Abstract
OBJECTIVE
To determine to what extent plasma C-reactive protein (CRP) values influence 5-year all-cause and cardiovascular mortality in type 2 diabetic individuals, independently of albumin excretion rate (AER) and other cardiovascular risk factors, and its incremental usefulness for predicting individual risk of mortality.
RESEARCH DESIGN AND METHODS
Measurements of CRP were performed in 2,381 of 3,249 (73.3%) subjects as part of the population-based Casale Monferrato Study. Its association with 5-year all-cause and cardiovascular mortality was assessed with multivariate Cox proportional hazards modeling. The C statistic and measures of calibration and global fit were also assessed.
RESULTS
Results are based on 496 deaths in 11.717 person-years of observations (median follow-up 5.4 years). With respect to subjects with CRP ≤3 mg/l, those with higher values had an adjusted hazard ratio (HR) of 1.51 (95% CI 1.18–1.92) for all-cause mortality and 1.44 (0.99–2.08) for cardiovascular mortality. In normoalbuminuric subjects, respective HRs of CRP were 1.56 (1.13–2.15) and 1.65 (1.00–2.74), AER being neither a modifier nor a confounder of CRP association. In analysis limited to diabetic subjects without cardiovascular disease (CVD), adjusted HRs were 1.67 (1.24–2.24) for all-cause mortality and 1.36 (0.83–2.24) for cardiovascular mortality. The improvement in individual risk assessment was marginal when measured with various statistical measures of model discrimination, calibration, and global fit.
CONCLUSIONS
CRP measurement is independently associated with short-term mortality risk in type 2 diabetic individuals, even in normoalbuminuric subjects and in those without a previous diagnosis of CVD. Its clinical usefulness in individual assessment of 5-year risk of mortality, however, is limited.
Inflammation is a crucial factor in the atherosclerotic disease process (1). High-sensitivity C-reactive protein (CRP) is an acute-phase response protein that is considered both a marker of inflammation and a predictor of cardiovascular events, including myocardial infarction, stroke, peripheral arterial disease, and sudden cardiac death (2–4). CRP may also act directly on atherosclerosis, promoting endothelial cell activation and adhesion molecule expression, resulting in endothelial dysfunction (5,6).
Studies have pointed out that diabetic individuals have higher concentrations of CRP than nondiabetic individuals, suggesting an increased role of inflammation in the accelerated atherosclerosis seen in these patients (7–14). As CRP has been reported to correlate with cardiovascular risk factors, such as hyperglycemia, hypertension, and lipids, its predictive role on cardiovascular events in individuals with type 2 diabetes could be merely due to the effect of associated factors (7–9). Population-based studies examining the association between CRP and cardiovascular disease in diabetic people are limited (4,10). Most studies have been hampered by 1) the recruitment of clinic-based rather than population-based cohorts of subjects, which limits the extrapolation of results to the whole diabetic population (2,11); 2) the cross-sectional rather than prospective study design, which limits the identification of temporal relationship between CRP and events (7–9,12–14); and 3) the spectrum of risk factors assessed simultaneously, not including microalbuminuria, the main predictor of cardiovascular events in diabetic people, thus limiting the assessment of the interrelationship between markers of inflammation and markers of endothelial dysfunction (4,10). Moreover, conflicting results on the incremental usefulness of CRP measurement for cardiovascular prediction have been provided in nondiabetic subjects (15–18), whereas no data are available in subjects with type 2 diabetes.
The aims of this report of the ongoing population-based Casale Monferrato Study (19–21) were to determine 1) to what extent a CRP value influences 5-year all-cause and cardiovascular mortality in type 2 diabetic subjects, independently of other cardiovascular risk factors; 2) whether microalbuminuria acts as a confounder or modifier of this association; and 3) the incremental mortality prediction using CRP in subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The study base was composed of 3,249 patients with known type 2 diabetes who in 2000 were residents of the town of Casale Monferrato in northwestern Italy (93,477 inhabitants) (19). They were identified in the second prevalence survey of the Casale Monferrato Study, using independent data sources (diabetes clinics; administrative data sources), obtaining a high degree of ascertainment (80%). The study was approved by the institutional review committee. As described in detail elsewhere, at baseline all patients were interviewed and examined by trained investigators after having provided informed consent (19). All laboratory determinations were centralized. Venous blood samples were collected after overnight fasting for determination of triglycerides, total cholesterol, HDL cholesterol (enzymatic colorimetric method after precipitation with Mn2+), apolipoprotein (apo) A1, apoB (turbidimetric method, BM/Hitachi 717; BBR, Tokyo, Japan), and A1C (high-performance liquid chromatography; Daiichi, Menarini, Japan) (laboratory reference range 3.8–5.5%). LDL cholesterol was calculated from Friedewald's formula for all subjects in the cohort whose triglyceride values were <4.48 mmol/l. One overnight timed urine collection was obtained, after exclusion of urinary tract infection, congestive heart failure, and other known causes of nondiabetic renal disease, and the urinary albumin concentration was measured by using the nephelometric method (coefficient of variation [CV] = 4%) (Behring Nephelometer Analyzer; Behring Institute, Marburg, Germany). The albumin excretion rate (AER) was then calculated. Fibrinogen levels were measured in 1,399 of 2,381 subjects also having CRP measurements (59%). High-sensitivity CRP levels were measured using an immunoturbidimetric method (CV = 0.5%) (Roche Diagnostic). Hypertension was defined as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg or treatment with antihypertension drugs. Central obesity was defined as waist circumference ≥102 cm in men and ≥88 cm in women. Cardiovascular disease (CVD) was defined as physician-diagnosed myocardial infarction, angina, coronary artery bypass graft, stroke, arterial disease of lower-limb, or epiaortic arterial trunks. The Rose questionnaire was also admistered. Coronary heart disease was also defined on the basis of electrocardiographic abnormalities according to the Minnesota code, as probable (major Q and QS items, codes 1.1 and 1.2) or possible (minor Q and QS items, S-T/T items, codes 1.3, 4.1–4.4, and 5.1–5.3). Smoking habit was classified into one of three categories (never smoker, ex-smoker if patient stopped smoking at least 1 month before the visit, and smoker). We estimated glomerular filtration rate (GFR) by using the four-component abbreviated equation from the Modification of Diet in Renal Disease Study (all patients were Euripids): estimated GFR = 186 × (serum creatinine [mg/dl])1.154 × age0.203 (× 0.742 if female) (22). For all patients enrolled the date of diagnosis was retrieved and recorded.
