Abstract
Neuropeptide Y (NPY) functions as a peptide neurotransmitter and as a neuroendocrine hormone. The active NPY peptide is generated in secretory vesicles by proteolytic processing of proNPY. Novel findings from this study show that cathepsin L participates as a key proteolytic enzyme for NPY production in secretory vesicles. Notably, NPY in cathepsin L knockout (KO) mice were substantially reduced in brain and adrenal medulla by 80% and 90%, respectively. Participation of cathepsin L in producing NPY predicts their colocalization in secretory vesicles, a primary site of NPY production. Indeed, cathepsin L was colocalized with NPY in brain cortical neurons and in chromaffin cells of adrenal medulla, demonstrated by immunofluorescence confocal microscopy. Immunoelectron microscopy confirmed the localization of cathepsin L with NPY in regulated secretory vesicles of chromaffin cells. Functional studies showed that coexpression of proNPY with cathepsin L in neuroendocrine PC12 cells resulted in increased production of NPY. Furthermore, in vitro processing indicated cathepsin L processing of proNPY at paired basic residues. These findings demonstrate a role for cathepsin L in the production of NPY from its proNPY precursor. These studies demonstrate the novel biological role of cathepsin L in the production of NPY, a peptide neurotransmitter and neuroendocrine hormone.
Introduction
Neuropeptide Y (NPY) functions as a peptide neurotransmitter in the nervous system to mediate cell-cell communication for neurotransmission in the regulation of feeding behavior (Wieland et al. 2000; Gehlert 1999). In the periphery, NPY of the sympathetic nervous system participates in the control of blood pressure (Walker et al. 1991; Kennedy et al. 1997). With the important physiological actions of NPY, it is important to understand the proteolytic mechanisms that are utilized for NPY biosynthesis.
The role of cysteine protease activity for NPY production has been demonstrated by the ‘prohormone thiol protease’ (PTP), which shows high activity for cleaving proNPY (Hook et al. 1996). ProNPY consists of the NPY domain and the COOH-terminal (CT) peptide domain; these two domains are liberated by proteolytic processing at a dibasic processing site (Lys-Arg) located between the NPY and the CT-peptide (Higuchi et al. 1988). The cysteine protease activity of PTP has been shown to process proNPY, however, the identity of the cysteine protease responsible for NPY production has not yet been defined. While the PC1/3 and PC2 prohormone convertases have been suggested to participate in NPY production in in vitro cellular expression studies (Brakch et al. 1997; Paquet et al. 1996), in vivo studies in PC2 knockout mice show little change in NPY (Miller et al. 2003b). The status of NPY in PC1/3 knockout mice has not been reported. Clearly, proteases responsible for NPY production have not yet been fully defined. It is possible that the cysteine protease activity of PTP represents a key proNPY processing enzyme. Therefore, the goal of this study was to identify the cysteine protease that produces NPY.
Cathepsin L has been identified as the cysteine protease component of purified PTP (Yasothornsrikul et al. 2003). Therefore, this study sought to assess the role of cathepsin L for NPY production. Results showed that cathepsin L gene knockout mice possess major reductions in NPY levels in brain and adrenal gland, compared to wild-type controls. Cellular localization studies demonstrate the colocalization of cathepsin L with NPY at the primary site of proneuropeptide processing in secretory vesicles of brain cortical neurons and in adrenal medullary chromaffin cells. Coexpression of cathepsin L with proNPY in PC12 cells, neuroendocrine cells from adrenal medulla, resulted in increased production of NPY. Moreover, in vitro proNPY processing assays demonstrated that cathepsin L cleaves proNPY at its dibasic processing site. These findings demonstrate a novel biological role of cathepsin L for production of NPY in neuroendocrine tissues.
