Abstract
Axonal action potentials initiate the cycle of inter-neuronal, synaptic communication that is key to our understanding of nervous system functioning. The field has accumulated vast knowledge of the signature action potential waveform, firing patterns, and underlying channel properties of many cell types, but in most cases this information comes from somatic intracellular/whole-cell recordings, which necessarily measure a mixture of the currents compartmentalized in the soma, dendrites, and axon. Because the axon in many neuron types appears to be the site of lowest threshold for action potential initiation, the channel constellation in the axon is of particular interest. However, the axon is more experimentally inaccessible than the soma or dendrites. Recent studies have developed and applied single-fiber extracellular recording, direct intracellular recording, and optical recording techniques from axons toward understanding the behavior of the axonal action potential. We are starting to understand better how specific channels and other cellular properties shape action potential threshold, waveform, and timing: key elements contributing to downstream transmitter release. From this increased scrutiny emerges a theme of axons with more computational power than in traditional conceptualizations.
Introduction
Glutamate transmission, like most chemical neurotransmission, typically begins with the initiation of an action potential near the soma of the presynaptic cell and axonal propagation of the impulse toward presynaptic terminals. The fidelity, timing, and waveform of action potentials as they propagate and arrive at the presynaptic terminal help dictate important features of synchrony and efficacy of synaptic communication at glutamate synapses. The disruption of normal (a)synchrony and efficacy of glutamate transmission likely participates in clinical disturbances of CNS function. Therefore, it is important to understand the factors shaping action potential initiation and propagation in glutamatergic neurons.
Much of what we know about differing action potential properties among neurons has come from somatic intracellular and whole-cell recordings. Recent reviews and indeed much of the last several decades of research on the excitable properties of CNS neurons have focused on different classes of channels that mediate the variety of action potential waveforms and firing properties, almost always monitored with somatic intracellular recordings (Connors and Gutnick, 1990; Bean, 2007). A wealth of new information has also been gathered recently on the active properties of dendrites (Johnston et al., 1996; London and Hausser, 2005) and the role of these properties in modulating synaptic information transfer (Magee and Johnston, 2005; Kampa et al., 2007). Although instructive, somatic and dendritic recordings beg the question of events in the largely inaccessible axonal compartment, where presumably action potentials are initiated and where critical “decisions” (determinations of spike threshold and waveform) are made by the local constellation of ion channels.
Classical work from motor neurons and peripheral axons informs our current understanding of action potential initiation and propagation, including effects of myelination and fiber size on conduction properties (Gasser and Erlanger, 1927; Huxley and Stampfli, 1949). On the topic of action potential initiation, intracellular recordings from motor neuron somas and initial segments showed that the action potential waveform, whether driven synaptically or antidromically, contains multiple components. These components include an initial segment action potential which always precedes a somatic action potential (Coombs et al., 1957). From this work arose the hypothesis, now well established for many neuron types, that the action potential normally initiates in the axon initial segment. Subsequent modeling proposed that a reason for preferential axonal initiation is a high density of sodium channels in the axon initial segment (Dodge and Cooley, 1973), a proposal that is still being actively investigated in various cell types (Wollner and Catterall, 1986; Colbert and Johnston, 1996; Jenkins and Bennett, 2001; Colbert and Pan, 2002; Komada and Soriano, 2002; Naundorf et al., 2006; Meeks and Mennerick, 2007; Kole et al., 2008). There is also a long history of work on peripheral axons that serves as our foundation for understanding action potential fidelity, timing, and the role of axon dysfunction in various disease states (Swadlow et al., 1980; Waxman, 2006).
Our review focuses on developments using techniques to explore the behavior of single fibers in the central nervous system. Recently several groups have combined conventional somatic intracellular recordings with direct, single-axon recordings from the same neuron to increase our understanding of action potential initiation and propagation in principal cells of the hippocampus and cortex. From these studies a picture emerges of axons with a different collection of ion channels than the somatodendritic compartment. This in turn can lead to dichotomies in the behavior of the somatodendritic compartment versus the axon. By contrast, other recent studies have shown that the somatic membrane potential can have a significant impact on the behavior of the axon, and on proximal synapses, through passive, electrotonic influences (Alle and Geiger, 2006; Shu et al., 2006). Together, these experimental lines suggest more computational power present in axons than traditionally assumed. Recent studies have encompassed experiments on both myelinated fibers (e.g. subicular, layer 5 rat neocortical) and unmyelinated fibers (e.g. young rat CA3 pyramidal neurons, ferret layer 5 neocortical pyramidal neurons). Accordingly, our review encompasses both fiber types.
Sodium channels
In neurons, voltage-gated sodium conductances play an essential role in action potential initiation and propagation (Hodgkin and Huxley, 1952). Voltage-gated sodium channels activate and inactivate within milliseconds. As the cell membrane is depolarized, sodium channels activate, resulting in the influx of sodium ions to further depolarize the membrane. This inward current produces the upstroke of the action potential. Along with the gating of potassium channels, sodium channel inactivation participates in the action potential downstroke. Although variations in many ion channels likely participate in the diversity of action potential waveforms observed in neurons (Bean, 2007), differences in sodium channel subunit composition, localization, and modulation may participate in shaping a neuron’s action potential.
Sodium channels are found on the soma, dendrite, and axon of a neuron. The predominant sodium channel subunits found on the CNS neuronal soma are Nav1.1 and Nav1.3 (Westenbroek et al., 1989). In addition Nav subunits 1.1, 1.3, and 1.6 have been identified in dendrites (Westenbroek et al., 1989; Westenbroek et al., 1992; Caldwell et al., 2000). Recently, sodium channels on dendritic spines of neocortical pyramidal neurons were found to participate in the amplification and effectiveness of dendritic action potential back propagation (Araya et al., 2007). In most cells examined, Nav1.2 and/or Nav1.6 are clustered at the axon initial segment. This clustering of Nav channels is thought to represent the site of lowest spike threshold in most neurons. In myelinated axons, these same subunits, especially Nav1.6 in the mature animal, cluster at nodes of Ranvier (Caldwell et al., 2000) in the central and peripheral nervous systems.
In some cases, unmyelinated fibers may also possess regions of high-density sodium channel clustering responsible for regenerating the action potential. Engel and Jonas (Engel and Jonas, 2005) recorded directly from mossy fiber terminals, a large, specialized en passant synapse made by small diameter, unmyelinated intrahippocampal mossy fiber axons of dentate granule neurons. These boutons contain approximately 2000 sodium channels per bouton (Engel and Jonas, 2005), and these presynaptic sodium channels have faster inactivation kinetics than dentate granule somatic sodium channels. Models of mossy fiber boutons suggest the high density of sodium channels on the bouton helps regenerate the action potential at a location that otherwise, because of impedence mismatch with the axon, might prove a liability for propagation (Engel and Jonas, 2005). Therefore, the strategic placement of voltage-gated sodium channels on an unmyelinated axon may increase conduction reliability in at least some specialized unmyelinated axons. The high sodium channel density may also participate in ensuring maxiumum presynaptic Ca2+ influx, which in turn may promote the strong presynaptic frequency-dependent, short-term synaptic enhancement characteristic of the mossy fiber synapse (Langdon et al., 1995; Salin et al., 1996).