Statistical analysis.
Analyses were performed using baseline variables. Variables distributed normally are presented as means ± SD, whereas variables with skewed distribution were analyzed after logarithmic transformation (triglycerides, AER, creatinine, and CRP) and results presented as geometric means and 95% CIs. Differences in clinical characteristics of patients were assessed using the t test for continuous variables and χ2 test for ordinal variables. Pearson correlations between CRP and continuous variables were also performed. ANCOVA was performed to adjust CRP values for potential confounders. Information as to which subjects had died up to 31 December 2006 was obtained from the demographical files of towns of residence, hospital discharges, and autopsy records. Underlying causes of death were derived and coded by two authors (G.B. and O.S.), according to the ICD-9. Mortality rates were calculated by dividing the number of deaths occurring during the study period by the number of person-years of observation. We performed mortality analyses of the cohort using multivariate Cox proportional hazards modeling to estimate the hazard ratios (HRs) of cardiovascular and all-cause mortality by CRP values, independently of AER and other risk factors commonly examined in diabetological clinical practice in Italy (23,24). Results are reported as HRs with 95% CIs for tertiles 2 and 3 of CRP values compared with tertile 1. Models for either logCRP as a continuous measure, assessing the multiplicative increase of each increment of CRP to mortality risk, or clinically relevant cutoff points of CRP (<1.0, 1–3, or >3 mg/l) were also constructed. As HRs of CRP values 1–3 mg/l and <1 mg/l were similar, they were aggregated as the reference category and compared with CRP values >3 mg/l.
To assess the incremental usefulness of CRP with respect to traditional cardiovascular risk factors, all models were adjusted for age, sex, diabetes duration (model 1), hypertension, A1C, smoking, LDL cholesterol, HDL cholesterol, CVD, and diabetes treatment (model 2). Central obesity (yes versus no) and treatment with statins (yes versus no) were also included in models 2 and 3, as they might provide residual confounding of the relationship between CRP and mortality. The role of AER was examined in model 3 using clinically relevant strata (<20 vs. ≥20 μg/min). Models were constructed with variables as continuous measures to provide maximum power for detecting an association between CRP and mortality. In addition, we allowed any of the following variables to enter the model if they add significantly or modified HRs of CRP (uric acid, apoA1/apoB, and estimated GFR). The interrelationship between CRP and fibrinogen with respect to mortality was further assessed including a categorical variable with two strata of fibrinogen with cutoff point defined by its median value (3.5 g/l). Stratified mortality analysis by sex, CVD at the baseline examination, median values of A1C, diabetes duration, LDL cholesterol, and HDL cholesterol have also been performed. The likelihood ratio test was used to assess the statistical significance of examined variables in nested models. We also carried out analyses that included terms for the interaction between CRP and sex, A1C, BMI, waist, AER, and CVD. Tests for nonproportional hazard using Schoenfeld residuals resulted in nonsignificant findings in all analyses. The P value was two sided; a P value of <0.05 was considered to indicate statistical significance.
As statistical significance does not imply either clinical significance or improvement in model performance, we also examined discrimination and calibration of the model including CRP with respect to the model without CRP in estimating individual mortality risk. In our analysis, discrimination is the ability to separate diabetic subjects of the Casale Monferrato cohort who will die and those who will survive during the 5-year follow-up period. This issue is typically described by the concordance or C statistic, which is equivalent to the area under the receiver-operating characteristic curve, and is calculated as the fraction of pairs of subjects (one who has an event and one who does not) for which the probability of the event is higher in the subject who has the event. The C index can range from 0.5 (no predictive ability) to 1 (perfect discrimination). In our analysis, the C statistic shows the proportion of pairs for which the model assigns higher probability to the subject who has died than to the subject who has survived. As the C statistic is a measure of discrimination based on ranks, however, it is still debatable whether it is the best way to assess the power of prediction for a given risk factor (16). Therefore, we also provide as measure of model fit the Bayes information criterion, a likelihood-based measure that adds a penalty for model complexity, with lower values indicating better fit (25).
The second component of model performance is calibration. In our analysis, it shows how close the probability of diabetic subjects dying as predicted by the model including CRP is to the observed probability of dying in this cohort. To assess this issue, we used the Grønnesby and Borgan calibration test, which compares the number of events that are observed with those that are expected on the basis of the estimation from the Cox models, with four groups (26). Finally, to assess the impact of CRP values on clinical reclassification of mortality risk, we examined how many individuals would be reclassified using the final model with and without CRP. We categorized the predicted probability of surviving into deciles (0–0.10, 0.10–0.20,…0.90–1.00) to assess the number of people who were reclassified by CRP into different categories of probability, separately among those who survived and those who died. Analyses were performed with Stata software version 10.0.
