Abstract
OBJECTIVE
Non–Fc-binding anti-CD3–specific antibodies represent a promising therapy for preserving C-peptide production in subjects with recent-onset type 1 diabetes. However, the mechanisms by which anti-CD3 exerts its beneficial effect are still poorly understood, and it is questionable whether this therapeutic approach will prove durable with regard to its ability to impart metabolic preservation without additional actions designed to maintain immunological tolerance. We used the NOD mouse model to test whether rapamycin, a compound well-known for its immunomodulatory activity in mice and humans, could increase the therapeutic effectiveness of anti-CD3 treatment in type 1 diabetes.
RESEARCH DESIGN AND METHODS
Rapamycin was administered to diabetic NOD mice simultaneously with anti-CD3 or to NOD mice cured by anti-CD3 therapy. The ability of this combined therapy to revert type 1 diabetes and maintain a state of long-term tolerance was monitored and compared with that of anti-CD3 therapy alone.
RESULTS
Rapamycin inhibited the ability of anti-CD3 to revert disease without affecting the frequency/phenotype of T-cells. Rapamycin also reinstated diabetes in mice whose disease was previously reversed by anti-CD3. Withdrawal of rapamycin in these latter animals promptly restored a normoglycemic state.
CONCLUSIONS
Our findings indicate that, when combined with anti-CD3, rapamycin exerts a detrimental effect on the disease outcome in NOD mice for as long as it is administered. These results suggest strong caution with regard to combining these treatments in type 1 diabetic patients.
The NOD mouse is widely used as a model of human type 1 diabetes (1). Whereas a large number of therapeutic approaches have shown success in preventing type 1 diabetes in NOD mice, agents demonstrating the clear ability to reverse established disease and restore self-tolerance in this animal model have been far more difficult to identify (2). Among the limited number of treatments demonstrated to revert established disease in diabetic NOD mice is the non–Fc-binding anti-CD3ε antibody (anti-CD3) (3). Indeed, a short-term treatment with anti-CD3 at the time of diabetes onset is sufficient to reverse the disease, induce long-term remission, and prevent recurrent immune responses, including those against transplanted syngeneic pancreatic islets (4). The exact mechanism of action by which anti-CD3 provides this beneficial effect is still not fully known, but it is clear that its tolerogenic capacity develops in two consecutive phases. The first phase, known as the induction phase, occurs concomitantly with antibody administration via three distinct nonmutually exclusive mechanisms: 1) antigenic downmodulation of the T-cell receptor–CD3 complex, 2) induction of apoptosis that preferentially affects activated T-cells, and 3) induction of anergy in T-cells (5). The second phase, known as maintenance phase, is long-term in its mode of action and involves the generation of inducible TGF-β–dependent CD4+ regulatory T-cells (Tregs) that coexist with pathogenic T-cells (6). Tregs are a specialized T-cell subset essential for maintaining peripheral tolerance and preventing autoimmune disease (7). CD4+ Tregs are often categorized into two major subgroups based on their ontogeny. The first, naturally occurring CD4+CD25+FOXP3+ Tregs (nTregs), originate from the thymus. The second, so-called inducible Tregs (iTregs), are generated in the periphery. Each of these Treg subsets has been shown to be required for tolerance induction to self- and nonself-antigens (7).
Given the therapeutic effectiveness of anti-CD3 in reversing type 1 diabetes in NOD mice, the clinical efficacy of this drug was tested in two independent clinical trials conducted in new-onset type 1 diabetic patients. Anti-CD3 treatment was shown to be effective in preventing loss of insulin production for at least 1 year following diagnosis, but its long-term efficacy was only evident in a limited group of patients (8,9). Given this finding, it was hypothesized that the effectiveness of anti-CD3 therapy might be improved by its use in combination with other tolerogenic treatments (10).
