Abstract
Neuroattenuated herpes simplex virus ICP34.5 mutants slow progression of preformed tumors and lead to complete regression of some tumors. Although this was previously thought to be due to viral lysis of infected tumor cells, it is now understood that there is an immune component to tumor destruction. We have previously shown that no difference in survival is seen in lymphocyte-depleted mice after viral or mock therapy of syngeneic intracranial melanomas. We have also demonstrated the presence of a wide spectrum of immune cells following viral therapy, including larger percentages of CD4+ T cells and macrophages. In this paper, the contribution of the immune system to tumor destruction has been further delineated. Viral therapy of intracranial melanoma induces a tumor-specific cytotoxic and proliferative T cell response. However, there is no increase following viral therapy in either serum tumor antibody levels or viral-neutralizing antibodies. Thus specific T cell responses appear to mediate viral-elicited prolongation in survival. These data suggest that designing new viruses capable of augmenting T cell responses may induce stronger tumor destruction upon viral therapy.
Keywords: melanoma, HSV, immune response
Introduction
At least 20–40% of cancer patients are expected to develop brain metastases [1,2]. Among these, melanoma has the highest frequency of brain metastases in adults [3], with asymptomatic cerebral metastases found in 50–75% of melanoma patients at autopsy [4]. Furthermore, with 41,600 cases diagnosed in 1998, melanoma has the fastest increasing cancer incidence rate in the United States [5]. Although many successful therapeutic agents have been developed for treatment of noncerebral metastases, few have been efficacious in the treatment of brain metastases, which often impact quality of life and long-term survival. These therapeutic agents are ineffective for treatment of cerebral metastases because they are unable to cross the blood–brain barrier. Therapy for brain metastases remains mainly palliative, with aggressive therapy, such as surgery and steroids, prolonging survival to 1 year [6]. Thus, there is great interest in alternative therapies, such as neuroattenuated herpes simplex virus 1 (HSV-1), for intracranial therapy of brain metastases.
Neuroattenuated HSV-1, with a deletion in the RL1 gene, is able to prolong survival of mice bearing various tumors [7–17]. The HSV-1 RL1 gene confers neurovirulence [18–20]. Deletion or mutation of the RL1 gene, which encodes the ICP34.5, or γ-34.5, protein, results in HSV variants that are incapable of replicating in the central nervous system (CNS) of mice and are also incapable of causing encephalitis [19,21]. Previous work with the S91 Cloudman M3 melanoma model in DBA/2 mice demonstrated that neuroattenuated HSV-1 prolongs survival of intracranial tumor-bearing mice following direct intra-neoplastic viral delivery [11,12]. However, the innate immunogenicity of this tumor cell line has limited studies into the role of the immune response in viral-mediated tumor cell killing. Although HSV-1 was thought to infect many different cell types derived from many animals, early work showed that several murine tumor lines, such as B16 and K-1735, are resistant to HSV-1 infection and killing [11]. Although several murine melanoma cell lines are resistant to HSV-1 infection, over 40 human melanoma primary cell lines and established human tissue culture lines were shown to be susceptible to infection [11].
Because of the paucity of available HSV-susceptible murine tumor cell lines, new murine melanoma cell lines were developed to study the role of the immune response in HSV-1-mediated tumor destruction. These cell lines are murine B78H-1 melanoma cells, a nonmelanotic sub-clone of the murine B16 melanoma cell line, transfected with the HSV-1 entry coreceptors, HVE A and C [13]. The B78H1-A10 (A10) and B78H1-C10 (C10) cells are susceptible to HSV infection, with growth rates comparable to those of other cell lines [13]. Thus the A10 and C10 cell lines allow for the investigation of host and viral factors that influence the outcome of HSV-based therapy. When HSV-1 1716 is administered to intracranial melanoma-bearing mice at the midpoint of survival, survival is prolonged twofold [11]. Furthermore, when virus is administered even earlier, there is a 60% long-term survival rate [11]. However, in immunodeficient mice bearing intracranial syngeneic murine melanomas, HSV-1 1716 is unable to prolong survival [12], demonstrating the contribution of the immune response to viral-mediated tumor destruction. An earlier onset of the immune response following viral therapy was also seen in immunocompetent syngeneic mice [12]. This response was characterized by an early influx of immune cells, mainly of CD4+ T cells, NK cells, and macrophages (although most types of immune cells are found in the tumor mass following viral therapy) [12]. CD8+ T cells were present sparingly in tumor masses. In our model, viral activation of the immune response within the CNS is the preliminary trigger for recruiting an anti-tumor response. Thus, nonspecific macrophages and natural killer cells enter the area and release cytokines, recruiting and activating lymphocytes. These activated lymphocytes are then exposed to tumor antigens from virally lysed cells and alert the immune system to the presence of the tumor. In this model, active viral replication and an active immune response are necessary for tumoricidal activity.
This paper explores more fully the activation and contribution of a tumor-specific immune response following neuroattenuated HSV-1 therapy of intracranial melanoma. The data demonstrate that while an integrated, complete immune response is necessary for viral-mediated prolongation in survival, this effect is mediated mainly by a tumor-specific cytotoxic and proliferative T cell response. Furthermore, significant levels of serum antibodies to tumor and virus antigens are not present and a neutralizing antibody response does not develop following intracranial viral therapy. These data predict that a more vigorous, specific T cell immune response to tumor antigens during neuroattenuated HSV-1 tumor therapy should result in an improved therapeutic outcome.