RESULTS
Measurements of CRP values were available in 2,381 of 3,249 (73.3%) diabetic subjects of the population-based Casale Monferrato Study. With respect to subjects who were not recruited, those who were selected were younger (67.6 ± 10.4 vs. 73.2 ± 12.9 years, P < 0.0001), had lower duration of the disease (10.7 ± 7.9 vs. 14.0 ± 9.4 years, P < 0.0001), and had lower A1C values (7.02 ± 1.8 vs. 7.6 ± 1.8, P = 0.0004) but similar systolic (145.7 ± 16.4 vs. 145.9 ± 19.0 mmHg, P = 0.93) and diastolic blood pressure (82.8 ± 8.2 vs. 82.6 ± 8.0 mmHg, P = 0.80). At the baseline examination, most of diabetic subjects were elderly, 1,505 of 2,381 (63.2%) being ≥65 years at recruitment. A1C <7% was obtained in 57.4% of the overall cohort (A1C interquartile range 5.7–8.1).
The distribution of CRP was right skewed, with a median value of 2.6 mg/dl (interquartile range 1.3–5.8). Sex differences in CRP values were found, with significantly higher values (P < 0.0001) in women than in men (3.1 mg/l [1.4–6.9] vs. 2.2 mg/l [1.1–4.9]), even after adjustment for age, BMI, waist, and blood pressure. CRP values were positively correlated with BMI (r = 0.30, P < 0.0001), waist circumference (r = 0.27, P < 0.0001), systolic blood pressure (r = 0.10, P < 0.0001), diastolic blood pressure (r = 0.08, P = 0.0006), plasma glucose (r = 0.15, P < 0.0001), A1C (r = 0.13, P < 0.0001), total cholesterol (r = 0.10, P < 0.0001), LDL cholesterol (r = 0.10, P < 0.0001), apoB (r = 0.16, P < 0.0001), fibrinogen (r = 0.35, P < 0.0001), uric acid (r = 0.15, P < 0.0001), and AER (r = 0.10, P < 0.0001) and negatively correlated with HDL cholesterol (r = −0.13, P < 0.0001) and apoA1 (r = −0.16, P < 0.0001).
As shown in Table 1, with respect to people with CRP values in the lowest tertile, those with CRP values in the highest tertile had similar age, duration of diabetes, smoking habits, and glucose-lowering and statin treatments but significantly higher values of BMI, waist circumference, blood pressure, and AER and plasma values of A1C, lipids, fibrinogen, and uric acid, as well as higher prevalence of CVD.
TABLE 1.
Baseline characteristics of subjects with type 2 diabetes of the Casale Monferrato Study, by CRP values
| CRP tertiles |
P | |||
|---|---|---|---|---|
| <1.6 mg/l | 1.6–4.4 mg/l | >4.4 mg/l | ||
| n | 759 | 834 | 788 | |
| Men | 434 (57.2) | 422 (50.6) | 328 (41.6) | <0.0001 |
| Age (years) | 67.1 ± 10.6 | 68.1 ± 9.9 | 67.7 ± 10.7 | 0.16 |
| Duration of diabetes (years) | 11.2 ± 8.4 | 10.3 ± 7.4 | 10.5 ± 8.0 | 0.06 |
| BMI (kg/m2) | 26.7 ± 4.0 | 28.7 ± 4.5 | 30.6 ± 6.0 | <0.0001 |
| Waist circumference (cm) | 93.9 ± 11.0 | 98.8 ± 11.6 | 101.9 ± 12.5 | <0.0001 |
| Systolic blood pressure (mmHg) | 143.2 ± 15.9 | 146.7 ± 15.9 | 147.1 ± 17.0 | <0.0001 |
| Diastolic blood pressure (mmHg) | 81.8 ± 7.9 | 82.2 ± 8.1 | 83.4 ± 8.5 | <0.0001 |
| Hypertension | 653 (86.3) | 740 (89.1) | 718 (91.1) | 0.01 |
| Glucose (mg/dl) | 163.0 ± 48.7 | 172.9 ± 52.9 | 182.6 ± 61.0 | <0.0001 |
| A1C (%) | 6.8 ± 1.7 | 6.9 ± 1.7 | 7.3 ± 1.9 | <0.0001 |
| Total cholesterol (mg/dl) | 205.6 ± 40.6 | 213.8 ± 38.7 | 215.4 ± 40.6 | <0.0001 |
| LDL cholesterol (mg/dl) | 123.0 ± 35.5 | 128.7 ± 31.9 | 130.9 ± 35.0 | <0.0001 |
| HDL cholesterol (mg/dl) | 57.2 ± 16.1 | 54.5 ± 14.8 | 52.7 ± 14.2 | <0.0001 |
| Triglycerides (mg/dl) | 113 (79–155) | 134 (96–178) | 141 (101–192) | <0.0001 |
| ApoA1 (mg/dl) | 160.5 ± 39.5 | 154.7 ± 37.8 | 147.1 ± 38.4 | <0.0001 |
| ApoB (mg/dl) | 96.1 ± 26.0 | 103.5 ± 25.9 | 106.7 ± 27.4 | <0.0001 |
| ApoB/apoA1 | 0.65 ± 0.31 | 0.72 ± 0.31 | 0.79 ± 0.37 | <0.0001 |
| Creatinine (mg/dl) | 0.84 (0.70–0.97) | 0.84 (0.71–0.98) | 0.86 (0.69–1.02) | 0.16 |
| Fibrinogen (g/l) | 353.5 ± 66.5 | 371.8 ± 68.9 | 413.1 ± 96.2 | <0.0001 |
| Uric acid (mg/dl) | 5.22 ± 1.44 | 5.57 ± 1.55 | 6.08 ± 2.53 | <0.0001 |
| AER (μg/min) | 10.9 (4.0–22.6) | 13.1 (4.6–27.7) | 16.3 (4.9–40.7) | <0.0001 |
| 20–200 | 137 (22.9) | 169 (25.7) | 184 (29.8) | <0.0001 |
| >200 | 24 (4.0) | 31 (4.7) | 46 (7.5) | |
| CVD | 136 (17.9) | 180 (21.6) | 194 (24.6) | 0.006 |
| Smoking | ||||
| Former smoker | 243 (32.9) | 236 (29.7) | 221 (28.9) | 0.28 |
| Smoker | 112 (15.2) | 130 (16.3) | 109 (14.2) | |
| Glucose-lowering treatment | ||||
| Diet | 128 (17.0) | 135 (16.4) | 102 (13.1) | 0.21 |
| Oral drugs | 549 (73.0) | 605 (73.7) | 585 (75.4) | |
| Insulin | 75 (10.0) | 81 (9.9) | 89 (11.5) | |
| Statins | 163 (21.5) | 163 (19.5) | 151 (19.2) | 0.77 |
Data are means ± SD, n (%), or geometric means (95% CI).