We previously demonstrated that rapamycin, a non–calcineurin-based inhibitor used to prevent acute graft rejection following allogeneic transplantation (11), allows for in vitro expansion of murine (12) and human (13) CD4+CD25+FOXP3+ nTregs. Rapamycin also expands CD4+CD25+FOXP3+ nTregs in vivo in pre-diabetic NOD mice and has a synergistic effect with interleukin (IL)-10 in blocking disease development and restoring self-tolerance (14). In addition, rapamycin monotherapy in patients with long-lasting type 1 diabetes patients improves CD4+CD25+FOXP3+ nTreg function (15). These data provide strong evidence that rapamycin is, in fact, a protolerogenic compound that could be used to boost the tolerogenic activity previously ascribed to anti-CD3 treatment in vivo. Therefore, we tested whether rapamycin could be combined with anti-CD3 therapy in curing type 1 diabetes and reinforcing the long-term tolerance in NOD mice. Surprisingly, we observed that rapamycin therapy not only blocks the ability of anti-CD3 treatment to cure type 1 diabetes in NOD mice but also reverts its curative effect once established. These previously unreported and unexpected results raise serious questions regarding the effectiveness of combining rapamycin and anti-CD3 therapy to induce tolerance in type 1 diabetes patients.
RESEARCH DESIGN AND METHODS
Animals.
NOD/LtJ female mice were purchased from Charles River (Calco, Italy). All mice were maintained under specific pathogen-free conditions. Animal care procedures were performed according to protocols approved by the Hospital San Raffaele Institutional Animal Care and Use Committee (IACUC no. 350).
Blood glucose monitoring.
Blood glucose was measured in the morning three times a week using a Glucometer Ascensia Breeze 2 Glucose Meter (Bayer, Leverkusen, Germany). A diagnosis of diabetes was made after a glucose measurement of ≥300 mg/dl to ensure no spontaneous disease reversal. A relapse of disease in treated animals was considered following two consecutive glucose measurements of ≥200 mg/dl, levels at which spontaneous diabetes reversal never occurred in any of the tested mice (data not shown).
Treatment.
After diabetes onset, female NOD mice (aged 22 ± 7 weeks) were treated with varying doses of the non–Fc-binding anti-CD3ε F(ab′)2 clone 145-2C11 (Bio Express, West Lebanon, NH) or mAb isotype control (Golden Syrian Hamster IgG; eBioscience, San Diego, CA), according to the glycemia levels, the same day they were found to have diabetes. Rapamycin (Rapamune; Wyeth Europe, Taplow, U.K.) was diluted in water and administered by gavage once a day at 1 mg/kg, a dose that we and others previously demonstrated not to be toxic to pancreatic islets (14,16). Recombinant human IL-10 (BD Biosciences, Mountain View, CA) was diluted in PBS and administered twice a day at a dose of 0.05 mg/kg i.p. (14).
Intraperitoneal glucose tolerance test.
Mice were fasted 16 h before receiving 2 g/kg glucose i.p. (30% glucose solution). Glucose tolerance was monitored via tail-vein sampling at time 0 (just before glucose solution injection) and 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, and 120 min after glucose solution injection. All statistical analyses were performed using a two-tailed Student's t test. A P value of <0.05 was deemed significant.
RESULTS
To define a suboptimal dose of anti-CD3 amenable to combinational therapy studies (i.e., having a second agent that improves the action of the first) and to identify the influence of starting glycemia on the ability to reverse disease, we first grouped NOD mice based on degree of hyperglycemia and treated with various dosages of anti-CD3 (Fig. 1A). Using this strategy, several suboptimal anti-CD3 dosages leading to a wide range of diabetes reversal rates (i.e., from 35 to 75%) were identified. The efficacy of the anti-CD3 treatment was strictly dependent on the dosage, as previously shown (17,18), but was also influenced by glucose levels at time of treatment. Figure 1B shows the glucose levels of each of the animals treated with the best effective anti-CD3 dosage (i.e., 50 μg × 3 doses in mice with 300–349 mg/dl glycemia levels), demonstrating a rapid and uniform diabetes reversal in six of eight animals treated (Fig. 1B).
FIG. 1.
Anti-CD3 and rapamycin monotherapy in diabetic NOD mice. Female diabetic NOD mice were grouped based on glycemia levels (300–349 [gray bar] and 350–400 mg/dl [white bar]) and treated with various anti-CD3 dosages. Percentages of diabetes reversal and number of mice in each group are shown (A). Glucose levels of the eight NOD mice with 300–349 mg/dl glycemia and treated with three 50-μg doses of anti-CD3 (75% diabetes reversal) are shown (B). Female diabetic NOD mice with 300–400 mg/dl glycemia were treated with rapamycin alone (1 mg/kg daily during the entire time of observation [□]) or anti-CD3 alone (50 μg × 3 doses at days 0, 1, and 2 [♦]). Percentage of diabetes reversal and number of mice are shown (C).