Results
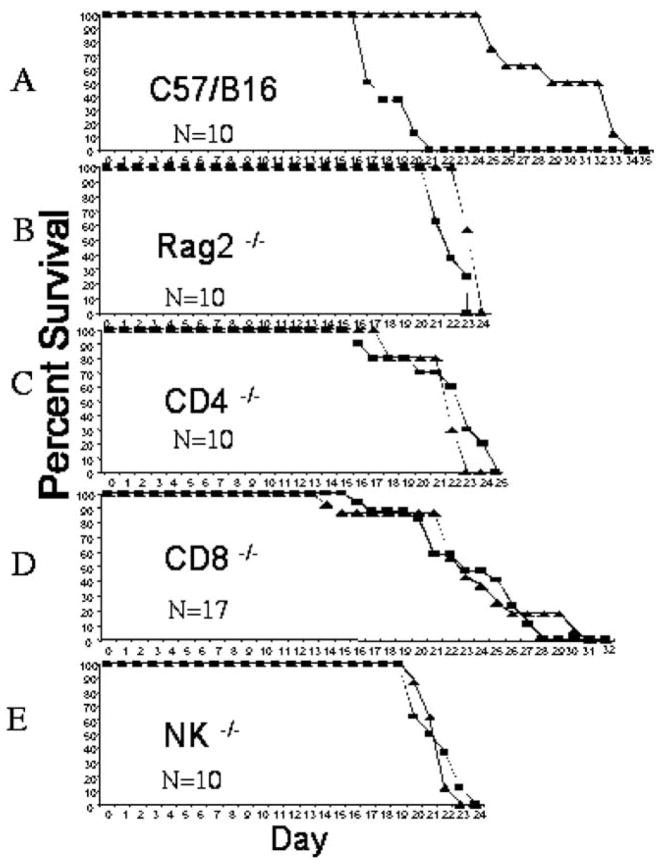
The Immune System Is Required for Viral Therapy
Although the contribution to the therapeutic effect of an active immune response (i.e., capability to respond to viral therapy of intracranial melanomas) has been demonstrated previously [12], problems of tumor immunogenicity exist with the models used in these experiments. Thus, we repeated the importance of the immune response using the B78H1-A10 and B78H1-C10 tumor cell lines [13] in syngeneic C57/Bl6 RAG2−/− knockout mice. We implanted both RAG2−/− and immunocompetent C57/Bl6 mice intracranially with tumors and followed 10 days later with either mock or HSV-1 1716 therapy. There was no increase in survival following viral therapy in immunodeficient, RAG2−/− knockout mice (Fig. 1B) compared to the significant prolongation in immunocompetent C57/Bl6 mice (Fig. 1A). This response is independent of tumor line, either A10 (Fig. 1) or C10 (data not shown). The mean average survival time following viral therapy is 28.6 ± 1.26 days for immunocompetent C57/Bl6 mice compared to 22.5 ± 0.2 days for Rag2−/− mice. To delineate further which immune cells contribute to viral-mediated prolongation in survival, we implanted CD4+ and CD8+ T cell-knockout as well as NK-deficient beige mice with tumors and followed 10 days later with viral therapy. As can be seen in Figs. 1C, 1D, and 1E, no prolongation in survival occurred following viral therapy in any of the immunodeficient strains. Mean survival for CD4−/− treated mice is 20 ± 0.5 days, for CD8−/− mice is 22.5 ± 1.25 days, and for NK mice is 20.5 ± 0.3 days. However, the range of days over which death occurred differs for the different immunodeficient strains; thus CD8−/− mice have a range of 15 days, whereas CD4−/− mice have a range of 10 days, and NK and Rag2−/− mice have a narrow range of 4 days. These data demonstrate that a complete and integrated immune response is necessary for viral-mediated prolongation of survival.
FIG. 1.
An immune response is necessary for viral-mediated prolongation in survival. Mice were treated with direct intratumoral injections of either mock viral culture medium (square) or HSV-1 1716 (5 × 104 pfu, triangle) 10 days following tumor implantation (see Materials and Methods). Significant prolongation in survival is seen in C57/Bl6 immunocompetent mice (A) following viral therapy, 17.4 ± 0.5 vs 28.6 ± 1.26, P > 0.001. No significant prolongation in survival is seen following viral therapy in Rag2−/− immunodeficient mice, 21.2 ± 0.4 vs 22.5 ± 0.2 (B), CD4−/− immunodeficient mice, 20.7 ± 1.13 vs 20 ± 0.5 (C), CD8−/− immunodeficient mice, 21.4 ± 1.2 vs 22.5 ± 1.25 (D), or NK−/− beige immunodeficient mice, 20.4 ± 0.5 vs 20.5 ± 0.3 (E). N = 10 for C57/Bl6, N = 10 for Rag2−/−, N = 10 for CD4−/−, N = 17 for CD8−/−, and N = 10 for NK−/−.