In addition to localization differences among sodium channel subunits, there are differential expression profiles during development. During early development, sodium channel subunit Nav1.2 is found at the axon initial segment (Westenbroek et al., 1989). At maturing initial segments and nodes of Ranvier, Nav1.6 replaces Nav1.2. (Boiko et al., 2001; Kaplan et al., 2001). This switch occurs at both myelinated axons, coincident with the onset of myelination, and in unmyelinated proximal segments of retinal ganglion cell axons (Boiko et al., 2003; Van Wart et al., 2007). If Nav1.6 is genetically deleted, cell-specific compensation occurs, with other isoforms increasing their prominence at the axon initial segment (Van Wart and Matthews, 2006).
When comparing the response of Nav1.2 and Nav1.6 sodium currents to rapid repetitive depolarizations, Nav1.6 sodium currents show use-dependent potentiation while Nav 1.2 sodium currents show use-dependent reduction (Zhou and Goldin, 2004). Additionally, Nav1.6 currents are more resistant to inactivation when compared to Nav1.2 (Zhou and Goldin, 2004). These data suggest the sodium channel subunit composition at the axon initial segment contributes to the firing properties of neurons, particularly the characteristic maximum firing frequency of a particular cell class. Thus, at nodes of Ranvier the sodium channel subunit composition may contribute to a high safety factor for action potential propagation fidelity.
Many of the identified neuronal protein components of nodes of Ranvier are also found at the axon initial segment (Poliak and Peles, 2003; Salzer, 2003). This suggests these two axon regions may share common protein sorting mechanisms and functions. Nodes of Ranvier have three distinct domains. The nodal domain is characterized by a high density of voltage-gated sodium channels, primarily of Nav1.6 subunit composition (Boiko et al., 2001); clustering of potassium channels, including KCNQ2/3 (Devaux et al., 2004; Lai and Jan, 2006; Pan et al., 2006) and Kv3.1b (Devaux et al., 2003; Lai and Jan, 2006); cell adhesion molecules; and several cytoskeletal and scaffolding proteins such as ankyrinG and βIV spectrin (Kordeli et al., 1995; Berghs et al., 2000). The paranodal domain, a region adjacent to nodes of Ranvier and below the myelin sheath, contains cell adhesion molecules primarily thought to aid in the attachment of myelin to the axon plasma membrane. The third domain, the juxtaparanodal domain adjacent to the paranodes, is characterized by high density potassium channels primarily of Kv1.1 and Kv1.2 subunit composition (Lai and Jan, 2006), several cell adhesion molecules, and scaffolding proteins. The consequences of potassium channel subunit localization at the nodal and juxtaparanodial domains will be discussed in subsequent sections. In unmyelinated axons, which by definition are devoid of nodes of Ranvier, clustering of channels and other proteins at the axon initial segment appears broadly similar to those of myelinated fibers. Thus, myelin itself appears unnecessary for instructing the clustering of initial segment proteins.
Many neurons capable of firing sustained, high-frequency trains (fast-spiking neurons) possess substantial resurgent sodium currents (Raman and Bean, 1997, 1999). This includes cerebellar Purkinje neurons, with spontaneous firing rates of ~ 50 Hz (Hausser and Roth, 1997; Smith and Otis, 2003). Resurgent sodium currents are voltage-dependent and tetrodotoxin sensitive. Channels open briefly and then become blocked by a voltage-dependent, open-channel blocker that unbinds rapidly at negative potentials (Raman and Bean, 2001). As a result, the resurgent sodium current flows briefly as channels unblock during repolarization. Following deactivation, channels are readily available to reactivate, resulting in short refractory periods. Recent evidence suggests the cytoplasmic tail of the β4 accessory subunit is responsible for the properties of the resurgent sodium current in Purkinje cells (Grieco et al., 2005). These resurgent currents are prominent in many classes of fast-spiking neurons (Bean, 2007), but pyramidal neurons of the hippocampus and cortex, which typically are not fast spiking, apparently do not have significant resurgent sodium current. An exception appears to be layer 3 perirhinal cortex pyramidal cells, which may have resurgent sodium currents localized specifically to the axon initial segment (Castelli et al., 2007).
Steady-state persistent sodium currents can contribute to excitability and to the shape of an action potential. These sodium channels are active near rest (−65 mV) and do not inactivate even with quite strong depolarization. Therefore, these currents can participate in cellular excitability and in setting action potential threshold. There remains controversy over whether the same channels mediate persistent and transient sodium currents (Crill, 1996; Ptak et al., 2005; Bean, 2007); a persistent sodium current is predicted by Hodgkin-Huxley models over the voltage range in which steady-state activation and inactivation of currents overlap. Persistent sodium currents have been observed both in the soma and dendrites (Crill, 1996). Recently, persistent sodium currents have been blocked by local tetrodotoxin application near the initial portion of the axon 10–40 μm from layer 5 neocortical pyramidal neuron somas (Astman et al., 2006). This result suggests persistent sodium currents on the axon initial segment can modulate spiking behavior in particular cell types. Persistent sodium currents are also thought to be important for the firing properties of at least some principal cell types within the hippocampus (Yue et al., 2005; Carlier et al., 2006; Golomb et al., 2006; Vervaeke et al., 2006a).
Our focus here has been on voltage-gated sodium channel composition and localization. It is also worth noting that sodium channels can be part of protein-protein signaling complexes, which may alter the functional properties of the channels (Catterall et al., 2006; Kole et al., 2008). Likewise, sodium channels can be the target of second messenger modulation (e.g. phosphorylation), which sculpts voltage-gated sodium channel functioning and therefore excitability (Scheuer and Catterall, 2006). It remains unknown whether channels at the initial segment and axon might be specific targets of these forms of modulation. Such modulation could underlie hyperpolarizing shifts in the activation of initial segment sodium channels proposed to be important for action potential initiation (Colbert and Pan, 2002).
Potassium channels
Potassium channels are the most structurally and functionally diverse of voltage-gated ion channels and accordingly play a major role in characteristic spiking patterns and spike waveforms. Potassium channels modulate the resting membrane potential, action potential threshold, spike shape, afterhyperpolarization, and interspike interval. A diverse group of voltage-gated potassium channel subunits have been identified (Doyle et al., 1998; Dodson and Forsythe, 2004; Trimmer and Rhodes, 2004; Gutman et al., 2005). Our emphasis is on the functional classification of channels that are localized to the axons of myelinated and unmyelinated CNS axons.