Although rapamycin has a strong immunomodulatory capacity in pre-diabetic NOD mice (14,16), rapamycin monotherapy did not lead to disease reversal in any of the diabetic mice (Fig. 1C). To test whether rapamycin cooperates with anti-CD3 to reverse diabetes and reinforce the development of long-term tolerance, diabetic NOD mice were treated with rapamycin and the anti-CD3 dosage that showed the weaker ability to reverse disease (i.e., 35% diabetes reversal). The concomitant administration of rapamycin and anti-CD3 did not lead to enhanced diabetes reversal in any of the animals tested (Fig. 2A); rather, the inclusion of rapamycin blocked the ability of anti-CD3 to impart its beneficial effect. The deleterious influence of rapamycin on the anti-CD3 reversal capacity was even observed when rapamycin was coadministered with dosages of anti-CD3 able to cure 65% of diabetic NOD mice (Fig. 2B). Of 10 diabetic NOD mice, 9 remained diabetic when treated with rapamycin and anti-CD3 at the highest effective dose (i.e., 70% diabetes reversal) (Fig. 2C).
FIG. 2.
Simultaneous administration of anti-CD3 and rapamycin or IL-10 in diabetic NOD mice. Female diabetic NOD mice were treated with anti-CD3 alone or in conjunction with rapamycin (1 mg/kg) or IL-10 (0.05 mg/kg) during the entire time of observation. NOD mice were treated with various doses of anti-CD3. Mice with 350–400 mg/dl glycemia were treated with 25 μg anti-CD3 (one dose) alone (♦) or in conjunction with rapamycin (□) (A). Mice with 300–349 mg/dl glycemia were treated with 18 μg anti-CD3 (one dose) alone (♦) or in conjunction with rapamycin (□) (B). Mice with 300–400 mg/dl glycemia were treated with 50 μg anti-CD3 (three doses) alone (♦) or in conjunction with rapamycin (□) (C). Mice with 300–400 mg/dl glycemia were treated with 50 μg anti-CD3 (three doses) alone (♦) or in conjunction with IL-10 (□) (D). Percentages of diabetes reversal and number of mice are shown.
To evaluate whether other pro-tolerogenic compounds acted similarly to rapamycin when combined with anti-CD3, we tested IL-10, an immunomodulatory cytokine with known tolerogenic potential in vivo (14,19). When coadministered with anti-CD3 in diabetic NOD mice, the addition of IL-10 did not interfere with the anti-CD3 therapeutic activity (Fig. 2D). These data demonstrate that the tolerogenic capacity of anti-CD3 during the first induction phase is completely halted by rapamycin, but not by IL-10 therapy, in diabetic NOD mice.
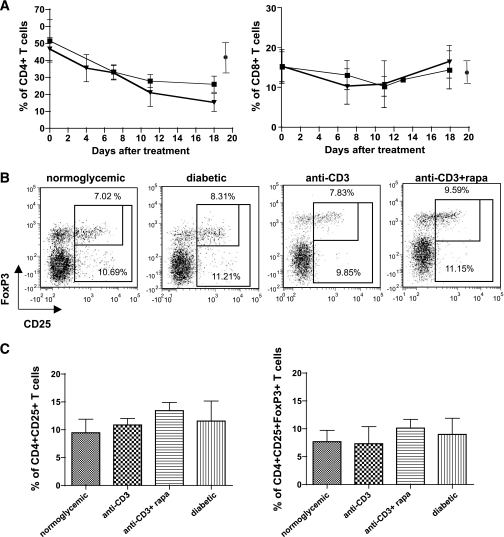
To define whether the detrimental effect of rapamycin on anti-CD3 therapy was related to changes in the T-cell compartment, we tested the percentages of circulating CD4+ and CD8+ T-cells and the frequency of CD4+CD25+FoxP3+ T-cells in the pancreatic lymph nodes of NOD mice treated with anti-CD3 alone or in combination with rapamycin. Circulating CD4+ T-cells were depleted upon anti-CD3 treatment irrespective of the presence of rapamycin, whereas CD8+ T-cells were only partially affected (Fig. 3A). Similarly, rapamycin did not alter the frequency of CD4+CD25+FoxP3+ T-cells in the pancreatic lymph nodes 2 weeks after anti-CD3 treatment (Fig. 3B–C). Contrary to what was previously demonstrated (6), anti-CD3–treated NOD mice did not show a selective increase of CD4+CD25+ T-cells in the pancreatic lymph nodes compared with control NOD mice. This might be due to the reduced anti-CD3 dosage used in our study (i.e., 50 μg × 3 doses) compared with that used by Belghith et al. (i.e., 50 μg × 5 doses) (6), which might lead to different kinetics in the expansion of CD4+CD25+ T-cells.