Experimental Procedure
NPY in cathepsin L knockout mice
Cathepsin L deficient mice were generated by gene targeting in mouse embryonic stem cells as described previously (Reinheckel et al. 2001). Genotyping established wild-type (+/+) and cathepsin L gene knockout (−/−) mice. Cathepsin L and age-matched wild-type control mice were generated in the C57BL/6J mouse strain. The absence of cathepsin L in the knockout mice has been confirmed by anti-cathepsin L western blots (Roth et al. 2000). Brain tissues from adult mice (approximately 3 months of age) were collected and homogenized in 1 N acetic acid, heated at 95% for 10 min., centrifuged (15,000 × g for 15 min.), and the supernatant was analyzed for NPY by radioimmunoassays (RIA) as described previously (Miller et al. 2003). The NPY RIA has been characterized and it does not detect recombinant proNPY. Furthermore, the NPY RIA detects NPY and NPY-Gly with similar levels of detection (Hook et al., unpublished observations). Protein concentration in tissue extracts were measured (DC protein assay kit, Biorad, CA). Tissue content of NPY per unit amount of protein was calculated.
Primary cultures of brain cortical neurons and adrenal medullary chromaffin cells
Colocalization studies of cathepsin L and NPY were conducted in primary cultures of brain cortical neurons and in neuroendocrine adrenal medullary chromaffin cells. Primary cultures of cortical neurons were prepared from CD1 swiss mice at gestation day E18 (Charles River Laboratories). Neurons from embryonic brain cortex were dissociated with trypsin (0.5% trypsin in HBSS, Hank’s balanced salt solution), collected by centrifugation, plated on poly-D-L-ornithine/laminin coated german glass coverslips (Bellco Glas, NJ), and cultured in neurobasal medium with glutamine (2 mM) and supplement B27 (Invitrogen, CA) in a humidified chamber at 37°C with 95% air and 5% CO2. Primary cultures of adrenomedullary chromaffin cells were prepared from fresh bovine adrenal medulla as described previously (Yasothornsrikul et al. 2003; O’Connor et al. 2007).
Cellular colocalization of cathepsin L with NPY assessed by confocal immunofluorescence microscopy
Brain cortical neurons were fixed in 4% paraformaldehyde (PFA), permeabilized with 0.1% Triton X-100. Adrenal medullary chromaffin cells were fixed as described previously (Yasothornsrikul et al. 2003). Cortical neurons and chromaffin cells were incubated with primary antibodies anti-NPY-sheep (1:100, Chemicon, CA) or anti-cathepsin L-rabbit (1:100, Athens Research & Technologies, GA) in PBS containing 3% bovine serum albumin (PBS-BSA 3%) for 2 hours at room temperature. The anti-cathepsin L serum detects procathepsin L (bovine and human) and active cathepsin L (unpublished observations). After washing with PBS, cells were then incubated with secondary goat anti-sheep Alexa Fluor 594 and/or goat anti-rabbit Alexa Fluor 488 (1:200, red and green fluorescence labels, respesctively, Molecular Probes, OR), respectively, in PBS-BSA 3%. Colocalization of cathepsin L and NPY was observed by merged images, utilizing the Olympus IX70 microscope or Nikon Eclipse 800 microscope coupled to a BCM confocal system. Images were analyzed with Delta Vision Spectris Image Deconvolution System and SIMPLE PCI software. Nuclei were observed by DAPI staining for cellular analyses in selected experiments.
Immunoelectron microscopy of cathepsin L with NPY in secretory vesicles
Secretory vesicles were isolated from adrenal medulla by differential sucrose density centrifugation (Yasothornsrikul et al. 1998), and were fixed in 0.2% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Samples were osmicated in 2% osmium tetroxide in 0.1 M cacodylate and embedded in Epon 812. Ultrathin sections were partially deosmicated through 1% periodic acid/9% sodium periodate, and incubated in 3% normal goat serum in 1X TBS. Cathepsin L was visualized by anti-cathepsin L (rabbit) detected by 15 nm gold conjugated to anti-rabbit IgGs. NPY is visualized by anti-NPY (mouse) detected by 6 nM gold conjugated to anti-mouse IgGs. Controls without primary antibodies resulted in the absence of immunostaining. Sections were examined in a Tecnai-12 transmission electron microscope using a CCD camera and Digital Micrograph Software (Gatan Inc., CA).