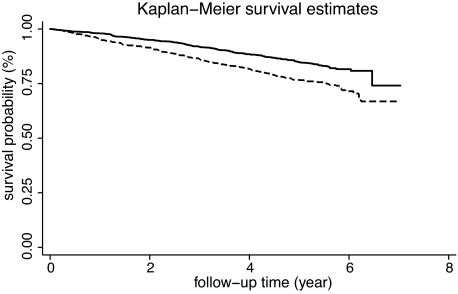
During the 5-year follow-up period (median 5.4 years [range 0.1–7.0]), 496 diabetic subjects died, of 11,716 person-years, giving an all-cause mortality rate per 1,000 person-years of 42.3 (95% CI 38.8–46.2). Table 2 shows that with respect to people who survived, those who died had higher values of CRP and other risk factors, such as duration of diabetes, prevalence of hypertension, micro- and macroalbuminuria, and CVD. Figure 1 shows the Kaplan-Meier curves depicting the survival curves of the cohort by CRP levels (log-rank test P < 0.001).
TABLE 2.
Baseline characteristics of people with type 2 diabetes of the Casale Monferrato Study, by living status on 31 December 2006
| Survived | Deceased | P | |
|---|---|---|---|
| n | 1,885 | 496 | |
| Men | 922 (48.9) | 262 (52.8) | 0.12 |
| Age (years) | 65.9 ± 10.2 | 74.1 ± 8.5 | <0.0001 |
| Duration of diabetes (years) | 10.2 ± 7.8 | 12.6 ± 8.4 | <0.0001 |
| CRP (mg/dl) | 2.5 (1.2–5.3) | 3.7 (1.6–9.1) | <0.0001 |
| <1.6 | 642 (34.1) | 117 (23.6) | <0.0001 |
| 1.6–4.4 | 671 (35.6) | 163 (32.8) | |
| >4.4 | 572 (30.3) | 216 (43.6) | |
| BMI (kg/m2) | 28.9 ± 5.0 | 28.0 ± 5.4 | 0.0009 |
| Waist circumference (cm) | 98.2 ± 12.2 | 98.3 ± 12.8 | 0.82 |
| Systolic blood pressure (mmHg) | 145.5 ± 16.2 | 146.7 ± 17.1 | 0.14 |
| Diastolic blood pressure (mmHg) | 83.0 ± 8.2 | 82.3 ± 8.4 | 0.10 |
| Hypertension | 1,653 (87.9) | 458 (92.3) | 0.005 |
| Glucose (mg/dl) | 171.5 ± 53.5 | 178.3 ± 60.0 | 0.014 |
| A1C (%) | 7.0 ± 1.7 | 7.3 ± 1.9 | 0.0003 |
| Total cholesterol (mg/dl) | 213.1 ± 40.0 | 206.3 ± 40.2 | 0.001 |
| LDL cholesterol (mg/dl) | 129.0 ± 34.2 | 122.3 ± 33.9 | 0.0002 |
| HDL cholesterol (mg/dl) | 54.7 ± 14.8 | 55.0 ± 16.6 | 0.70 |
| Triglycerides (mg/dl) | 129.6 (92–177) | 127.7 (87–175) | 0.57 |
| ApoA1 (mg/dl) | 154.9 ± 38.8 | 150.7 ± 39.1 | 0.03 |
| ApoB (mg/dl) | 102.8 ± 26.3 | 100.0 ± 28.3 | 0.04 |
| ApoB/apoA1 | 0.72 ± 0.33 | 0.72 ± 0.34 | 0.99 |
| Creatinine (mg/dl) | 0.83 (0.69–0.97) | 0.93 (0.73–1.11) | <0.001 |
| Fibrinogen (g/l) | 372.1 ± 68.5 | 406.9 ± 110.4 | <0.0001 |
| Uric acid (mg/dl) | 5.52 ± 1.73 | 6.04 ± 2.51 | <0.0001 |
| AER (μg/min) | 12.1 (4.2–25.5) | 21.3 (6.4–49.3) | <0.0001 |
| 20–200 | 377 (24.3) | 113 (35.3) | |
| >200 | 73 (4.7) | 28 (8.8) | |
| CVD | 353 (18.7) | 157 (31.6) | 0.0001 |
| Smoking | |||
| Former smoker | 534 (29.3) | 166 (34.9) | 0.03 |
| Smoker | 291 (16.0) | 60 (12.6) | |
| Glucose-lowering treatment | |||
| Diet | 303 (71.0) | 62 (12.7) | <0.0001 |
| Oral drugs | 1,402 (75.4) | 337 (68.9) | |
| Insulin | 155 (8.3) | 90 (18.4) | |
| Statins | 379 (20.1) | 98 (19.8) | 0.98 |
Data are means ± SD, n (%), or geometric means (95% CI).