FIG. 3.
T-cell frequency and phenotype in NOD mice treated with anti-CD3 or anti-CD3 and rapamycin. Diabetic NOD mice were treated with anti-CD3 alone (50 μg × 3 doses, n = 6, [■]) or in combination with rapamycin (1 mg/kg per day, n = 5, [▾]). Peripheral blood was collected at different time points after treatment, and circulating CD4+ (left) and CD8+ (right) T-cells were analyzed by a fluorescence-activated cell sorter. Percentages of CD4+ and CD8+ T-cells within the CD45+ cells are shown. (●), mean ± SD of peripheral CD4+ and CD8+ T-cells in nondiabetic NOD mice within CD45+ cells (n = 10) (A). Pancreatic lymph nodes from normoglycemic untreated (n = 4), diabetic untreated (n = 5), anti-CD3–treated (50 μg × 3 doses) (n = 2), and anti-CD3– (50 μg × 3 doses) plus rapamycin treated (n = 5) NOD mice were analyzed by a fluorescence-activated cell sorter 3 weeks after treatment. One representative plot for each group, after CD4+ T-cell gating, is shown. The big gate includes all CD25+ T-cells, whereas the small gate includes only CD25+FoxP3+ T-cells. Numbers indicate the relative percentages in each gate (B). Percentages of means ± SD of CD25+ T-cells (left panel) and CD25+FoxP3+ T-cells (right panel) within CD4+ T-cells in the pancreatic lymph nodes are shown (C).
The anti-CD3 maintenance phase in NOD mice is a stable condition of tolerance that is no longer dependent on the presence of the antibody. Given our previous results, we tested whether rapamycin negatively affects this stable condition of reversed type 1 diabetes. Five weeks after anti-CD3–mediated diabetes reversal, normoglycemic NOD mice were treated with rapamycin. Quite remarkably, all previously cured mice returned to a state of hyperglycemia within 7 weeks of rapamycin administration, whereas rapamycin-untreated animals showed no signs of diabetes recurrence (Fig. 4A). To further delay the interval between anti-CD3 intervention and rapamycin administration, normoglycemic NOD mice were treated with rapamycin 30 weeks after anti-CD3–mediated diabetes reversal. Consistent with the aforementioned observations, two of three anti-CD3–cured NOD mice returned to a diabetic state within 10 weeks of rapamycin administration, whereas all rapamycin-untreated animals remained normoglycemic (Fig. 4B). This phenomenon was reversible and strictly dependent on the presence of rapamycin, as all mice treated for 230 days with rapamycin promptly returned to a normoglycemic state upon drug removal.
FIG. 4.
Delayed rapamycin administration in anti-CD3–treated NOD mice. According to their glycemia levels, 28 female NOD mice were previously cured with anti-CD3 (♦). Thirty-five days after anti-CD3 mAb–mediated diabetes reversal, four mice were treated with rapamycin (■) and 24 were left untreated (♦). Rapamycin was administered during the entire time of observation. Percentages of diabetes reversal and number of mice are shown (A). According to their glycemia levels, 24 female NOD mice were previously cured with anti-CD3 (♦). One hundred and eighty days after anti-CD3–mediated diabetes reversal, three mice were treated with rapamycin (□) and 21 were left untreated (♦). Rapamycin was administered for 230 days and then withdrawn. Percentages of diabetes reversal and number of mice are shown (B). Intraperitoneal glucose tolerance test was performed in three normoglycemic untreated NOD mice, three diabetic NOD mice, six normoglycemic anti-CD3–cured mice, and three mice previously cured with anti-CD3 but again diabetic upon rapamycin treatment. Intraperitoneal glucose tolerance test was performed in the latter group during rapamycin treatment (i.e., 320 days after anti-CD3 mAb treatment). The average glucose levels per each time point and the best-fitting curve for each group are shown (C). The area under the curve (AUC) from time 0 to 120 min after glucose injection for all the animals included in panel B is shown (D).