The Kv3 family of potassium channels has interesting roles in the soma, axon, and presynaptic terminals. Kv3.1 currents are noninactivating, rapidly activating delayed- rectifiers with a high threshold for activation (Yokoyama et al., 1989). Because of their signature kinetics, Kv3 currents minimize the refractory period following individual action potentials. In the CNS, Kv3 channels help define a “fast-spiking” phenotype, a firing pattern characterized by narrow action potential waveforms, short refractory periods, and little adaptation in firing frequency to sustained current injection. For instance, prominent Kv3.1/Kv3.2 expression is found in fast spiking interneurons of the hippocampus and cortex. This current is not prominent in pyramidal neurons, a non-fast spiking phenotype. Instead, an A-type current (moderately rapidly activating, moderately rapidly inactivating potassium current) mediated by a Kv4 family member is responsible for the adapting firing pattern of pyramidal cells (Lien et al., 2002; Lien and Jonas, 2003). Other fast spiking cells expressing Kv3 family members include neurons of the striatum and auditory system (Perney et al., 1992; Gan and Kaczmarek, 1998; Wang et al., 1998b; Lien and Jonas, 2003). These results emphasize the importance of particular classes of potassium channels to signature action potential waveform and firing patterns of neurons.
Kv3 channels also influence axon behavior and subsequent neurotransmitter release. On myelinated axons, Kv3.1b channels are found on the nodal and juxatanodal domains (Lai and Jan, 2006) and generate a fast axonal potassium current (Corrette et al., 1991; Devaux et al., 2003). Kv3 channels also appear to be prominent on axons of the fast-spiking cortical interneuron classes mentioned above, and these channels directly affect GABA release from the presynaptic terminals of these axons (Goldberg et al., 2005). At the calyx of Held, a specialized glutamate presynaptic terminal in the auditory brainstem, blocking Kv3.1 with 1 mM tetraethylammonium results in axonal action potential broadening with subsequent augmentation of neurotransmitter release (Wang et al., 1998b; Ishikawa et al., 2003). Together, these data suggest that Kv3.1 influences action potential propagation fidelity and waveform in multiple neuronal compartments and may have a direct role at axon terminals in influencing neurotransmitter release.
Other potassium channels are important to the behavior of axons. Located in the juxatanodal domain are Kv1 channels (Rasband et al., 1998; Trimmer and Rhodes, 2004). These channels primarily produce a sustained outward potassium current activated with modest depolarization and exhibit fast activation and slow inactivation kinetics. In some cells these channels’ primary influence occurs after the first action potential to increase the action potential threshold for subsequent action potentials (Wu and Barish, 1992; Dodson et al., 2002; Brew et al., 2003). Other evidence suggests that the first action potential of a train can be influenced by Kv1 channels (Bekkers and Delaney, 2001). Blocking calyx of Held channels containing Kv1.2 subunit with dendrotoxin increases the incidence of aberrant action potentials (Dodson et al., 2003). Therefore, these presynaptic Kv1.2 subunits limit axonal hyperexcitabilty and help maintain a high level of action potential propagation fidelity within the neuron’s firing frequency range.
During high-frequency stimulation in the hippocampal mossy fiber bouton, axonal action potential broadening occurs due to the fast inactivation properties of Kv1.1 and Kv1.4-containing channels (Geiger and Jonas, 2000). This axonal action potential broadening leads to an increase in presynaptic calcium concentration that in turn augments neurotransmitter release (Geiger and Jonas, 2000). These data suggest that the Kv1.1 and/or Kv1.4 channels on mossy fiber boutons influence action potential repolarization, thereby affecting high frequency action potential propagation fidelity.
Because the axon initial segment seems to represent a preferred region for action potential initiation in cells studied to date, the mix of potassium channels here is of particular interest. Channels in this region should have particular importance in setting characteristic firing patterns, action potential threshold, and the shape of the initiating impulse. Using whole-cell axonal recordings, two recent studies probed the function of Kv1 channels on the axon initial segment and main axon of cortical pyramidal neurons (Kole et al., 2007; Shu et al., 2007a). These two studies reported a low threshold, rapidly activating, and slowly inactivating potassium current, possibly representing Kv1.1 and especially Kv1.2 subunits on layer 5 neocortical pyramidal axons (Inda et al., 2006; Kole et al., 2007; Shu et al., 2007a). This potassium channel subtype influences the axonal action potential duration. Blocking Kv1 channels with α-dendrotoxin broadens the axonal spike width, suggesting these voltage-gated potassium currents are responsible for axonal spike repolarization. Because of activation near resting membrane potentials, this current could influence spike threshold and the timing of action potential initiation.
Also striking were gradients of channel density within the axon initial segment. The soma contains few dendrotoxin-sensitive channels, and the somatic action potential waveform is relatively unaffected by α-dendrotoxin (Kole et al., 2007; Shu et al., 2007a). The proximal initial segment contains a moderate channel density, and the distal initial segment contains a high channel density, with moderately high density persisting more distally along the axon (Kole et al., 2007). The density of current accounts for a steep change in action potential waveform across the axon initial segment, with distal initial segment and the downstream axon producing waveforms considerably narrower, with larger undershoots, than proximal initial segment and soma.
Subthreshold somatic depolarization is transmitted to these Kv channels, resulting in spike waveform changes at the initiation site (~35–40 μm from the soma), and in changes to the waveform of the propagating action potential. This modulation appears to influence release of glutamate from proximal synapses (Kole et al., 2007). The data suggest that the constellation of potassium channels near the initiation site and in the distal axon can produce spikes of varied waveforms and have a significant impact on subsequent propagation characteristics and downstream synaptic transmission.
These recent studies of initial segment potassium currents have several interesting technical and functional implications. First, because dendrotoxin-sensitive Kv1 currents arising from the initial segment were not detectable in somatic recordings, the results highlight the need for methods to examine axonal function directly. Similarly, the results show that the waveform of the somatic and axonal action potential can differ considerably (see also (Engel and Jonas, 2005), with the latter presumably most relevant to subsequent transmitter release. Finally, the results show that channels other than voltage-gated sodium channels can be strategically localized to the initial segment to influence action potential threshold, axon action potential waveform, and downstream transmitter release.
Interestingly, the gradient of Kv1 channel density from the proximal to distal initial segment observed in these recent studies is similar to a gradient in sodium channels observed on retinal ganglion cell initial segment (Van Wart et al., 2007) and the compartmentalization of sodium channel clustering in the distal hippocampal CA3 initial segments (Meeks and Mennerick, 2007). On the other hand, human neocortical pyramidal neurons demonstrate an asymmetrical distribution of sodium and potassium (Kv1.2) channels, with Kv1.2 channels localized to the distal initial segment and Nav channels showing dense labeling throughout the length of initial segment (Inda et al., 2006). Although these results differ in details across different channels and cell types, the observations suggest that functional sub-specialization within the initial segment may be a common feature among axons. The functional implications of this subspecialization are unknown.