Cytotoxic T Cell Response
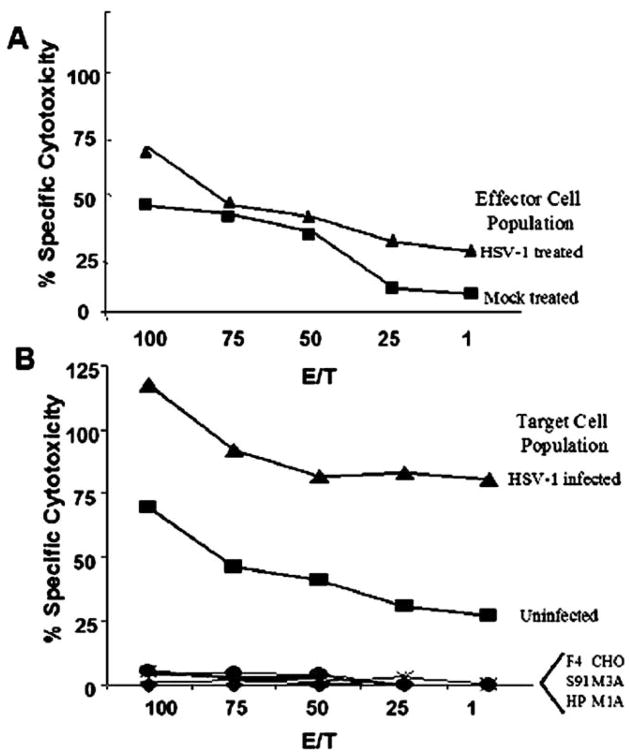
The above data along with previous work [12] have demonstrated that an active immune response is necessary for efficacy of viral therapy. To explore more fully how the immune components mediate prolongation in survival and whether tumor or viral antigens are being recognized, we performed a cytotoxic T lymphocyte (CTL) assay. This assay was performed even though CD8+ T cells are not present in high levels following viral therapy [12], as the above data demonstrate that CD8+ T cells are necessary for prolongation in survival. The assay was designed to determine if tumor-specific and/or viral-specific immune responses develop following viral therapy of intracranial tumors. We implanted immunocompetent mice intracranially with tumors and followed 10 days later with either mock or HSV-1 1716 therapy. We isolated splenocytes 20 days following therapy and determined specific cytotoxicity toward syngeneic tumor cells, nonsyngeneic tumor cells (irrelevant H2 tumor controls), and syngeneic non-tumor cells (H2-matched nontumor controls). The data demonstrate that a tumor-specific immune response develops following tumor implantation alone (Fig. 2A); however, this response is increased in animals receiving viral therapy (Fig. 2A). No specific cytotoxicity toward nonsyngeneic tumor cells (irrelevant H2-matched tumor control) or syngeneic nontumor cells (H2-matched non-tumor control) was observed (data not shown). Interestingly, when target cells are infected with virus prior to determining CTL-specific response, a viral-specific response develops in animals receiving viral therapy that is significantly higher than that seen for non-viral-infected target cells (Fig. 2B). However, no specific anti-tumor response is seen for nonsyngeneic infected tumor cells (irrelevant H2-matched tumor control) or syngeneic infected nontumor cells (H2-matched nontumor control) (Fig. 2B). Thus this response requires both viral and tumor antigens to be present and may be directed toward novel antigens expressed only upon viral infection of A10 and C10 tumor cells. This response is not directed toward the viral coreceptors expressed on the cell surface as no specific response is seen when the target cells are viral-infected Hve A- or Hve C-expressing CHO cells (data not shown). Also, the CTL response toward A10 and C10 target cells is similar whether the effector cells are isolated from either A10 or C10 tumor-bearing treated mice.
FIG. 2.
Viral therapy of intracranial melanoma increases both tumor- and viral-specific CTL activity. Effector lymphocytes were isolated from mock- and HSV-1 1716-treated tumor-bearing animals 20 days post-viral therapy and incubated for 3 days. Splenocytes were then examined for specific killing of target cells, either uninfected or infected tumor cells, using the Promega CytoTox96 nonradioactive cytotoxicity assay. (A) A tumor-specific CTL response develops following viral therapy. Splenocytes isolated from either mock-treated tumor-bearing mice (square) or HSV-1 1716-treated tumor-bearing mice (triangle) were examined for specific killing of target uninfected tumor cells. (B) A viral-specific CTL response develops following viral therapy. Splenocytes isolated from HSV-1 1716-treated tumor-bearing animals were examined for specific killing of target cells, either uninfected (square) or HSV-1 infected (triangle) tumor cells. No response is seen toward the control targets, including H2-matched cells (F4) (circle), H2-unmatched melanoma (S91 and HP) (diamond and plus sign), or Hve-expressing CHO cells (M1A, M3A, and CHO) (x, dash, and asterisk). Each line represents the average percentage of specific killing as a function of maximum LDH release at various E/T ratios for N = 5 mice per group.