To evaluate the metabolic parameters underlying this finding, a glucose tolerance test was performed in NOD mice previously treated with anti-CD3 and under rapamycin treatment. Although two of three rapamycin-treated mice were hyperglycemic at the time of analysis (as shown in Fig. 4B), all three demonstrated a glucose response similar to that observed in diabetic untreated NOD mice, while control anti-CD3–cured NOD mice showed a glucose response superimposable to that of normoglycemic untreated animals (Fig. 4C–D). These data proved the inability of active rapamycin treatment to control glucose homeostasis. Overall, rapamycin reverts the stable tolerance condition established in anti-CD3–cured NOD mice while it is administered.
DISCUSSION
With prior data demonstrating that rapamycin is a protolerogenic compound both in vitro and in vivo (12,13,15) as well as information from the first clinical trials with anti-CD3 in recent-onset type 1 diabetic patients suggesting that this form of therapy can be improved (8,9), we tested the specific hypothesis that rapamycin would augment the therapeutic effectiveness of anti-CD3–mediated type 1 diabetes reversal in NOD mice. Against all expectations, we observed that rapamycin not only blocks the ability of anti-CD3 treatment to cure overt hyperglycemia in NOD mice but also breaks its curative effect while it is administered.
Previous data demonstrate that cyclosporine A (20), anti–TGF-β, and anti–CTLA-4 neutralizing antibodies (6) block the reversal capacity of anti-CD3 in the induction phase while cyclophosphamide breaks anti-CD3–mediated tolerance during the maintenance phase (20). The mechanisms by which these compounds counteract the anti-CD3 effect have been elucidated. Cyclosporine A blocks T-cell activation and T-cell depletion mediated by activation-induced cell death, and anti–TGF-β and anti–CTLA-4 monoclonal antibodies impede the generation and/or function of inducible TGF-β–dependent Tregs, whereas cyclophosphamide depletes Tregs. Rapamycin inhibits both phases of anti-CD3–induced tolerance. In addition, rapamycin allows T-cell activation and activation-induced cell death (11), permits generation of inducible Tregs (21–23), and does not selectively deplete Tregs (12,24,25). In fact, T-cell frequency and phenotype in anti-CD3–rapamycin–treated mice were identical to those of anti-CD3–treated NOD mice. It is therefore unlikely that rapamycin alters the anti-CD3 activity through the same inhibitory mechanisms demonstrated for the abovementioned compounds.
It has been recently proposed that the reduced IL-2 production by T effector cells in NOD mice is the root cause of the progressive loss of Treg–T effector cell balance in the islets, leading to β-cell destruction (26). Anti-CD3 therapy might restore IL-2 production, leading to stable disease reversal. Rapamycin, by inhibiting signal transduction delivered by IL-2, might directly interfere with this pathway, nowadays considered so crucial for maintaining immunological tolerance in NOD mice. Experiments are currently ongoing to test this hypothesis.
Alternative mechanisms that can explain the unique effect of rapamycin in anti-CD3–treated NOD mice may be related to β-islet physiology. It has recently been demonstrated that rapamycin induces fulminant diabetes in the Psammomys obesus mouse model of nutrition-dependent type 2 diabetes by increasing insulin resistance and reducing β-cell function and mass through increased apoptosis (27). The fundamental function of mammalian target of rapamycin–signaling in β-cells, which is blocked by rapamycin, has been confirmed by others (28,29). Rapamycin might therefore have a negative effect directly on the islets rather than blocking the activity of anti-CD3 in NOD mice. However, this hypothesis is in contrast to previous observations by our group (14) and others (16) in pre-diabetic NOD mice wherein rapamycin monotherapy significantly protected animals from disease development. In addition, diabetic NOD mice treated with rapamycin did not develop a more aggressive disease, in terms of glycemia, than untreated mice (A.V., unpublished data).
An alternative hypothesis is that rapamycin interferes with β-cell proliferation, as demonstrated in specific experimental settings such as pregnancy (30) and transgenic mice (31). However, at this time, there are no data indicating that anti-CD3 leads to β-cell proliferation. Indeed, currently available data suggest the opposite: recovery of metabolic control following anti-CD3 therapy may be due to mending of β-cells that had been already present but not functional in the pancreas at the moment of hyperglycemia rather than β-cell proliferation (18,32). Future experiments will investigate the pancreata of NOD mice treated with rapamycin, with or without anti-CD3, in order to further understand the mechanisms underlying its deleterious action.
