to INK4 inhibitors. EMBO J 20: 6637– 6647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croce CM: Oncogenes and cancer. N Engl J Med 358: 502– 511, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.