The M-current is a voltage-gated potassium current that has been a biophysical and physiological curiosity since its discovery in sympathetic neurons more than two decades ago. It was coined the M-current for its inhibition by muscarinic acetylcholine receptor agonists (Brown and Adams, 1980; Marrion, 1997). The current is active near resting potentials, and is more slowly activated than most voltage-gated potassium currents. The voltage range over which the current is active suggests importance in setting spike threshold and overall excitability. The M-current channel subunits belong to the Kv7 (KCNQ) gene family, consisting of five members, four of which are expressed in the nervous system (Wang et al., 1996; Wang et al., 1998a; Selyanko et al., 1999; Jentsch, 2000). The most common subunit composition of the channels underlying the M-current appears to be a heteromeric complex of KCNQ2 and KCNQ3 subunits (Wang et al., 1998a). Some studies show homometric KCNQ1–4 channels produce M-current-like kinetic and pharmacological properties (Brown and Adams, 1980; Selyanko et al., 1999). Surprisingly, a mutation in the KCNQ2 gene that produces a 25% reduction of M-current amplitude results in a form of human juvenile epilepsy (Jentsch et al., 2000). In the brain, KCNQ2 and KCNQ3 are strongly expressed within the hippocampus and cortex, with weaker expression in the cerebellum (Wang et al., 1998a). The clustering of KCNQ2 and KCNQ3 is seen on the axon initial segment of both myelinated and unmyelinated axons and at the nodal region of myelinated axons (Cooper et al., 2001; Devaux et al., 2004; Peters et al., 2005; Rasmussen et al., 2007). Debate continues about whether dendritic M-currents play a prominent role in CA1 pyramidal neurons (Yue and Yaari, 2006) or whether the primary localization is perisomatic/axonal (Devaux et al., 2004; Pan et al., 2006; Vervaeke et al., 2006b).
A striking effect of the M-current on axonal action potentials and synaptic physiology was recently demonstrated (Vervaeke et al., 2006b). In two afferent pathways to CA1 hippocampal pyramidal cells, mild depolarization enhanced neurotransmitter release in a manner sensitive to pharmacological M-channel blockers (Vervaeke et al., 2006b). This suggests the counterintuitive finding that a voltage-gated potassium conductance enhances transmitter release. Modeling these axonal channels suggests that M-current activation by depolarization decreases sodium channel inactivation, leading to the strengthening of neurotransmission. The results suggest that both action potential initiation and action potential propagation fidelity can be affected by the M-current in unmyelinated axons. Several studies implicate second messenger influences on M-channel properties (Delmas and Brown, 2005). It has yet to be investigated how these modulators can influence M-channels located specifically on the axon.
The M-current activator, retigabine [N-(2-amino-4-(4-fluorobenzylamino)-phenyl)carbamic acid ethyl ester], is in phase three clinical trials for controlling neuronal hyperexcitability in patients with partial-onset seizures. The drug’s mechanism of action was investigated only after the genetic link between M-channel subunit mutations and seizure disorders was discovered. Retigabine was found to have novel potentiating actions on KCNQ2–5 voltage-activated potassium channels (Main et al., 2000; Wickenden et al., 2000; Blackburn-Munro et al., 2005; Porter et al., 2007). Other studies demonstrate the usefulness of retigabine in animal models of neuropathic pain and anxiety (Munro et al., 2007). These results stress the major influence of the M-current in controlling pathophysiologically relevant neuronal excitability.
The role of Ca2+-activated potassium conductances in spiking behavior has recently been investigated and reviewed (Bean, 2007; Gu et al., 2007). The localization of Ca2+ activated potassium conductances is uncertain in principal cells. The channels are known to affect action potential afterhyperpolarization and spike frequency adaptation in several cell types. If the primary localization of these conductances is near the axon initial segment, they may have a direct role in setting the action potential threshold and the repolarization rate at the site of first-threshold crossing. If their localization is primarily somatodendritic, they may have a less direct role, shunting depolarizing currents and making it less likely that these currents reach the initial segment at sufficient amplitude to trigger a spike.
Future research will undoubtedly uncover the presence other specific axonal potassium channel subunits as well as signaling pathways regulating the function of these channels and, in turn, neuronal excitability (Levitan, 2006). Particularly interesting will be to explore further how this extremely diverse family of voltage-gated channels alters excitability during development, maturation, and in disease pathology.
Action potential initiation and propagation
As noted above, a characteristic of nearly all neurons studied is preferential initiation in the axon, with subsequent development/backpropagation into the somatodendritic compartment. Direct recordings from single axons yield direct, quantitative information regarding the initiation and propagation of the action potential. Action potential propagation has been studied most extensively in the peripheral nervous system because these fibers are easily accessible, often large and myelinated, and are more amenable to experimental manipulation compared to CNS nerve fibers. Other studies of transmission rely on population measures obtained from fiber tracts, where behavior of individual axons must be inferred. Recently, several studies have probed action potential properties of CNS neurons through the use of either dual soma-axon recording or with fast voltage sensitive dyes (Clark et al., 2005; Khaliq and Raman, 2005; Meeks et al., 2005; Monsivais et al., 2005; Palmer and Stuart, 2006; Shu et al., 2006; Kole et al., 2007; Meeks and Mennerick, 2007; Scott et al., 2007; Shu et al., 2007b; Shu et al., 2007a; Kole et al., 2008). These studies have been able to test the general applicability of conclusions first established in larger fibers and fiber tracts to small CNS fibers.
During axonal action potential initiation, the active depolarization propagates both towards the soma (antidromic) and down the axon (orthodromic). Because of very different passive and active membrane properties of the soma compared with the axon, the conduction velocity in the two directions is likely different in most cells (Clark et al., 2005; Kole et al., 2007; Meeks and Mennerick, 2007; Shu et al., 2007b). The conduction velocity of the antidromic action potential may have a significant impact on dendritic backpropagation. This in turn will affect spike-timing dependent plasticity, synaptic plasticity sensitive to the timing of dendritic action potentials relative to incoming synaptic information (Sjostrom and Nelson, 2002). The orthodromic velocity will affect the degree of synchrony of arrival of information at different postsynaptic targets of the same axon.
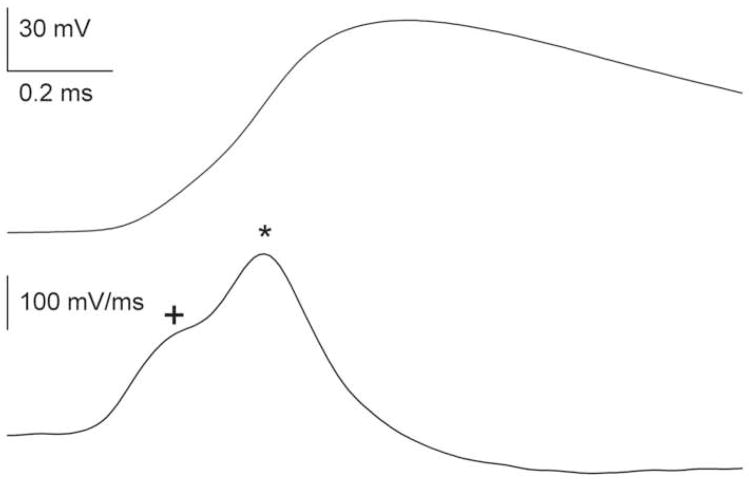
A complication in measuring antidromic versus orthodromic propagation latency is the altered action potential shape as it develops antidromically and orthodromically from the site of initial threshold crossing. The most accurate measurements of antidromic conduction velocity should account for the lag between hillock invasion and full invasion of the somadendritic compartment (Coombs et al., 1957; Colbert and Johnston, 1996; Meeks and Mennerick, 2007). This lag can be appreciated by differentiating the raw somatic spike waveform. An example from a CA3 neuron is shown in Figure 1. The first and second derivative waveforms of somatic membrane potential contain inflections, the first of which represents the axonal spike, the second of which represents the time lag between action potential development in the axon versus full invasion of the somatodendritic compartment (Coombs et al., 1957; Colbert and Johnston, 1996).
