Abstract
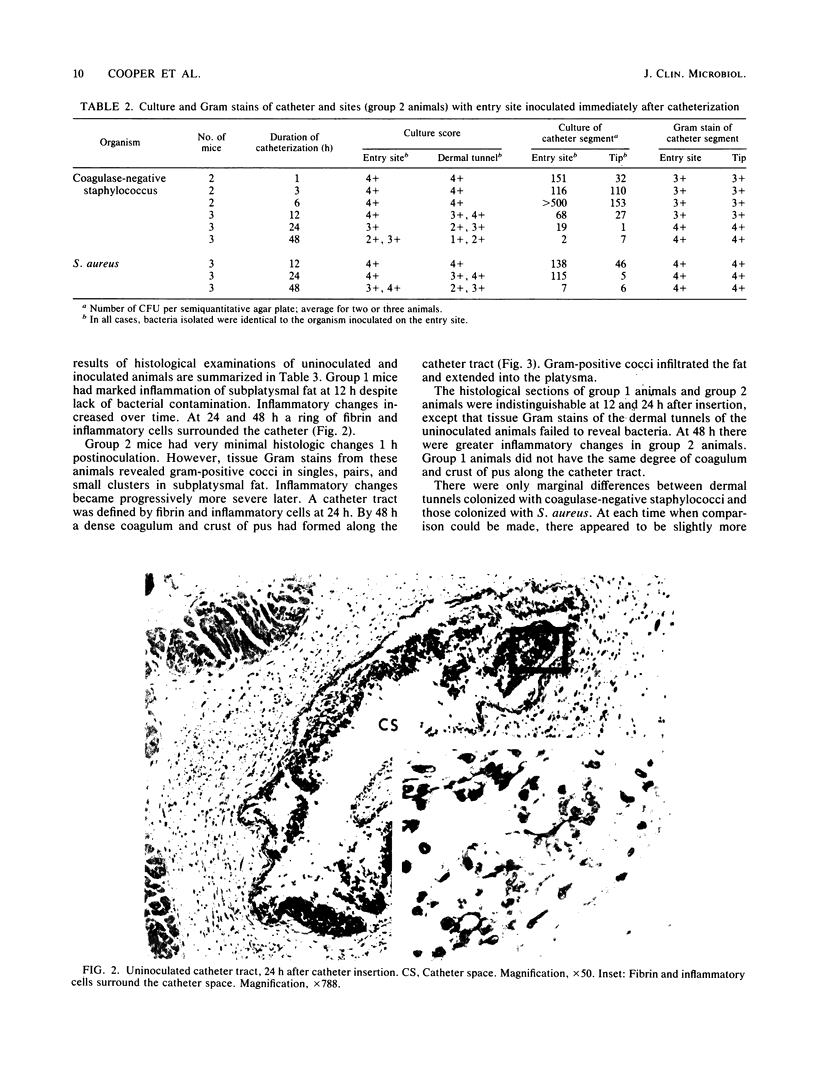
An animal model of vascular-catheter-associated dermal tunnel infections was developed to study the pathogenesis of such infections. Bacteria inoculated onto entry sites of catheters into skin could be identified by culture and Gram stain on the tips of plastic catheters (4 cm from the entry site) within 1 h of inoculation, whether the animal was inoculated at the time of insertion of the catheter or 1 week afterwards. Histological examination of dermal tunnels revealed that the introduction of bacteria preceded the development of tissue inflammation. Bacteria on entry sites of percutaneous catheters moved rapidly from the entry site into the dermal tunnel along the external catheter surface, perhaps suspended in a fluid phase and propelled by capillary action.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. L., Hopkins C. C. Rapid diagnosis of intravascular catheter-associated infection by direct Gram staining of catheter segments. N Engl J Med. 1985 May 2;312(18):1142–1147. doi: 10.1056/NEJM198505023121802. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Lichtenberg D. A., Kunches L. M., McDonough A. T., Gonzalez M. I., Heeren T. C., McCabe W. R. A randomized study comparing a transparent polyurethane dressing to a dry gauze dressing for peripheral intravenous catheter sites. Infect Control. 1985 Sep;6(9):361–366. doi: 10.1017/s0195941700063323. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katich M., Band J. Local infection of the intravenous-cannulae wound associated with transparent dressings. J Infect Dis. 1985 May;151(5):971–972. doi: 10.1093/infdis/151.5.971-a. [DOI] [PubMed] [Google Scholar]
- Liñares J., Sitges-Serra A., Garau J., Pérez J. L., Martín R. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol. 1985 Mar;21(3):357–360. doi: 10.1128/jcm.21.3.357-360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki D. G., Band J. D. A comparative study of polyantibiotic and iodophor ointments in prevention of vascular catheter-related infection. Am J Med. 1981 Mar;70(3):739–744. doi: 10.1016/0002-9343(81)90605-7. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Goldman D. A., Rhame F. S. Infection control in intravenous therapy. Ann Intern Med. 1973 Dec;79(6):867–887. doi: 10.7326/0003-4819-79-6-867. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Jarrett F., Sarafin H. W. A semiquantitative culture method for identification of catheter-related infection in the burn patient. J Surg Res. 1977 May;22(5):513–520. doi: 10.1016/0022-4804(77)90034-8. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Weise C. E., Sarafin H. W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977 Jun 9;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- Rumpf K. W., Seubert S., Seubert A., Lowitz H. D., Valentin R., Rippe H., Ippen H., Scheler F. Association of ethylene-oxide-induced IgE antibodies with symptoms in dialysis patients. Lancet. 1985 Dec 21;2(8469-70):1385–1387. doi: 10.1016/s0140-6736(85)92555-3. [DOI] [PubMed] [Google Scholar]