Proliferative T Cell Response
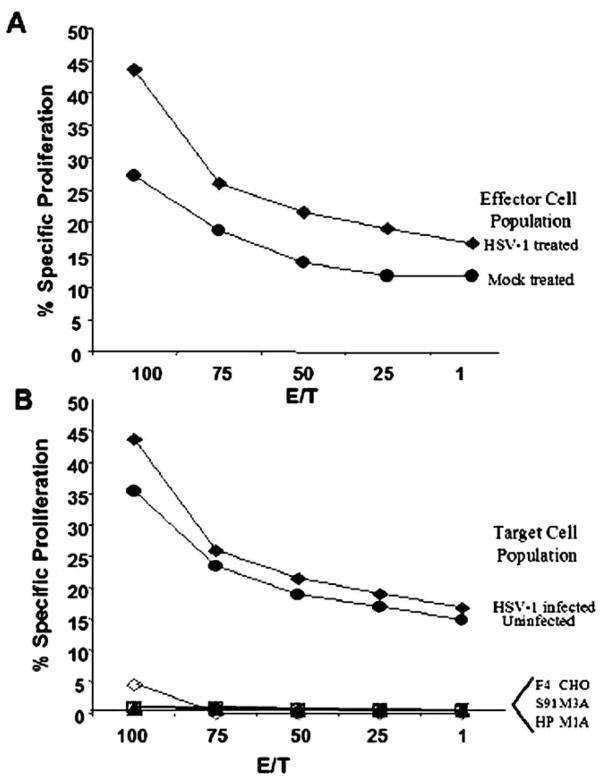
Because the above data, in combination with previous results demonstrating higher percentages of CD4+ T cells following viral therapy [12], suggest an active role of CD4+ helper T cells in viral-mediated tumor destruction, we performed a proliferative T cell assay. In this assay, we isolated splenocytes from either mock or tumor-bearing animals that had been either mock or virally treated. We incubated the cells together with tumor cells or UV-inactivated HSV-1-infected tumor cells, as well as nontumor syngeneic and nonsyngeneic tumor cells, to determine the response to both tumor and viral antigens. As can be seen in Fig. 3A, an increase in the proliferative T cell response occurs in HSV-1 1716-treated tumor-bearing animals compared to mock-treated tumor-bearing animals. No response was seen when cells were incubated with nonsyngeneic tumor cells (irrelevant H2-matched tumor control) or syngeneic nontumor cells (H2-matched nontumor cells) (data not shown). Additionally, unlike the CTL response, viral therapy did not induce a viral-specific proliferative response (Fig. 3B). Use of infected H2-matched or irrelevant H2-matched control cells as stimulation cells also did not induce cell proliferation. Thus, only a tumor-specific proliferative T cell response develops following HSV-1 1716 therapy of intracranial melanomas.
FIG. 3.
Viral therapy of intracranial melanoma increases tumor-specific proliferation activity but not viral-specific activity. Effector lymphocytes were isolated from mock- and HSV-1 1716-treated tumor-bearing animals 20 days post-viral therapy and incubated for 3 days with mock- or viral-infected tumor cells as targets and examined for specific proliferation with the Promega Cell Titer 96 aqueous nonradioactive proliferation assay. (A) A tumor-specific proliferation occurs following viral therapy. Splenocytes isolated from either mock- (circle) or HSV-1 1716-treated (diamond) tumor-bearing animals were examined for specific proliferation following incubation with target syngeneic tumor cells. (B) No viral-specific proliferation occurs following viral therapy. Splenocytes isolated from HSV-1 1716-treated tumor-bearing animals were examined for specific proliferation following incubation with target cells, either uninfected (closed circle) or HSV-1-infected (diamond) tumor cells. No response is seen following stimulation with the infected control cells, including H2-matched cells (F4) (open circle), H2-unmatched melanoma (S91 and HP) (open diamond and open square), and Hve-expressing CHO cells (M1A, M3A, and CHO) (x, triangle, and plus sign). Each line represents the average percentage of specific killing as a function of maximum formazan release at various E/T ratios for N = 5 mice per group.
Serum Antibody Levels
B cells are present sparingly in intracranial tumor masses [12], but antigen-presenting cells traveling from the tumor site to regional lymph nodes and the spleen may activate B cells distally; thus we assayed the presence of serum antibodies against tumor cells following mock and HSV-1 1716 therapy. We collected sera from animals 20 days following mock or viral therapy and used an ELISA to determine the presence of tumor-specific antibodies. Although an increase in tumor-specific antibody levels was detected, it was not significant over levels detected for nonspecific staining from sera collected from mock tumor-bearing animals (data not shown). Additionally, there was no increase in antibody levels toward tumor cells or HSV-1-infected cells from sera collected from virally treated animals. Thus, a significant humoral response could not be detected following viral therapy and does not seem to play a significant role in viral-mediated prolongation in survival.
Neutralizing Antibody Levels
As stated above, B cells are present sparingly in intracranial tumor masses [12]. Thus, the absence of tumor-specific antibodies is not unexpected. Subsequently, we determined the presence of serum virus-neutralizing antibodies, following mock and HSV-1 1716 therapy. We incubated sera collected for the above experiments with virus and determined the ability to neutralize virus. No neutralizing antibodies were detected from any tumor-bearing group (data not shown). Thus serum-neutralizing antibodies are not produced following viral therapy of intracranial tumors.