Figure 1.
Multiple components in somatically recorded action potentials. The waveforms are taken from a somatic whole-cell recording of a hippocampal CA3 pyramidal neuron in an acutely prepared slice of juvenile rat hippocampus. The top trace represents the raw somatic waveform of an action potential elicited by direct current injection to the soma. The bottom trace is the first derivative of the waveform with respect to time. Note the two inflections in the bottom trace not easily evident from inspection of the rising phase of the raw voltage waveform. The two components result from the axonally initiated action potential registered by the somatic recording (plus sign) followed by the eventual recruitment of the voltage-gated channels of the large somatodendritic compartment (asterisk) (Coombs et al., 1957; Colbert and Johnston, 1996; Meeks and Mennerick, 2007).
The most direct measurements of action potential latencies and conduction velocities come from dual somatic and axonal intracellular recordings. Because of the small size of most CNS glutamatergic axons, such intracellular recordings have been possible only in the axon initial segment region or in distal axon by recording from axon “blebs,” the balled ends of axons created by the slicing procedure. These recordings have allowed direct identification of the arrival/development of the action potential peak in the soma and at various points along the axon of layer 5 neocortical neurons. The recordings thus predict conduction velocities, measured as the arrival of the action potential peak, that are slower in the antidromic direction than in the orthodromic direction (Shu et al., 2006; Kole et al., 2007; Shu et al., 2007b; Shu et al., 2007a) (Table 1). The recordings also verify axonal initiation, showing that the action potential normally develops first in the initial segment of the axon (Shu et al., 2007b; Schmidt-Hieber et al., 2008).
Table 1.
Axonal action potential properties from recent studies of cell types in three major brain regions.
| Brain Area | Cell Type | Tissue Species | Animal Age | Temp (°C) | Axon Myelination Status | Conduction Velocity (m/s) | Est. AP Initation Site (μm from soma) | Recording Configuation | Reference |
|---|---|---|---|---|---|---|---|---|---|
| neocortex | layer 5 pyr | Rat | P26-30 | 35 | ? | antidromic 0.3 –0.4 | ≥ 30 | dual whole-cell soma and axon initial segment | Stuart et al., 1997 |
| layer 5 pyr | Rat | P14-24 | 23 | ? | ? | ≥ 30 | axon initial segment patches, whole-cell soma | Colbert and Pan, 2002 | |
| layer 5 pyr | Rat | P21-35 | 34 | starting at ~40 um | orthodromic ~0.4 | ~35 | whole-cell soma, voltage sensitive dyes | Palmer Stuart, 2006 | |
| layer 5 pyr | Rat | P12-60 | 34 | starting at ~50 um | orthrodromic 2.9 | ~38 | dual whole-cell soma and axon bleb | Kole et al., 2007 | |
| layer 5 pyr | ferret | P49-56 | 36.5 | starting at ~200 um | orthrodromic 0.83 antidromic 0.77 | 40–55 | dual whole-cell soma and axon bleb | Shu et al., 2007 | |
| cerebellum | Purkinje | Rat | P13-31 | 22 | Yes | ? | ≥ 7 | dual whole-cell soma and axon initial segment | Stuart and Hausser, 1994 |
| Purkinje | mouse | P15-29 | 33 | yes | orthrodromic 1 | ~30 | dual whole-cell soma and axon loose-seal | Khaliq and Raman, 2006 | |
| Purkinje | Rat | P18-26 | 34 | starting at ~20 um | orthrodromic 0.77 antidromic 0.56 | 75+−11 | dual soma attached recording and axon loose-seal | Clark et al., 2005 | |
| hippocampus | CA3 pyr | Rat | P 18-22 | 25 | not within 1 mm | orthrodromic 0.3 | 35–40 | dual whole-cell soma and axon loose-seal | Meeks and Mennerick, 2007 |
| CA3 pyr | Rat | P21-84 | 23 | unmyelinated | estimated ~0.23 | NA | Antidromic stimulation, CA3 unit | Soleng et al., 2003a | |
| dentate granule | Rat | P17-23 | 25 | not within 1 mm | orthrodromic 0.25 | ~40 | dual whole-cell soma and axon loose-seal | Kress et al., 2007 | |
| dentate granule | Rat | P30-50 | 33 | ? | antidromic 0.67 | ? | antidromic population spike | Langdon et al. 1993 | |
| subicular pyr | Rat | P14-56 | 24 | yes | ? | 30–60 | dual whole-cell soma and axon initial segment | Colbert and Johnston 1996 |
Est estimate, AP action potential, Temp temperature, Pyr Pyramidal.
Direct intracellular axonal measurements have also allowed the discovery of an unanticipated form of analogue signal propagation along axons. Two recent studies have shown that subthreshold synaptic potentials can reach a significant length down the axon and influence glutamate release from the affected presynaptic terminals (Alle and Geiger, 2006; Shu et al., 2006). These studies stress that the axon does not carry only digital signals and that analogue and digital axonal signals can interact to influence neurotransmitter release under certain conditions.
