Abstract
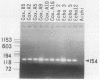
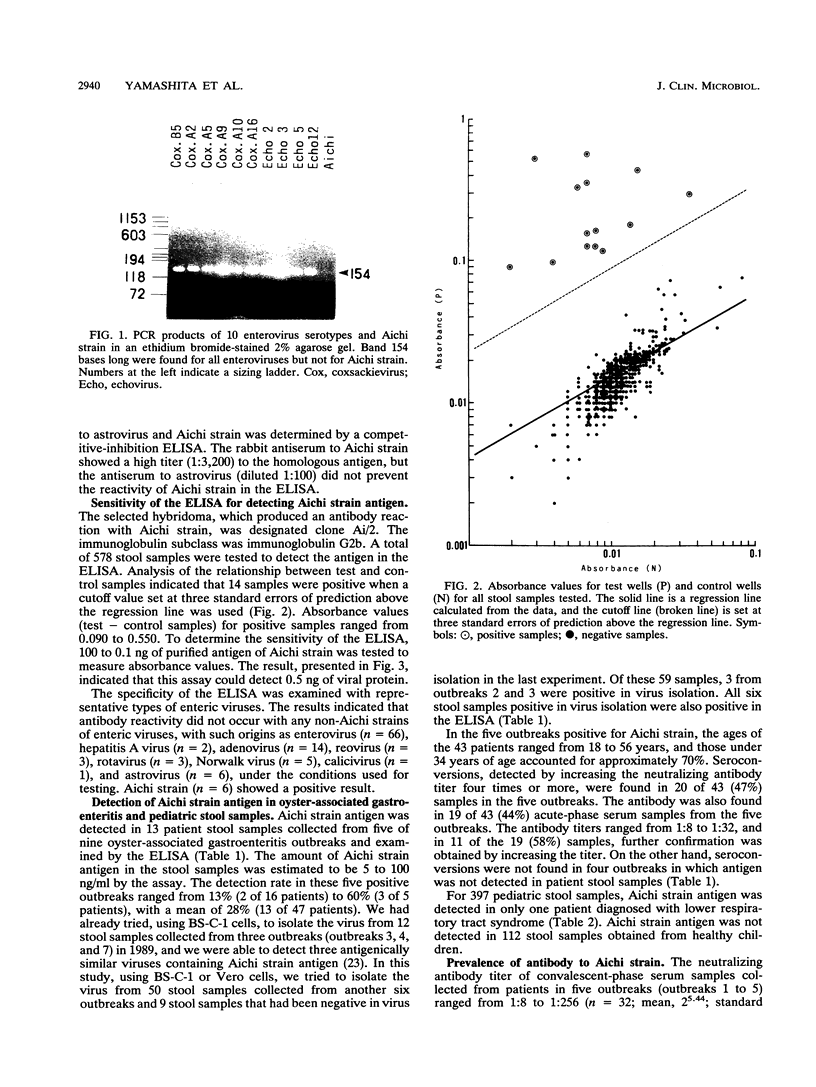
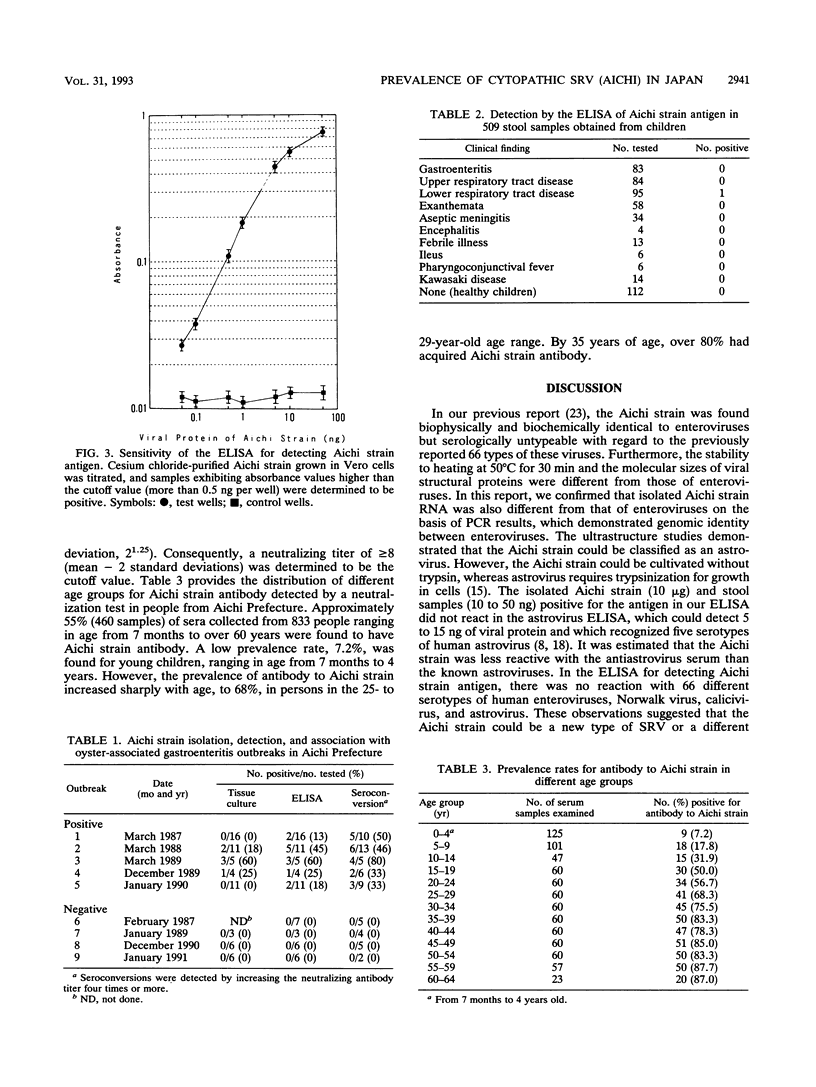
Cytopathic small round virus (Aichi strain), isolated from a patient with oyster-associated gastroenteritis, showed no reaction in the polymerase chain reaction method for enteroviruses or in the enzyme-linked immunosorbent assay (ELISA) for the five serotypes of astroviruses. Our ELISA was sensitive in detecting the Aichi strain antigen in stool samples, but there was no reaction in this ELISA with any non-Aichi strains of enteric viruses, with such origins as enterovirus, rotavirus, Norwalk virus, calicivirus, or astrovirus. In the ELISA, 13 of 47 stool samples from adult patients in five of nine oyster-associated gastroenteritis outbreaks were positive, but only 1 of 397 pediatric stool samples in Aichi Prefecture was positive. The prevalence rate for Aichi strain antibody was found to be 7.2% for persons aged 7 months to 4 years. The prevalence rate for antibody to Aichi strain increased with age, to about 80% in persons 35 years old. On the basis of the results of the present study, it was hypothesized that Aichi strain could be a new type of small round virus that mainly produces diarrhea in patients in the 15- to 34-year-old age group, 50 to 76% of whom possess neutralizing antibody.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. R., Caul E. O., Paver W. K. Astrovirus-associated gastroenteritis in children. J Clin Pathol. 1978 Oct;31(10):939–943. doi: 10.1136/jcp.31.10.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J. R., Bartlett A. V., Herrmann J. E., Cáceres P., Blacklow N. R., Cano F. Astrovirus-associated diarrhea among Guatemalan ambulatory rural children. J Clin Microbiol. 1992 May;30(5):1140–1144. doi: 10.1128/jcm.30.5.1140-1144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt W. D., Blacklow N. R., Herrmann J. E., Nowak N. A., Nakata S., Chiba S. Antigenic relationships between human caliciviruses and Norwalk virus. J Infect Dis. 1987 Nov;156(5):806–814. doi: 10.1093/infdis/156.5.806. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D. The candidate caliciviruses. Ciba Found Symp. 1987;128:126–143. doi: 10.1002/9780470513460.ch8. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Treanor J. J., Madore H. P. Novel agents of viral enteritis in humans. J Infect Dis. 1987 Mar;155(3):365–376. doi: 10.1093/infdis/155.3.365. [DOI] [PubMed] [Google Scholar]