Discussion
Brain tumors, both primary and metastatic, require the development of innovative new therapeutic agents due to the increase in their frequency and the inability of current therapies to provide beneficial results. Different replication-competent and noncompetent viral therapies for tumors located within the central nervous system are currently being studied, including herpes simplex viruses, adenoviruses, poxviruses, retroviruses, and Newcastle disease virus [7,8,26–32]. Of these, both HSV-1 and adeno-viruses have mounting potentials as therapeutic agents. Replication-competent adenoviruses are currently being used for squamous cell carcinomas of the head and neck [33] and are being explored as therapeutic agents for many other types of tumors [34–39]. Neuroattenuated HSV-1 has the ability to replicate in dividing cells within the CNS and is unable to replicate or cause disease in nondividing cells [40,41]. Neuroattenuated HSV-1 is also unable to cause encephalitis [42] and is avirulent in SCID mice [43]. Thus, neuroattenuated HSV-1 is a leading candidate for intracranial tumor therapy and is currently being used in clinical trials [44,45]. Previous studies have demonstrated the proof of the principal that neuroattenuated HSV-1 is able to prolong survival of intracranial tumor-bearing animals [7,8,11,15,16,46–48]. The study described herein was undertaken to define more fully the role of specific immune components in viral therapy.
To determine the specific immune components involved in viral-mediated prolongation in survival, a new syngeneic tumor model was needed. The natural host for HSV-1 is humans; however, the majority of the tumor models studied are in rodents. HSV-1 does not naturally infect rats or mice and thus it is not surprising that some murine and rodent tumor cell lines are resistant to HSV-1 infection [11]. This resistance to infection is at the level of coreceptor expression in murine B16 melanoma cells [13]. Thus, we reported previously the development of two new murine melanoma cell lines, B78H1-A10 and B78H1-C10, that have been stably transfected with human HSV-1 coreceptors [13]. These cells are syngeneic in C57/Bl6 mice and develop tumors normally with a mean survival of 3 weeks. HSV-1 1716 replicates to levels similar to those of wild-type parental strains in these cell lines [13]. Viral therapy prolongs survival significantly and the tumors remain modestly immunogenic [13].
To determine further the specific components of the immune response important to viral therapy, different immunodeficient and knockout strains of mice were implanted with tumors, followed by either mock or viral therapy. Similar to earlier reports [13], in immunodeficient RAG2−/− mice the viral therapy was unable to prolong survival over that of mock-treated animals. Interestingly, in CD4−/− and CD8−/− mice viral therapy was also unable to prolong survival. Also, viral therapy was unable to prolong survival in NK-deficient beige mice. Thus an organized, balanced immune response is necessary to mediate the prolongation in survival seen following viral therapy. Interestingly, the survival of the CD8−/− was not confined to a narrow range as with other immunodeficient strains. CD8−/− mice succumbed to the tumor burden over a range of approximately 15 days, while RAG2−/−, CD4−/−, and NK-deficient mice succumbed over a range of 5–10 days. However, the median survival for all groups was 20–21 days. This difference in survival of CD8−/− mice may indicate that CD8+ T cells are important during later stages of viral-mediated tumor destruction and that CD4 T+ cells and NK cells are more important earlier. This correlates with previous immuno-histochemistry data demonstrating increases in CD4+ T cells and NK cells early following viral therapy [12].
We next determined the ability of viral therapy to induce either a tumor- or a viral-specific CTL or proliferative T cell response. Previous reports have demonstrated that neuroattenuated HSV-1 is able to induce a tumor-specific CTL response in a flank model of tumor therapy; however, the ability of neuroattenuated HSV-1 to induce a tumor-specific CTL or proliferative response after intracranial therapy has not been demonstrated. In this paper, we utilized the newly created, lowly immunogenic B78H1-A10 and B78H1-C10 tumors to demonstrate that viral therapy of intracranial tumors induces both a CTL and a proliferative T cell response toward tumor antigens. The CTL response induced following viral therapy is both tumor- and viral-specific; however, the proliferative response is toward tumor antigens and not viral antigens. Thus, although a tumor-specific proliferative T cell response is induced earlier in viral-mediated tumor destruction, only a viral-specific CTL response develops following viral therapy. Accordingly, following viral therapy the lysis of tumor cells recruits CD4+ T cells to the tumor mass, where the cells are triggered to respond to tumor antigens and not viral antigens. This may in part be due to the ability of the virus to block immune recognition of infected cells, through modulation of both MHC classes I and II [49–51]. CD4−/−, CD8−/−, and NK−/− mice are unable to mount an immune response to prolong survival after viral therapy. Taken together, these data point to a model in which CD4+ T cells are recruited to the tumor mass following viral therapy, as are NK cells. CD4+ T cells secrete cytokines to recruit and activate CD8+ T cells. CD8+ T cells, along with NK cells, lyse both infected and uninfected tumor cells, leading to tumor destruction and the development of a tumor-specific CTL response. The proliferation of T cells in response to tumor antigen alone combined with the specific CTL response may translate to better recognition of distant, untreated metastases, which may in turn lead to better destruction of the metastases.