FIG. 1.
Kaplan-Meier curves depicting the survival curves of the cohort by CRP levels (dotted line CRP ≤3 mg/l, solid line >3 mg/ml). Log-rank test P < 0.001.
Table 3 shows that HRs of all-cause mortality were significantly higher in people with CRP values in the highest tertile compared with people with lower values and that this excess was only slightly reduced after multiple adjustment for age, sex, diabetes duration, hypertension, LDL and HDL cholesterol, smoking, AER, A1C, glucose-lowering treatment, CVD, waist circumference, and treatment with statins. When this final model was run with clinically relevant CRP strata, we found that with respect to CRP values <1 mg/l, HRs provided by CRP values 1–3 mg/l and >3 mg/l were 1.03 (95% CI 0.72–1.47) and 1.49 (1.00–2.05), respectively. Results were virtually unmodified after further adjustment for fibrinogen values; indeed, in a fully adjusted model including both variables, fibrinogen values >3.5 g/l provided an HR of 1.30 (0.95–1.77), whereas CRP >3 mg/l conferred an HR of 1.57 (1.15–2.15).
TABLE 3.
HRs (95% CI) of 5-year mortality in people with type 2 diabetes of the Casale Monferrato Study
| All-cause mortality |
Cardiovascular mortality |
|||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| CRP tertiles (mg/dl) | ||||||
| <1.6 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1.6–4.4 | 1.27 (1.00–1.62) | 1.27 (0.98–1.64) | 1.35 (0.98–1.87) | 1.18 (0.82–1.68) | 1.07 (0.73–1.56) | 1.22 (0.74–2.00) |
| >4.4 | 1.99 (1.58–2.49) | 1.81 (1.41–2.32) | 1.65 (1.20–2.28) | 1.94 (1.39–2.71) | 1.62 (1.13–2.33) | 1.76 (1.09–2.82) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.006 | 0.015 |
| CRP (mg/l) | ||||||
| ≤3 mg/l | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| >3 mg/l | 1.64 (1.37–1.96) | 1.49 (1.22–1.82) | 1.51 (1.18–1.92) | 1.53 (1.17–1.99) | 1.32 (0.99–1.77) | 1.44 (0.99–2.08) |
| AER | ||||||
| <20 μg/min | 1.00 | 1.00 | ||||
| ≥20 μg/min | 1.47 (1.15–1.87) | 1.47 (1.01–2.14) | ||||
| Hypertension | 1.02 (0.64–1.63) | 0.69 (0.36–1.34) | ||||
| LDL cholesterol (1 SD) | 0.89 (0.79–1.01) | 1.07 (0.89–1.28) | ||||
| HDL cholesterol (1 SD) | 0.99 (0.88–1.13) | 0.92 (0.76–1.12) | ||||
| A1C (1 SD) | 1.10 (0.97–1.26) | 1.19 (0.97–1.44) | ||||
| CVD | 1.70 (1.31–2.22) | 2.56 (1.74–3.76) | ||||
| Antidiabetes treatment | ||||||
| Diet | 1.00 | 1.00 | ||||
| Oral drugs | 1.09 (0.76–1.57) | 1.24 (0.68–2.24) | ||||
| Insulin | 1.81 (1.12–2.94) | 2.29 (1.08–4.84) | ||||
| Smoking | ||||||
| Never | 1.00 | 1.00 | ||||
| Former smoker | 1.34 (0.96–1.86) | 1.24 (0.74–2.06) | ||||
| Current smoker | 1.42 (0.94–2.15) | 0.74 (0.33–1.65) | ||||
| Central obesity | 0.87 (0.67–1.12) | 0.92 (0.62–1.37) | ||||
| Statins | 1.02 (0.75–1.37) | 1.31 (0.86–1.99) | ||||
Model 1: adjusted for age, sex, diabetes duration. Model 2: model 1 adjustments plus hypertension, LDL and HDL cholesterol, A1C, smoking status, CVD, diabetes treatment, waist circumference, and statins. Model 3: model 2 adjustments plus AER.
Cardiovascular mortality (ICD 390–459) accounted for 223 of 496 (45.0%) deaths, giving a mortality rate per 1,000 person-years of 19.0 (95% CI 16.7–21.7); 100 deaths occurred among subjects with CRP ≤3.0 mg/l (rate 15.5 per 1,000 person-years [12.8–18.9]) and 123 among subjects with CRP >3 mg/l (rate 23.3 per 100,000 person-years [19.5–27.8]), giving an unadjusted HR of 1.53 (1.17–1.99) and a fully adjusted HR of 1.44 (0.99–2.08) (Table 3).
In the analyses treating CRP as a continuous variable after log transformation, fully adjusted models showed 22% (95% CI 10–36) increment of all-cause mortality and 17% (1–38) of cardiovascular mortality for each increment of CRP (one natural logarithm = 3.6 mg/l). In the final model, patterns of risk were similar in both sexes and in strata defined by median values of A1C, diabetes duration, LDL and HDL cholesterol, presence or absence of central obesity, and CVD at the baseline examination (Table 4).
TABLE 4.