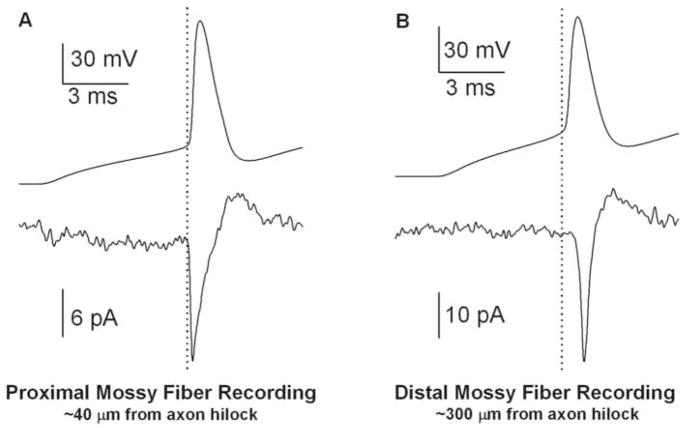
A potentially somewhat less invasive approach to recording axonal action potentials (but inappropriate for analogue signal measurement) has employed intact axons and local loose-seal extracellular recordings of axonal signals (Clark et al., 2005; Khaliq and Raman, 2005; Meeks et al., 2005; Monsivais et al., 2005; Meeks and Mennerick, 2007). In this case, because of the extracellular recording configuration, the axonal signal corresponds best to the first time derivative (slope) of membrane voltage (Meeks et al., 2005). Accordingly, the signal amplitude readily identifies the time of maximum rate of depolarization. Two representative dual recordings from dentate granule neuron soma (whole cell) and axon (loose seal extracellular) are shown in Figure 2A, B. Figure 2A represents an axon recording close to the soma, and it can be appreciated that the axonal spike nearly coincides with threshold (dotted vertical line in Figure 2A), defined in the somatic record by the dotted vertical line. By contrast, a more distal axon recording location in another dentate granule cell (Figure 2B) shows an axonal action potential arising later with respect to threshold. Many such recordings can be analyzed to produce estimates of conduction velocity (Clark et al., 2005; Meeks et al., 2005; Meeks and Mennerick, 2007; Shu et al., 2007b).
Figure 2.
Dual whole-cell somatic and loose-seal extracellular axon recordings from dentate granule neurons. A. Example of an axonal recording performed near the hillock. The axonal action potential occurs nearly coincident with the onset of the somatically recorded action potential. The dotted vertical line indicates action potential threshold measured at the soma, calculated as 5% of maximum voltage acceleration (Meeks and Mennerick, 2007). B. Example from another dentate granule cell of an axonal recording performed more distally. The axonal spike occurs with a longer latency relative to the onset of the somatically recorded action potential. Axon recordings were performed in the voltage-clamp mode of a patch amplifier. The extracellular axon signal corresponds most closely to the first derivative of intracellularly recorded membrane potential (Meeks et al., 2005). Action potentials were elicted by direct current injection through the whole-cell somatic recording pipette.
Loose-seal axonal recordings also verify an axonal action potential initiation site. In hippocampal CA3 pyramidal neuron axons, although the site that reaches threshold first is located approximately 35 μm from the soma, the zone of axon stretching to 100 μm distal from the soma reaches a maximum rate of depolarization nearly synchronously by the influx of sodium from the high-density sodium channels on the axon’s distal initial segment (Meeks and Mennerick, 2007). In these cells, the site of first-threshold crossing corresponds to a region of high sodium channel density, assessed by antibody staining. Tetrodotoxin applied locally to this same region dramatically alters action potential threshold (Meeks and Mennerick, 2007). Dentate granule neurons also have a zone of dense axonal staining for voltage-gated sodium channels within the first 50 μm from the soma (Kress et al., 2007). Therefore, hippocampal unmyelinated fibers show evidence of axonal action potential initiation and are therefore similar in this respect to many myelinated fibers where axonal initiation was first demonstrated (Coombs et al., 1957; Colbert and Johnston, 1996; Stuart et al., 1997; Clark et al., 2005; Khaliq and Raman, 2006). Preferential axonal initiation appears common across myelinated and unmyelinated fiber types despite differences in the anatomical criteria (e.g. myelin onset) that help define the axon initial segment in the various axon classes.
The propagation time for an action potential traveling from the axonal action potential initiation site to a presynaptic bouton could be up to several milliseconds for both myelinated and unmyelinated axons. Table 1 shows the orthrodromic conduction velocities for a variety of neurons studied with recent single-fiber techniques. Prevoius studies of action potential propagation in PNS and CNS neurons, obtained with more traditional techniques of antidromic latency measurements or field measurements of axon volleys; are not included but have been previously reviewed (Swadlow et al., 1980). Purkinje neurons and cortical layer 5 pyramidal neurons, both possessing myelinated axons, have approximately the same orthrodromic conduction velocity of 0.5–0.6 m s−1. These figures are interesting because they fall into the range more typically associated with unmyelinated fibers (Swadlow et al., 1980), although one study estimated conduction velocity of layer 5 axons at 2.9 m s−1 (Kole et al., 2007). One recent study compared the conduction velocity of postnatal day 5 unmyelinated fibers and myelinated postnatal day 21–28 fibers from layer 5 cortical pyramidal neurons (Palmer and Stuart, 2006). Conduction velocity was significantly slower without myelination, but this result could also partly reflect a smaller diameter axon or a developmental change of sodium channel subtype expression and/or distribution. Through the use of newly synthesized fast voltage sensitive dyes, Palmer and Stuart visualized orthodromic action potential propagation in the myelinated axon, with the velocity varying in an apparently saltatory manner (Palmer and Stuart, 2006).
Propagation fidelity is another parameter important for information transfer that can be directly measured with dual soma/axon recording techniques. During spontaneous somatic action potentials and during short trains of action potentials, propagation fidelity towards the distal axon is nearly 100% in CA3 pyramidal cells, cerebellar Purkinje neurons, layer 5 cortical pyramidal neurons, and medial superior olive neurons (Khaliq and Raman, 2005; Meeks et al., 2005; Monsivais et al., 2005; Palmer and Stuart, 2006; Scott et al., 2007). During rapid firing rates of up to 250 Hz in Purkinje cells, or >500–1000 Hz in medial superior olive neurons, axonal propagation fidelity decreases substantially (Khaliq and Raman, 2005; Monsivais et al., 2005; Scott et al., 2007). Similarly, in CA3 pyramidal neurons, with very prolonged seizure-like firing, axonal action potentials become distorted, eventually resulting in failure toward the end of long bouts of firing. Interestingly, an envelope of sustained somatic depolarization appears critical for the axonal waveform changes; transient current injections eliciting a similar firing frequency does not produce the same alteration in axonal action potential waveform (Meeks et al., 2005). It is possible that potassium conductances specific to the axon contribute to this behavior (Pan et al., 2006; Kole et al., 2007; Shu et al., 2007a).
Several factors, including previous activity and release of neurotransmitters, can modulate spike propagation fidelity in certain axons. Branch point failures have been hypothesized and observed within axonal arbors (Krnjevic and Miledi, 1959; Parnas, 1972; Yau, 1976; Grossman et al., 1979b, a; Smith, 1980; Manor et al., 1991; Debanne et al., 1997; Segev and Schneidman, 1999; Soleng et al., 2003b), although high propagation fidelity has also been observed under many conditions (Mackenzie and Murphy, 1998; Cox et al., 2000).
Debanne and colleagues showed action potential failures after a brief depolarizing step from a hyperpolarizing potential (Debanne et al., 1997). Re-priming of inactivating IA-type potassium channels were proposed to explain these failures (Debanne et al., 1997). A similar phenomenon has been observed in cultured neurons (Thio and Yamada, 2004). Other conductances, including a hyperpolarizing cationic current, Ih, have been shown to influence activity-dependent excitability changes in axons of principal cells (Soleng et al., 2003a; Soleng et al., 2004). Although interneuron axons have not been explicitly investigated, our own work has shown that GABA release appears relatively more resistant to conduction changes than glutamate release in both hippocampal cultures and slices (Prakriya and Mennerick, 2000; He et al., 2002; Meeks and Mennerick, 2004). This raises the possibility that propagation fidelity may be more secure in at least some classes of interneuron axons than in principal neuron axons within the hippocampus, although this idea has not been directly tested.