As viral therapy of intracranial tumors depends on an active integrated immune response, viral therapies will depend on the immune status of the recipients. Results of phase I clinical trials of HSV-1 for malignant gliomas have been published, with viral therapy demonstrating potential therapeutic benefits in both trials [44,45]. No significant detrimental effects were observed from the viral therapy in either study. These studies have shown that neuroattenuated HSV-1 is a viable therapy for intracranial malignant gliomas and may be useful for other CNS neoplasms, both primary and metastatic. The demonstration of a significant immune component necessary for viral-mediated tumor destruction highlights the importance of determining the immune status of potential recipients of viral therapy and the importance of monitoring the immune status of patients entered into future clinical trials. The creation of new neuroattenuated HSV-1 therapeutic viruses designed to engage more fully the immune response in viral therapy may provide additional useful viral candidates for tumor therapy. New viruses that are able to activate more fully a complete and harmonized immune response may lead not only to better destruction of the local treated tumor, but also to immune-mediated destruction of distant metastases and to the destruction of migratory cells within the CNS.
Materials and Methods
Animals and viruses
Female C57/Bl6 and female B6.129S6-Rag2tm1 N12 mice (Rag2−/−) (4 – 6 weeks of age, weighing approximately 20 g) were obtained from Taconic (Germantown, NY). B6.129S2-Cd4tm1Mak (CD4−/−), B6.129S2-Cd8atm1Mak (CD8−/−), and C57Bl/6J-Lystbg-J/+ (beige NK−/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME).
To produce virus stocks, subconfluent monolayers of African green monkey kidney (Vero) cells were infected with HSV strain 1716 (stock titer 1 × 108 pfu/ml). HSV strain 1716 has a 759-bp deletion, which deletes part of the genes encoding ICP34.5, LAT, and orfP [22]. Virus was concentrated from the culture and titered by plaque assay as previously described [23]. All viral stocks were stored frozen in viral culture medium (DMEM containing penicillin and streptomycin) at −70°C and thawed rapidly just prior to use. Serum-free medium was used for control (mock) inoculation studies as negative controls.
Tumor cell lines
B78H1 cells [24] were obtained from Meenhard Herlyn (Wistar Institute, Philadelphia, PA). Generation and characterization of Hve-transfected cells are described elsewhere [13]. Briefly, B78H1 cells were transfected with the HveA- (pBec10), HveB- (pMW20), or HveC- (pBG38) containing vectors or empty vector (pcDNA3) (Invitrogen). Stable transfectants were selected after 14 days of antibiotic selection and then cloned for single-cell colonies by limiting dilution methods. FACS analysis was performed to select further for homogeneous receptor-expressing populations. Additional cell lines used include M-3 S91 Cloudman melanoma cells, Harding–Passey melanoma cells, F4 cells, and Hve-expressing Chinese hamster ovary cells, CHO-HveA-M1A, CHO-HveC-M3A, and parental CHO.
For preparation of tumor implantation, cells were grown using DMEM containing 0.05% penicillin, 0.05% streptomycin, and 10% calf serum. When originally obtained, cells were grown and then frozen in 95% calf serum/5% DMSO so that all experiments could be initiated with cells of a similar passage number. On the day of intracranial injection, cells in subconfluent monolayer culture were passaged with 0.25% trypsin solution in EDTA, washed 1× in cell culture medium, resuspended at the appropriate concentration in medium without serum, and held on ice.
Intracranial tumor production
Mice were anesthetized by interperitoneal injections of ketamine/xylazine (87 mg/kg ketamine and 13 mg/kg xylazine). The head was cleansed with 70% EtOH and betadine. A small midline incision was made in the skin of the head exposing the skull. Stereotactic injection of tumor cell suspension was performed using a small animal stereotactic apparatus (Kopf Instruments, Tujunga, CA). Injections were done with a Hamilton syringe through a 28-gauge needle. The needle was positioned at a point 2 mm caudal of the bregma and 1 mm left of midline. Using a separate 27-gauge needle the skull was breached at the appropriate coordinates. The injection needle was advanced through the hole in the skull to a depth of 2 mm from the skull surface and then extracted 0.5 mm to create a potential space. Cells (5 × 104) in a total volume of 10 μl were injected over 2 min. Following the injection, the needle was left in place for 2 min and then slowly withdrawn. The skin was sutured closed.
Viral inoculation
Mice were anesthetized by interperitoneal injections of ketamine/xylazine (87 mg/kg ketamine and 13 mg/kg xylazine) and the head was cleansed with 70% EtOH. Using a Hamilton syringe with a 28-gauge needle, the appropriate amount of virus was injected, in a volume of 10 μl, through a midline incision at the same stereotactic coordinates used for tumor cell injection. The injection was performed over 2 min, and following the injection the needle was left in place for 2 min and then slowly withdrawn. The amount of HSV-1 1716 used in all experiments (5 × 104 PFU/mouse) was the lowest PFU at which the longest survival was seen in previous experiments [11].
Cytotoxic T cell assay
Cytotoxic T cell activity was measured using the Promega CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Mad-ison, WI). Specifically, spleens were removed aseptically from either mock-or tumor-bearing animals, which had been either mock or virally treated, 20 days posttherapy. Spleens were teased into single-cell suspensions, red blood cells were lysed, and cells were resuspended in DMEM after viability was counted. Effector lymphocytes were incubated for 72 h prior to assay. CTL activity was determined per the manufacturer’s instructions. Target cells included syngeneic tumor cells, UV-infected tumor cells, nonsyngeneic tumor cells (irrelevant H2-matched tumor control—S91 melanoma and Harding–Passey melanoma cells), infected nonsyngeneic tumor cells, syngeneic nontumor cells (H2-matched controls—F4), and Hve receptor control cell lines (CHO-HveA-M1A, CHO-HveC-M3A, and CHO). Samples were processed in triplicate and averaged, and background lysis levels were subtracted. Data are representative of two separate experiments. Ratios of target-to-effector cell assays were 1:100 to 1:1.