HRs (95% CI) of 5-year mortality in people with type 2 diabetes of the Casale Monferrato Study
| All-cause mortality |
Cardiovascular mortality |
|||
|---|---|---|---|---|
| Number of deaths | HR (95% CI) for CRP >3.0 mg/l | Number of deaths | HR (95% CI) for CRP >3.0 mg/l | |
| Men | 262 | 1.41 (1.02–1.96) | 105 | 1.61 (0.94–2.74) |
| Women | 234 | 1.66 (1.14–2.41) | 118 | 1.51 (0.88–2.57) |
| Subjects without CVD at baseline | ||||
| All cohort | 339 | 1.67 (1.24–2.24) | 133 | 1.36 (0.83–2.24) |
| Men | 171 | 1.55 (1.03–2.32) | 57 | 1.11 (0.52–2.37) |
| Women | 168 | 1.71 (1.09–2.69) | 76 | 1.62 (0.82–3.22) |
| A1C (%) | ||||
| ≤6.7 | 216 | 1.54 (1.08–2.19) | 89 | 1.20 (0.67–2.14) |
| >6.7 | 268 | 1.51 (1.07–2.11) | 128 | 1.56 (0.94–2.57) |
| Diabetes duration (years) | ||||
| ≤8.7 | 202 | 1.60 (1.11–2.32) | 76 | 1.29 (0.70–2.40) |
| >8.7 | 291 | 1.48 (1.06–2.06) | 144 | 1.54 (0.94–2.51) |
| LDL cholesterol (mg/dl) | ||||
| ≤126 | 265 | 1.38 (1.00–1.90) | 95 | 1.41 (0.80–2.50) |
| >128 | 199 | 1.72 (1.18–2.50) | 110 | 1.49 (0.91–2.46) |
| HDL cholesterol (mg/dl) | ||||
| ≤51 | 237 | 1.55 (1.09–2.21) | 109 | 1.50 (0.88–2.54) |
| >51 | 242 | 1.52 (1.08–2.14) | 106 | 1.45 (0.84–2.49) |
| Central obesity | ||||
| No | 241 | 1.40 (0.98–1.99) | 102 | 1.46 (0.83–2.58) |
| Yes | 255 | 1.71 (1.20–2.43) | 121 | 1.48 (0.88–2.50) |
HRs adjusted for age, sex, diabetes duration, LDL and HDL cholesterol, A1C, smoking status, CVD, AER, diabetes treatment, waist circumference, and statins, apart from the stratification variable.
AER ≥20 μg/min and CRP >3 mg/l conferred similar magnitude of the associations with both all-cause and cardiovascular mortality, both of them providing higher HRs than other risk factors, including lipids, blood pressure, and glycemic control (Table 3). In normoalbuminuric subjects, all-cause and cardiovascular HRs of CRP were 1.56 (95% CI 1.13–2.15) and 1.65 (1.00–2.74), respectively. When fully adjusted models were fitted replacing CRP and AER terms with an indicator variable with four categories defined by their joined effects (Table 5), we found that with respect to the reference category (AER <20 μg/min and CRP ≤3 mg/l), HRs of both cardiovascular and all-cause mortality were significantly increased in all strata, although no evidence of an effect modification was found. Indeed, when an interaction term was added to the final models of Table 2, no significant interactions between AER and CRP values were found (P = 0.47 for all-cause mortality and P = 0.68 for cardiovascular mortality). Moreover, no effect modifications of the relationship between CRP and mortality were found for A1C, BMI, waist circumference, and sex.
TABLE 5.
HR (95% CI) of 5-year mortality in people with type 2 diabetes of the Casale Monferrato Study
| All-cause mortality |
Cardiovascular mortality |
|||
|---|---|---|---|---|
| HR (95% CI) |
HR (95% CI) |
|||
| AER <20 μg/min | AER ≥20 μg/min | AER <20 μg/min | AER ≥20 μg/min | |
| CRP ≤3 mg/l | 1.00 | 1.48 (1.03–2.13) | 1.00 | 1.74 (1.00–3.04) |
| CRP >3 mg/l | 1.52 (1.11–2.09) | 2.22 (1.60–3.09) | 1.64 (1.00–2.69) | 2.13 (1.26–3.61) |
HRs adjusted for age, sex, diabetes duration, LDL and HDL cholesterol, A1C, smoking status, CVD, diabetes treatment, waist circumference, and statins.
We then examined the discrimination effect of adding CRP measurement to classical cardiovascular risk factors and AER, comparing models with and without CRP (Table 6). The C statistic pointed out a very limited advantage in using CPR for both all-cause mortality (0.750 and 0.756 without and with CRP, respectively) and cardiovascular mortality (0.799 and 0.802 without and with CRP, respectively). The LR test comparing nested models with and without CRP were highly significant for all-cause mortality but not for cardiovascular mortality (P = 0.08). The Bayes information criterion indicated quite similar values with and without CRP. With regards to calibration, the P values for the Grønnesby and Borgan statistics indicate good calibration for both the models with and without CRP for all-cause mortality and cardiovascular mortality (P > 0.10 for all comparisons). Reclassification of subjects across deciles of estimated probability of survival according to models with and without CRP showed that among those who survived, 39 people shifted upward and 90 shifted downward by 1 decile of the surviving probability distribution after inclusion of CRP in multivariate model; respective figures among those who died were 2 and 5, giving a nonsignificant net reclassification improvement of only 0.02.
TABLE 6.