Rapamycin monotherapy in long-lasting type 1 diabetic patients does not aggravate the autoimmune disease but, rather, improves the suppressive function of nTregs (15). One should therefore expect that rapamycin behaves similarly in the case of new-onset type 1 diabetes. However, the disease in early-onset type 1 diabetic subjects appears both metabolically and immunologically different from that observed in long-lasting patients, and rapamycin might have a different outcome in diverse patient populations. A clinical trial combining rapamycin and IL-2 recently began with new-onset type 1 diabetic patients (http://www.clinicaltrials.gov/ct2/show/NCT00525889?term=rapamune+and+il-2&rank;=1). Of note, no preclinical studies demonstrating the curative potential of rapamycin–IL-2 therapy have been reported. Our data suggest caution in designing rapamycin-based combinational treatments because the addition of rapamycin to anti-CD3 therapy in early-onset diabetic NOD mice completely abolished the anti-CD3 therapeutic effect.
Acknowledgments
This work was supported by the Italian Telethon Foundation and the Juvenile Diabetes Research Foundation (GJT04014).
No potential conflicts of interest relevant to this article were reported.
We thank Nicola Gagliani and Giulia Barbagiovanni (San Raffaele Telethon Institute for Gene Therapy, HSR-TIGET) for discussing the data and Luca Guidotti and Marika Falcone (San Raffaele Diabetes Research Institute, HSR-DRI) for critical reading of this manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23: 447– 485, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT: A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 23: 115– 126, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Chatenoud L: CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 3: 123– 132, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chatenoud L, Thervet E, Primo J, Bach JF: Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 91: 123– 127, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatenoud L, Bluestone JA: CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol 7: 622– 632, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L: TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9: 1202– 1208, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Yamaguchi T, Nomura T, Ono M: Regulatory T cells and immune tolerance. Cell 133: 775– 787, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54: 1763– 1769, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 352: 2598– 2608, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bresson D, von Herrath M: Moving towards efficient therapies in type 1 diabetes: to combine or not to combine? Autoimmun Rev 6: 315– 322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponticelli C: The pleiotropic effects of mTor inhibitors. J Nephrol 17: 762– 768, 2004 [PubMed] [Google Scholar]
- 12.Battaglia M, Stabilini A, Roncarolo MG: Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105: 4743– 4748, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG: Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 177: 8338– 8347, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Battaglia M, Stabilini A, Draghici E, Migliavacca B, Gregori S, Bonifacio E, Roncarolo MG: Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 55: 1571– 1580, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo MG, Battaglia M: Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes 57: 2341– 23472008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R: Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 51: 638– 645, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M: Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 116: 1371– 1381, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC: Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148: 5136– 5144, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG: Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 55: 40– 49, 2006 [PubMed] [Google Scholar]
- 20.Chatenoud L, Primo J, Bach JF: CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J Immunol 158: 2947– 2954, 1997 [PubMed] [Google Scholar]
- 21.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB: Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant 7: 1722– 1732, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolaeva N, Bemelman FJ, Yong SL, van Lier RA, ten Berge IJ: Rapamycin does not induce anergy but inhibits expansion and differentiation of alloreactive human T cells. Transplantation 81: 445– 454, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M: Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol 177: 944– 949, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G: Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 18: 1007– 1018, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS: Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 108: 390– 399, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA: Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28: 687– 697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G: mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57: 945– 957, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Wang Q, Ling Z, Pipeleers D, McDermott P, Pende M, Heimberg H, Van de Casteele M: Akt activation protects pancreatic beta cells from AMPK-mediated death through stimulation of mTOR. Biochem Pharmacol 75: 1981– 1993, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E: Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci U S A 105: 9250– 9255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahr E, Molano RD, Pileggi A, Ichii H, San Jose S, Bocca N, An W, Gonzalez-Quintana J, Fraker C, Ricordi C, Inverardi L: Rapamycin impairs beta-cell proliferation in vivo. Transplant Proc 40: 436– 437, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553– 2561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ablamunits V, Sherry NA, Kushner JA, Herold KC: Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann N Y Acad Sci 1103: 19– 32, 2007 [DOI] [PubMed] [Google Scholar]