Axons may be modulated by ligand-gated ion channels. Axo-axonic GABA synapses offer one opportunity for distinct classes of interneurons to influence action potential threshold and possibly action potential waveform (Howard et al., 2005). In addition to GABA modulation of the initial segment mentioned above, neurotransmitter modulation of distal axonal action potentials, mediated through ionotropic receptors, has been shown in several systems (Kullmann et al., 2005). In the hippocampus, there has been interest in the presence of both GABAA receptors on dentate granule cell mossy fiber axons and kainate-type glutamate receptors on interneuron axons (Semyanov and Kullmann, 2001; Ruiz et al., 2003; Kullmann et al., 2005; Alle and Geiger, 2007). Both have been shown to modulate fidelity of antidromic spikes elicited with distal initiation and recorded somatically. Such receptors have the potential to modulate spike timing, fidelity, and waveform at the presynaptic terminal and thereby regulate neurotransmission.
Synaptic strength and synaptic transmitter release can be influenced by the shape of the action potential waveform within the presynaptic bouton (Lisman et al., 2007). The relationship between spike waveform and transmitter release has been studied most directly at specialized synapses (calyx of Held and hippocampal mossy fiber boutons) (Borst and Sakmann, 1999; Yang and Wang, 2006), optically in CA3-CA1 axons/synapses of the hippocampus (Qian and Saggau, 1999) and in parallel fibers of the cerebellum (Sabatini and Regehr, 1997). In most cases, transmitter release is particularly dependent on the width of the action potential. At hippocampal mossy fibers, Geiger and Jonas demonstrated frequency dependent broadening and amplitude reduction of action potentials but not at the dentate granule cell body (Geiger and Jonas, 2000). This broadening resulted in the greater presynaptic calcium influx that potentiated synaptic currents in a CA3 pyramidal dendrite. This broadening results from activity-dependent inactivation of a potassium conductance, reminiscent of recent findings at the initial segment/axon of layer 5 pyramidal neurons (Kole et al., 2007; Shu et al., 2007a).
Summary
The action potential is essential to our understanding of nervous system function. Its shape, velocity of conduction, and propagation fidelity are essential to the timing, synchrony, and efficacy of neuronal communication. As such, action potentials have been the subject of intense scrutiny for nearly a century. Nevertheless, axonal properties, particularly those of the vertebrate CNS, remain somewhat elusive, given the limited and rather indirect experimental tools that can be applied to the study of axonal action potentials. Improved imaging and direct electrophysiological recording methods are yielding new insights into the axons and action potentials of glutamatergic and other neuronal types. With increased resolution offered by these techniques comes increased realization that the action potential is not always digital and that the axon’s spike waveform and behavior can be somewhat divorced from that observed in the soma. Further, waveform, timing, and fidelity of the axonal action potential can be modulated, which leads to changes in presynaptic neurotransmitter release.
Acknowledgments
We thank laboratory members for advice and we acknowledge NIH grants MH78823 and NS54174 for support of work in our laboratory.
Abbreviations
- CNS
central nervous system
- Nav
voltage-gated sodium
- Kv
voltage-gated potassium
- GABA
gamma-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Alle H, Geiger JR. GABAergic spill-over transmission onto hippocampal mossy fiber boutons. J Neurosci. 2007;27:942–950. doi: 10.1523/JNEUROSCI.4996-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Nikolenko V, Eisenthal KB, Yuste R. Sodium channels amplify spine potentials. Proc Natl Acad Sci U S A. 2007;104:12347–12352. doi: 10.1073/pnas.0705282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neuocortical pyramidal neurons. J Neurosci. 2001;21:6553–35560. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Effect of changes in action potential shape on calcium currents and transmitter release in a calyx-type synapse of the rat auditory brainstem. Philos Trans R Soc Lond B Biol Sci. 1999;354:347–355. doi: 10.1098/rstb.1999.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier E, Sourdet V, Boudkkazi S, Deglise P, Ankri N, Fronzaroli-Molinieres L, Debanne D. Metabotropic glutamate receptor subtype 1 regulates sodium currents in rat neocortical pyramidal neurons. J Physiol. 2006;577:141–154. doi: 10.1113/jphysiol.2006.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli L, Biella G, Toselli M, Magistretti J. Resurgent Na+ current in pyramidal neurones of rat perirhinal cortex: axonal location of channels and contribution to depolarizing drive during repetitive firing. J Physiol. 2007;582:1179–1193. doi: 10.1113/jphysiol.2007.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Hulme JT, Jiang X, Few WP. Regulation of sodium and calcium channels by signaling complexes. J Recept Signal Transduct Res. 2006;26:577–598. doi: 10.1080/10799890600915100. [DOI] [PubMed] [Google Scholar]
- Clark BA, Monsivais P, Branco T, London M, Hausser M. The site of action potential initiation in cerebellar Purkinje neurons. Nat Neurosci. 2005;8:137–139. doi: 10.1038/nn1390. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The interpretation of spike potentials of motoneurones. J Physiol. 1957;139:198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrette BJ, Repp H, Dreyer F, Schwarz JR. Two types of fast K+ channels in rat myelinated nerve fibres and their sensitivity to dendrotoxin. Pflugers Arch. 1991;418:408–416. doi: 10.1007/BF00550879. [DOI] [PubMed] [Google Scholar]
- Cox CL, Denk W, Tank DW, Svoboda K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc Natl Acad Sci U S A. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action potential propagation gated by an IA-like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Cooley JW. Action potential of the motoneuron. IBM J Res Dev. 1973;17:219–229. [Google Scholar]
- Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–217. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J Physiol. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Gan L, Kaczmarek LK. When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons. J Neurobiol. 1998;37:69–79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gasser HS, Erlanger J. The role played by the sizes of the constituent fibers of a nerve trunk in determining the form of its action potential wave. Am J Physiol. 1927;80:522–547. [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Watanabe S, Chang SY, Joho RH, Huang ZJ, Leonard CS, Rudy B. Specific functions of synaptically localized potassium channels in synaptic transmission at the neocortical GABAergic fast-spiking cell synapse. J Neurosci. 2005;25:5230–5235. doi: 10.1523/JNEUROSCI.0722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D, Yue C, Yaari Y. Contribution of persistent Na+ current and M-type K+ current to somatic bursting in CA1 pyramidal cells: combined experimental and modeling study. J Neurophysiol. 2006;96:1912–1926. doi: 10.1152/jn.00205.2006. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Differential conduction block in branches of a bifurcating axon. J Physiol. 1979a;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Mechanisms involved in differential conduction of potentials at high frequency in a branching axon. J Physiol. 1979b;295:307–322. doi: 10.1113/jphysiol.1979.sp012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Hausser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. Journal of Physiology London. 1997;501:77–95. doi: 10.1111/j.1469-7793.1997.077bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zorumski CF, Mennerick S. Contribution of presynaptic Na+ channel inactivation to paired-pulse synaptic depression in cultured hippocampal neurons. J Neurophysiol. 2002;87:925–936. doi: 10.1152/jn.00225.2001. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Huxley A, Stampfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibers. J Physiol. 1949;108:315–339. [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J Neurosci. 2003;23:10445–10453. doi: 10.1523/JNEUROSCI.23-32-10445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Schroeder BC, Kubisch C, Friedrich T, Stein V. Pathophysiology of KCNQ channels: neonatal epilepsy and progressive deafness. Epilepsia. 2000;41:1068–1069. doi: 10.1111/j.1528-1157.2000.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30:456–463. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. J Neurosci. 2005;25:454–463. doi: 10.1523/JNEUROSCI.3045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–1944. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Meeks JP, Mennerick S. Properties of action potential initiation and propagation in rat hippocampal dentate granule neurons. Society for Neuroscience Meeting Planner Program No. 881.12 2007 [Google Scholar]