Proliferative T cell assay
Proliferative T cell activity was measured using the Promega 96 aqueous nonradioactive cell proliferation assay kit (Promega). Specifically, spleens were removed aseptically from either mock- or tumor-bearing animals, which had been either mock or virally treated, 20 days posttherapy. Spleens were teased into single-cell suspensions, red blood cells were lysed, and cells were resuspended in DMEM after viability was counted. Effector lymphocytes were incubated with stimulating cells for 48 h prior to assay. Proliferative T cell activity was determined per the manufacturer’s instructions. Stimulating cells included syngeneic tumor cells, UV-infected tumor cells, nonsyngeneic tumor cells (irrelevant H2-matched tumor control—S91 melanoma and Harding–Passey melanoma cells), infected nonsyngeneic tumor cells, syngeneic nontumor cells (H2 matched controls—F4), and Hve receptor control cell lines (CHO-HveA-M1A, CHO-HveC-M3A, and CHO). Samples were processed in triplicate and averaged, and background proliferation levels were subtracted. Data are representative of two separate experiments. Ratios of proliferating lymphocytes were 1:100 to 1:1.
Serum antibody assay
The presence of serum antibody against tumor antigens was determined by ELISA. Briefly, sera were collected from either mock- or tumor-bearing animals, which had been either mock or virally treated, 20 days posttherapy. Plates of confluent tumor cells (A10 and C10) and Vero cells (negative control) were fixed and blocked with 0.1% BSA in PBS. Sera were serially diluted and added to plates. Plates were incubated for 2 h, washed with PBS, and blocked with 0.1% BSA in PBS, and then biotinylated goat anti-mouse antibody (1:10,000) was added. Plates were again incubated for 1 h and then an avidin–biotin detection system (ABC and VIP; Vector Laboratories, Burlingame, CA) was used to determine serum antibody levels. Samples were processed in duplicate and averaged, and background binding was subtracted. ELISA was performed twice.
Neutralizing antibody levels
Neutralizing antibody levels were determined using previously published methods [25]. Briefly, sera collected from the previous experiment were heat inactivated for 1 h at 65°C. Sera were serially diluted and mixed in equal volumes with HSV-1 1716 for 20 min at 4°C. Sera and virus were then added to semiconfluent Vero cells in a 48-well plate. Plates were incubated for up to 72 h and neutralizing antibody levels were determined by calculating the concentration needed to reduce plaques by 50%. Data are the averages of samples processed in triplicate with background neutralizing activity subtracted. Two separate experiments were performed.
Acknowledgments
The authors thank Daniel Ruge for technical assistance and Katherine Molner-Kimber, Ph.D., for discussion. The work presented in this paper was supported by a public health services award (R01 NS37516) from the National Institutes of Health.
References
- 1.Cairncross J, Kim J, Posner J. Radiation therapy for brain metastasis. Ann Neurol. 1980;7:529–541. doi: 10.1002/ana.410070606. [DOI] [PubMed] [Google Scholar]
- 2.Posner JB. Management of brain metastases. Rev Neurol. 1992;148:477–487. [PubMed] [Google Scholar]
- 3.Chidel M, Suh J, Barnett G. Brain metastases: presentation, evaluation, and management. Cleveland Clin J Med. 2000;67:120–127. doi: 10.3949/ccjm.67.2.120. [DOI] [PubMed] [Google Scholar]
- 4.Akslen LA, Howe LM, Hartveit F. Metastatic distribution in malignant melanoma. Invasion Metastasis. 1987;7:253–263. [PubMed] [Google Scholar]
- 5.Landis S, Murray T, Bolden S, Wingo P. Cancer statistics. CA Cancer J Clin. 1998;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Vicioso E, Suh JH, Kupelian PA, Sohn JW, Barnett GH. Analysis of prognostic factors for patients with single brain metastasis treated with stereotactic radiosurgery. Radiat Oncol Invest. 1997;5:31–37. doi: 10.1002/(SICI)1520-6823(1997)5:1<31::AID-ROI5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Andreansky S, et al. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 8.Boviatsis EJ, et al. Long-term survival of rats harboring brain neoplasms treated with a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751. [PubMed] [Google Scholar]
- 9.Coukos G, et al. Multi-attenuated herpes simplex virus-1 mutant G207 exerts cytotoxicity against epithelial ovarian cancer but not normal mesothelium and is suitable for intraperitoneal oncolytic therapy. Cancer Gene Ther. 2000;7:275–283. doi: 10.1038/sj.cgt.7700130. [DOI] [PubMed] [Google Scholar]
- 10.Pyles RB, Warnick RE, Chalk CL, Szanti BE, Parsek LM. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 11.Randazzo BP, et al. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995;211:94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- 12.Miller C, Fraser N. Role of the immune response during neuro-attenuated herpes simplex virus-mediated tumor destruction in a murine intracranial melanoma model. Cancer Res. 2000;60:5714–5722. [PubMed] [Google Scholar]