Measures of discrimination and calibration in the Casale Monferrato Study, by selected variables
| All-causes mortality | Cardiovascular mortality | |
|---|---|---|
| Discrimination | ||
| C statistic | ||
| Nonmodifiable risk factors (age, sex, and diabetes duration) (model 1) | 0.706 | 0.717 |
| Established risk factors (model 2) | 0.744 | 0.793 |
| Established risk factors plus AER | 0.750 | 0.799 |
| Established risk factors plus AER and CRP | 0.756 | 0.802 |
| LR test | ||
| Established risk factors (model 2) | LR = 66, df = 11, P < 0.0001 | LR = 61.85, df = 11, P < 0.0001 |
| Established risk factors plus AER | LR = 10.76, df = 1, P = 0.001 | LR = 4.69, df = 1, P = 0.03 |
| Established risk factors plus AER and CRP | LR = 9.94, df = 1, P = 0.002 | LR = 3.03, df = 1, P = 0.08 |
| Bayes information criterion | ||
| Nonmodifiable risk factors (age, sex, and diabetes duration) (model 1) | 4,021.24 | 1,693.40 |
| Established risk factors | 4,036.78 | 1,713.18 |
| Established risk factors plus AER | 4,033.45 | 1,715.90 |
| Established risk factors plus AER and CRP | 4,030.93 | 1,720.29 |
| Calibration | ||
| Grønnesby and Borgan test | ||
| Nonmodifiable risk factors (age, sex, and diabetes duration) (model 1) | P = 0.28 | P = 0.10 |
| Established risk factors | P = 0.44 | P = 0.21 |
| Established risk factors plus AER | P = 0.42 | P = 0.27 |
| Established risk factors plus AER and CRP | P = 0.10 | P = 0.28 |
DISCUSSION
This study was conducted to investigate the association between plasma CRP values and 5-year mortality risk in subjects with type 2 diabetes recruited as part of the population-based Casale Monferrato Study, an ongoing survey examining the epidemiology of diabetes over time in Italy (19–21). We provide evidence that diabetic subjects with CRP values >3 mg/l have 51% higher risk of all-cause mortality and 44% higher risk of cardiovascular mortality than diabetic subjects of similar age and sex, independently of classical risk factors; the magnitude of this association is similar to that provided by microalbuminuria, the main predictor of death in diabetic individuals. Second, increased risk of mortality is evident even in people without CVD at baseline. Third, the role of CRP is not confounded nor modified by AER. These findings indicate that both markers of endothelial dysfunction (AER) and inflammation (CRP) are useful in defining mortality risk over a 5-year period in diabetic subjects. In our analyses, however, the C statistic failed to provide evidence of increased discrimination of models including CRP, and both models with and without CRP provide good calibration. Moreover, from a clinical point of view, CRP measurement allows the reclassification of a very limited proportion of patients on the basis of their 5-year probability of surviving; thus, the usefulness of this measurement in addition to other risk factors commonly used in clinical practice by diabetologists does not seem justified at present. Indeed, the apparent advantage of CRP does not translate into large improvement in 5-year mortality risk prediction when other well-known risk factors are utilized. Our findings are original, allowing us to point out that previously obtained data on clinical usefulness of CRP measurement in nondiabetic subjects do not extend to high-risk diabetic subjects (2,3,15,27). Even in elderly nondiabetic subjects recruited as part of the PROSPER Trial, CRP values did not provide further additional prediction of vascular events beyond established risk factors in a 3-year follow-up period (18).
Our results on the association between CRP and mortality are consistent with the few prospective studies examining either cardiovascular events (4) or mortality in diabetic subjects (10). The population-based study conducted in Finland was limited to diabetic subjects aged 45–64 years (10), whereas the subgroup of 746 diabetic subjects recruited as part of the Health Professional Follow-Up Study was limited to men only (4). Moreover, no study has examined the interrelationship between microalbuminuria, the main predictor of death in people with diabetes, and CRP (20). Our analyses pointed out that even in normoalbuminuric subjects, CRP values >3 mg/l are associated with a significant 64% increased risk of cardiovascular mortality, independently of classical risk factors. As no multiplicative effect was found between AER and CRP, it appears that both markers of endothelial dysfunction and inflammation independently contribute to increased mortality.
In our study, CRP was more strongly associated with death than other risk factors, apart from AER, and this association was found even in subjects with no evidence of CVD at the baseline examination. Indeed, when CRP was elevated, there was a significant 51% increased risk of all-cause mortality, which was virtually unaffected by adjustment for traditional and new risk factors, including AER and fibrinogen. With regards to cardiovascular mortality, there was a 44% significantly higher adjusted risk, which was marginally significant, probably due to the more limited number of person-years on which rates were based. A population-based study conducted in nondiabetic subjects aged ≥65 years with no evidence of baseline CVD showed that CRP was associated with neither all-cause nor cardiovascular mortality (17). This might suggest the hypothesis of a different strength of the association between death and elevated CRP in diabetic compared with nondiabetic subjects, probably reflecting the increased activation of inflammation seen in these individuals.
In a previous report of the Casale Monferrato Study, we found that fibrinogen was significantly and independently associated with mortality (20). In this report, carried out in a more recently recruited cohort with both CRP and fibrinogen measurements available in 60% of the cohort, we found that the association between fibrinogen and death was mainly mediated through the effect of CRP. A joint effect of A1C and CRP on cardiovascular event has also been suggested in a clinic-based prospective study including 454 people with advanced atherosclerosis, 40% of whom had diabetes, the highest risk being evident in people with CRP >4.4 mg/l and A1C >6.2% (28). In our cohort, only 27 subjects had CRP values >4.4, and 17 of them also had A1C >6.2%. Therefore, our study extends previous observations to the large majority of diabetic subjects in industrialized countries, who were mainly elderly with a good level of glycemic control. CRP and A1C were correlated, but neither confounding nor modification effect of A1C on the association between CRP and mortality risk was found over the range of examined values (interquartile range 5.7–8.1%).