- Haruki K., Seto Y., Murakami T., Kimura T. Pattern of shedding of small, round-structured virus particles in stools of patients of outbreaks of food-poisoning from raw oysters. Microbiol Immunol. 1991;35(1):83–86. doi: 10.1111/j.1348-0421.1991.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Hudson R. W., Perron-Henry D. M., Kurtz J. B., Blacklow N. R. Antigenic characterization of cell-cultivated astrovirus serotypes and development of astrovirus-specific monoclonal antibodies. J Infect Dis. 1988 Jul;158(1):182–185. doi: 10.1093/infdis/158.1.182. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Taylor D. N., Echeverria P., Blacklow N. R. Astroviruses as a cause of gastroenteritis in children. N Engl J Med. 1991 Jun 20;324(25):1757–1760. doi: 10.1056/NEJM199106203242501. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W. Astroviruses: human and animal. Ciba Found Symp. 1987;128:92–107. doi: 10.1002/9780470513460.ch6. [DOI] [PubMed] [Google Scholar]
- Kurtz J. B., Lee T. W., Pickering D. Astrovirus associated gastroenteritis in a children's ward. J Clin Pathol. 1977 Oct;30(10):948–952. doi: 10.1136/jcp.30.10.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J., Lee T. Astrovirus gastroenteritis age distribution of antibody. Med Microbiol Immunol. 1978 Nov 17;166(1-4):227–230. doi: 10.1007/BF02121154. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lee T. W., Kurtz J. B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J Gen Virol. 1981 Dec;57(Pt 2):421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- Madeley C. R., Cosgrove B. P. Letter: Viruses in infantile gastroenteritis. Lancet. 1975 Jul 19;2(7925):124–124. doi: 10.1016/s0140-6736(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Kim J. P., Greenberg H. B., Su W., Sun Q., Johnson P. C., DuPont H. L., Oshiro L. S., Reyes G. R. The isolation and characterization of a Norwalk virus-specific cDNA. J Clin Invest. 