- Krnjevic K, Miledi R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Langdon RB, Johnson JW, Barrionuevo G. Posttetanic potentiation and presynaptically induced long-term potentiation at the mossy fiber synapse in rat hippocampus. J Neurobiol. 1995;26:370–385. doi: 10.1002/neu.480260309. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9:305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci. 2003;23:2058–2068. doi: 10.1523/JNEUROSCI.23-06-02058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K(+) channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Raghavachari S, Tsien RW. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat Rev Neurosci. 2007;8:597–609. doi: 10.1038/nrn2191. [DOI] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Mackenzie PJ, Murphy TH. High safety factor for action potential conduction along axons but not dendrites of cultured hippocampal and cortical neurons. J Neurophysiol. 1998;80:2089–2101. doi: 10.1152/jn.1998.80.4.2089. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr Opin Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Manor Y, Koch C, Segev I. Effect of geometrical irregularities on propagation delay in axonal trees. Biophys J. 1991;60:1424–1437. doi: 10.1016/S0006-3495(91)82179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J Neurophysiol. 2007;97:3460–3472. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Jiang X, Mennerick S. Action potential fidelity during normal and epileptiform activity in paired soma/axon recordings from rat hippocampus. Journal of Physiology (London) 2005;566:425–441. doi: 10.1113/jphysiol.2005.089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais P, Clark BA, Roth A, Hausser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci. 2005;25:464–472. doi: 10.1523/JNEUROSCI.3871-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Naundorf B, Wolf F, Volgushev M. Unique features of action potential initiation in cortical neurons. Nature. 2006;440:1060–1063. doi: 10.1038/nature04610. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and Nav channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972;35:903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre-Botzinger complex region. J Neurosci. 2005;25:5159–5170. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Saggau P. Modulation of transmitter release by action potential duration at the hippocampal CA3-CA1 synapse. Journal of Neurophysiology. 1999;81:288–298. doi: 10.1152/jn.1999.81.1.288. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J. 2001;80:729–737. doi: 10.1016/S0006-3495(01)76052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. Journal of Neuroscience. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Scheuer T, Catterall WA. Control of neuronal excitability by phosphorylation and dephosphorylation of sodium channels. Biochem Soc Trans. 2006;34:1299–1302. doi: 10.1042/BST0341299. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J Physiol. 2008 doi: 10.1113/jphysiol.2007.150151. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LL, Hage TA, Golding NL. Weak action potential backpropagation is associated with high-frequency axonal firing capability in principal neurons of the gerbil medial superior olive. J Physiol. 2007;583:647–661. doi: 10.1113/jphysiol.2007.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol Paris. 1999;93:263–270. doi: 10.1016/s0928-4257(00)80055-8. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Delmas P, Buckley NJ, London B, Brown DA. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J Neurosci. 1999;19:7742–7756. doi: 10.1523/JNEUROSCI.19-18-07742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Kainate receptor-dependent axonal depolarization and action potential initiation in interneurons. Nat Neurosci. 2001;4:718–723. doi: 10.1038/89506. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A. 2007a;104:11453–11458. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol. 2007b;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Nelson SB. Spike timing, calcium signals and synaptic plasticity. Curr Opin Neurobiol. 2002;12:305–314. doi: 10.1016/s0959-4388(02)00325-2. [DOI] [PubMed] [Google Scholar]
- Smith DO. Mechanisms of action potential propagation failure at sites of axon branching in the crayfish. J Physiol (Lond) 1980;301:243–259. doi: 10.1113/jphysiol.1980.sp013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Otis TS. Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade. J Neurosci. 2003;23:367–372. doi: 10.1523/JNEUROSCI.23-02-00367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Chiu K, Raastad M. Unmyelinated axons in the rat hippocampus hyperpolarize and activate an H current when spike frequency exceeds 1 Hz. J Physiol (Lond) 2003a;552:459–470. doi: 10.1113/jphysiol.2003.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Raastad M, Andersen P. Conduction latency along CA3 hippocampal axons from rat. Hippocampus. 2003b;13:953–961. doi: 10.1002/hipo.10141. [DOI] [PubMed] [Google Scholar]
- Soleng AF, Baginskas A, Andersen P, Raastad M. Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons. J Physiol. 2004;560:491–503. doi: 10.1113/jphysiol.2004.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol (Lond) 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Kocsis JD, Waxman SG. Modulation of impulse conduction along the axonal tree. Annu Rev Biophys Bioeng. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- Thio LL, Yamada KA. Differential presynaptic modulation of excitatory and inhibitory autaptic currents in cultured hippocampal neurons. Brain Res. 2004;1012:22–28. doi: 10.1016/j.brainres.2004.02.077. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Hu H, Graham LJ, Storm JF. Contrasting effects of the persistent Na+ current on neuronal excitability and spike timing. Neuron. 2006a;49:257–270. doi: 10.1016/j.neuron.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006b;576:235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998a;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998b;509( Pt 1):183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Noebels JL, Catterall WA. Elevated expression of type II Na+ channels in hypomyelinated axons of shiverer mouse brain. J Neurosci. 1992;12:2259–2267. doi: 10.1523/JNEUROSCI.12-06-02259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anticonvulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58:591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci U S A. 1986;83:8424–8428. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Wang LY. Amplitude and kinetics of action potential-evoked Ca2+ current and its efficacy in triggering transmitter release at the developing calyx of Held synapse. J Neurosci. 2006;26:5698–5708. doi: 10.1523/JNEUROSCI.4889-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976;263:513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95:3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Goldin AL. Use-dependent potentiation of the Nav1.6 sodium channel. Biophys J. 2004;87:3862–3872. doi: 10.1529/biophysj.104.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]