The strengths of the study are the recruitment of a large, population-based cohort of diabetic subjects, the centralized measurement of risk factors and biomarkers, and the high degree of completeness of baseline and follow-up data. Several limitations of our analysis, however, deserve comment. Subjects included in this report (73% of the population-based cohort) were selected having a lower cardiovascular risk profile with respect to those who were not recruited. It is likely, therefore, that the true association between CRP and mortality is even higher than that reported. Our results are based on an observational prospective cohort. Although multivariate methods of analyses were used to control for the effect of known confounders of the relationship between CRP and mortality, we cannot rule out the possibility of residual or undetected confounding on our results. This study also used a single CRP measurement at baseline, and its stability over time has not been shown definitively.
In conclusion, our population-based study indicates that CRP value is independently associated with 5-year mortality risk in type 2 diabetic subjects, even in those who were normoalbuminuric and in those without a previous diagnosis of CVD. From a clinical point of view, however, the improvement in individual risk assessment was only marginal.
Acknowledgments
The Casale Monferrato Study is supported by grants from the Piedmont Region (Ricerca Sanitaria Finalizzata 2006) and the University of Turin (ex 60%).
No potential conflicts of interest relevant to this article were reported.
We thank the patients, the nurses at the diabetes clinic, the diabetologists, and the general practitioners for long-standing collaboration in this study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying original article, p. 798.
REFERENCES
- 1.Hansson GK: Inflammation, atheroclerosis and coronary artery disease. N Engl J Med 352: 1685– 1695, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB: Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599– 2610, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Wilson PW, Grundy SM: Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation 109: 2818– 2825, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ, Hu FB: C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care 27: 889– 894, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Pasceri V, Willersone JT, Yeh ET: Direct proinflamnatory effect of C-reactive protein on human endothelial cells. Circulation 102: 2165– 2168, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ: A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 106: 913– 919, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, Markiewicz W, Brook GJ, Levy Y: Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabet Med 21: 39– 44, 2004 [DOI] [PubMed] [Google Scholar]
- 8.de Rekeneire N, Peila R, Ding J, Colbert LH, Visser M, Shorr RI, Kritchevsky SB, Kuller LH, Strotmeyer ES, Schwartz AV, Vellas B, Harris TB: Diabetes, hyperglycemia, and inflammation in older individuals: the Health, Aging and Body Composition study. Diabetes Care 29: 1902– 1908, 2006 [DOI] [PubMed] [Google Scholar]
- 9.King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS: C-reactive protein and glycemic control in adults with diabetes. Diabetes Care 26: 1535– 1539, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T: High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 29: 329– 333, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Friedman AN, Hunsicker LG, Selhub J, Bostom AGthe Collaborative Study Group: C-reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int 68: 773– 778, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH: Insulin resistance, haemostatic and inflammatory markers and coronary heart disease risk factors in type 2 diabetic men with and without coronary heart disease. Diabetologia 47: 1557– 1565, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Vu JD, Vu JB, Pio JR, Malik S, Franklin SS, Chen RS, Wong ND: Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol 96: 655– 658, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Malik S, Wong ND, Franklin S, Pio J, Fairchild C, Chen R: Cardiovascular disease in U.S. patients with metabolic syndrome, diabetes, and elevated C-reactive protein. Diabetes Care 28: 690– 693, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Buring JE, Ridker PM: The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med 145: 21– 29, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Cook NR: Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115: 928– 935, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Wilson PWF, Nam B-H, Pencina M, D'Agostino RB, Benjamin EJ, O'Donnell CJ: C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med 165: 2473– 2478, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Sattar N, Murray HM, McConnachie A, Blauw GJ, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Murphy MB, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, Shepherd Jthe PROSPER Study Group: C-reactive protein and prediction of coronary heart disease and global vascular events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). Circulation 115: 981– 989, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bruno G, Merletti F, Bargero G, Melis D, Masi I, Ianni A, Novelli G, Pagano G, Cavallo-Perin P: Changes over time in the prevalence and quality of care of type 2 diabetes in Italy: the Casale Monferrato Surveys, 1988 and 2000. Nutr Metab Cardiovasc Dis 18: 39– 45, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Pagano G, Cavallo-Perin P: Fibrinogen and AER are major independent predictors of 11-year cardiovascular mortality in type 2 diabetes: the Casale Monferrato Study. Diabetologia 48: 427– 434, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, Perotto M, Pagano G, Cavallo-Perin P: Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato Study. Diabetologia 50: 941– 948, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lamb EJ, Tomson CR, Roderick PJ.Clinical Sciences Reviews Committee of the Association for Clinical Biochemistry: Estimating kidney function in adults using formulae. Ann Clin Biochem 42: 321– 345, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Diabete Italia, AMD (Associazione Medici Diabetologi), SID (Società Italiana di Diabetologia): Italian standards for diabetes mellitus 2007: executive summary. Acta Diabetol 45: 107– 127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi MC, Nicolucci A, Arcangeli A, Cimino A, De Bigontina G, Giorda C, Meloncelli I, Pellegrini F, Valentini U, Vespasiani Gthe Associazione Medici Diabetologi Annals Study Group: Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care 31: 2166– 2168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr: Regression Modeling Strategies New York, Springer-Verlag, 2001 [Google Scholar]
- 26.Grønnesby JK, Borgan O: A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal 2: 315– 328, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Zethelius B, Berglund L, Sundström , Ingelsson E, Basu S, Larsson A, Venge P, Ärnlöv J: Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 358: 2107– 2116, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schillinger M, Exner M, Amighi J, Mlekusch W, Sabeti S, Rumpold H, Wagner O, Minar E: Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation 108: 2323– 2328, 2003 [DOI] [PubMed] [Google Scholar]