1991 Apr;87(4):1456–1461. doi: 10.1172/JCI115152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe C. L., Allen J. R., Monroe S. S., Gary H. E., Jr, Humphrey C. D., Herrmann J. E., Blacklow N. R., Carcamo C., Koch M., Kim K. H. Detection of astrovirus in pediatric stool samples by immunoassay and RNA probe. J Clin Microbiol. 1991 Nov;29(11):2390–2395. doi: 10.1128/jcm.29.11.2390-2395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio O., Ishihara Y., Isomura S., Inoue H., Inouye S. Long-term follow-up of infants from birth for rotavirus antigen and antibody in the feces. Acta Paediatr Jpn. 1988 Aug;30(4):497–504. doi: 10.1111/j.1442-200x.1988.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Zeigler C. J., Martin G. M. Dimethyl sulfoxide enhances polyethylene glycol-mediated somatic cell fusion. Somatic Cell Genet. 1976 May;2(3):263–270. doi: 10.1007/BF01538964. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J. N., Graham D. Y., Wang K. N., Estes M. K. Norwalk virus genome cloning and characterization. Science. 1990 Dec 14;250(4987):1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Kasuya S., Noda S., Nagano I., Ohtsuka S., Ohtomo H. Newly isolated strains of Rickettsia tsutsugamushi in Japan identified by using monoclonal antibodies to Karp, Gilliam, and Kato strains. J Clin Microbiol. 1988 Sep;26(9):1859–1860. doi: 10.1128/jcm.26.9.1859-1860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Kobayashi S., Sakae K., Nakata S., Chiba S., Ishihara Y., Isomura S. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis. 1991 Nov;164(5):954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Ishihara Y., Isomura S. A 2-year survey of the prevalence of enteric viral infections in children compared with contamination in locally-harvested oysters. Epidemiol Infect. 1992 Feb;108(1):155–163. doi: 10.1017/s0950268800049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Sakae K., Ishihara Y., Isomura S., Totsuka A., Moritsugu Y. [Family-acquired hepatitis A--prevalence of hepatitis A among the family in Aichi Prefecture, 1990]. Kansenshogaku Zasshi. 1992 Jun;66(6):781–785. doi: 10.11150/kansenshogakuzasshi1970.66.781. [DOI] [PubMed] [Google Scholar]