- 13.Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther. 2001;3:160–168. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- 14.Yoon SS, Carroll NM, Chiocca EA, Tanabe KK. Cancer gene therapy using a replication-competent herpes simplex virus type 1 vector. Ann Surg. 1998;228:366–374. doi: 10.1097/00000658-199809000-00009. [Published erratum appears in Ann. Surg., 1998, 228(5): following table of contents]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yazaki T, Manz HJ, Rabkin SD, Martuza RL. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 16.Toda M, Martuza RL, Kojima H, Rabkin SD. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- 17.Todo T, Martuza R, Rabkin S, Johnson P. Oncolytic herpes simplex virus vector with enhanced mHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann M, Longnecker R, Roizman B, Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986;150:207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 19.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus neurovirulence to gamma 1 34.5, a gene nonessential for growth in culture. Science. 1990;252:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 20.McGeoch DJ, Barnett BC. Neurovirulence factor. Nature. 1991;353:609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- 21.MacLean AR, Ul-Fareed M, Robertson L, Harland J, Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 22.McLean CA, Efstathiou S, Elliot ML, Jamieson FE, McGeoch DJ. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J Gen Virol. 1991;72:897–906. doi: 10.1099/0022-1317-72-4-897. [DOI] [PubMed] [Google Scholar]
- 23.Spivack JG, Fraser NW. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987;61:3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [Published erratum appears in J. Virol., 1988, 62: 663]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasamura Y, Sato GH. Establishment of four functional, clonal strains of animal cells in culture. Science. 1966;154:1186–1189. doi: 10.1126/science.154.3753.1186. [DOI] [PubMed] [Google Scholar]
- 25.Molner-Kimber K, et al. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9:2121–2133. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- 26.Jia WWG, McDermott M, Goldie J, Cynader M, Tan J, Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type I. J Natl Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- 27.Toda M, Rabkin SD, Martuza RL. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 28.Chen B, et al. Evaluation of combined vaccinia virus-mediated antitumor gene therapy with p53, IL-2, and IL-12 in a glioma model. Cancer Gene Ther. 2000;7:1437–1447. doi: 10.1038/sj.cgt.7700252. [DOI] [PubMed] [Google Scholar]
- 29.Krauzewicz N, Griffin B. Polyoma and papilloma virus vectors for cancer gene therapy. In: Habib, editor. Cancer Gene Therapy: Past Achievements and Future Challenges. Kluwer Academic/Plenum; New York: 2000. pp. 73–82. [DOI] [PubMed] [Google Scholar]
- 30.Pizzato M, et al. Production and characterization of a bicistronic Moloney-based retroviral vector expressing human interleukin 2 and herpes simplex virus thymidine kinase for gene therapy of cancer. Gene Ther. 1998;5:1003–1007. doi: 10.1038/sj.gt.3300670. [DOI] [PubMed] [Google Scholar]
- 31.Nanda D, Vogels R, Havenga M, Avezaat C, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001;61:8743–8750. [PubMed] [Google Scholar]
- 32.Zheng H, Palese P, Garcia-Sastre A. Antitumor properties of influenza virus vectors. Cancer Res. 2000;60:6972–6976. [PubMed] [Google Scholar]
- 33.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 34.Reid T, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62:6070–6079. [PubMed] [Google Scholar]
- 35.Habib NA, et al. Assessment of growth inhibition and morphological changes in in vitro and in vivo hepatocellular carcinoma models post treatment with dl1520 adenovirus. Cancer Gene Ther. 2002;9:414–420. doi: 10.1038/sj.cgt.7700455. [DOI] [PubMed] [Google Scholar]
- 36.Johnson L, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 37.Portella G, Scala S, Vitagliano D, Vecchio G, Fusco A. ONYX-015, an E1B gene-defective adenovirus, induces cell death in human anaplastic thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2002;87:2525–2531. doi: 10.1210/jcem.87.6.8529. [DOI] [PubMed] [Google Scholar]
- 38.Petit T, et al. Efficient induction of apoptosis by ONYX-015 adenovirus in human colon cancer cell lines regardless of p53 status. Anti Cancer Drugs. 2002;13:47–50. [PubMed] [Google Scholar]
- 39.Geoerger B, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xeno-grafts. Cancer Res. 2002;62:764–772. [PubMed] [Google Scholar]
- 40.Brown SM, Harland J, MacLean AR, Podlech J, Clements JB. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J Gen Virol. 1994;75:2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- 41.Chou J, Roizman B. Herpes simplex virus 1 γ34.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, induced reactivation of herpes simplex virus γ134.5 deletion mutants in rodent models. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valyi-Nagy T, et al. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J Gen Virol. 1994;75:2059–2063. doi: 10.1099/0022-1317-75-8-2059. [DOI] [PubMed] [Google Scholar]
- 44.Markert J, et al. Conditionally replicating herpes simplex virus mutant G207 for the treatment of malignant glioma: results of a phase 1 trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 45.Rampling R, et al. Toxicity evaluation of replication competent herpes simplex virus (IC34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 46.Todo T, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 47.Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR, Molnar-Kimber KL. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Hum Gene Ther. 1999;10:3013–3029. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- 48.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 49.Goldsmith K, Chen W, Johnson DC, Hendricks RL. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill A, et al. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 51.Fruh K, et al. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]