Coexpression of proNPY and cathepsin L cDNAs in PC12 neuroendocrine cells
The rat adrenomedullary PC12 neuroendocrine cell line (obtained from Dr. Daniel O’Connor, Univ. of Calif., San Diego) was grown at 37°C with 6% CO2 in DMEM high glucose medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum, 10% horse serum, 100 U/ml of penicillin and 100 μg streptomycin, as described previously (Hwang et al. 2007). ProNPY cDNA/pcDNA3.1 (2 μg proNPY/pcDNA 3.1) and preprocathepsin L/pcDNA3.1 (2 μg) were transfected into PC12 cells, plated the previous day at 1.5 × 105 cells/well (70% confluency) in 6 well plates, with the Fugene transfection reagent using the protocol recommended by the manufacturer (Roche, Indianapolis, IN). Three days after transfection, cells were lysed in 0.1 N acetic acid for preparation of acid extracts, prepared as described previously (Miller et al. 2003). Acid extracts were analyzed for NPY levels by RIA (radioimmunoassay), expressed as the amount of NPY per unit amount of protein (measured by Biorad protein kit).
In vitro processing of recombinant proNPY by cathepsin L
Recombinant rat proNPY was expressed in E. coli and purifed by gel filtration chromatography, as described previously (Hook et al. 1996). ProNPY was incubated with cathepsin L (Athens Research and Technology, GA) at 37° C under assay conditions that have been reported previously (Yasothornsrikul et al. 1999). After incubation, proNPY-derived cleavage products were analyzed by MALDI-TOF mass spectrometry to assess cathepsin L processing products of proNPY.
Results
Reduction of NPY in cathepsin L knockout mouse brain and adrenal tissues
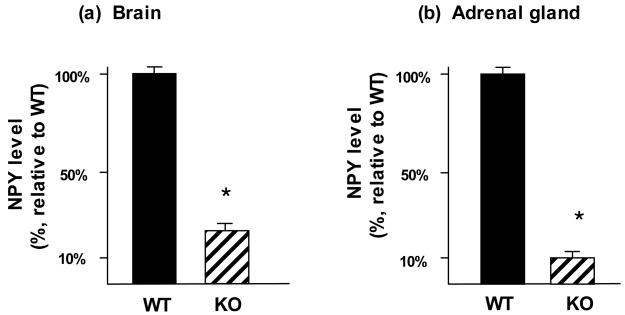
NPY levels were evaluated in brains and adrenal tissues of cathepsin L knockout mice. NPY levels in brain cortex were substantially reduced by 80% in cathepsin L knockout mice compared to wild-type controls (fig. 1a). Furthermore, in the peripheral adrenal gland tissue, NPY levels were reduced by 90% in cathepsin L knockout mice compared to control mice (fig. 1b). These results clearly demonstrate a functional role for cathepsin L in the production of NPY in neuronal and neuroendocrine tissues of brain and adrenal tissue regions, respectively.
Figure 1. Reduction of NPY levels in brain and adrenals of cathepsin L knockout mice.
NPY levels were measured in brain cortex (panel a) and adrenal (panel b) tissues from cathepsin L knockout mice and from wild-type control mice. Tissues were dissected and prepared as acid extracts for RIA quantitation of NPY by RIA (radioimmunoassay). NPY tissue levels were expressed as the mean ± s.e.m. are shown (n = 8–10 animals per group). Comparisons of cathepsin L knockout (KO) with wild-type (WT) mice showed significant reduction of NPY levels in the knockout mice compared to WT controls (*p < 0.0001, by student’s t-test).
Control experiments found that PC1/3 and PC2 were not altered in cathepsin L knockout mice (brain tissue). Therefore, the observed reduction of NPY in the cathepsin L knockout mice likely reflects the absence of cathepsin L.
Colocalization of cathepsin L and NPY in secretory vesicles of brain neurons and adrenal chromaffin cells
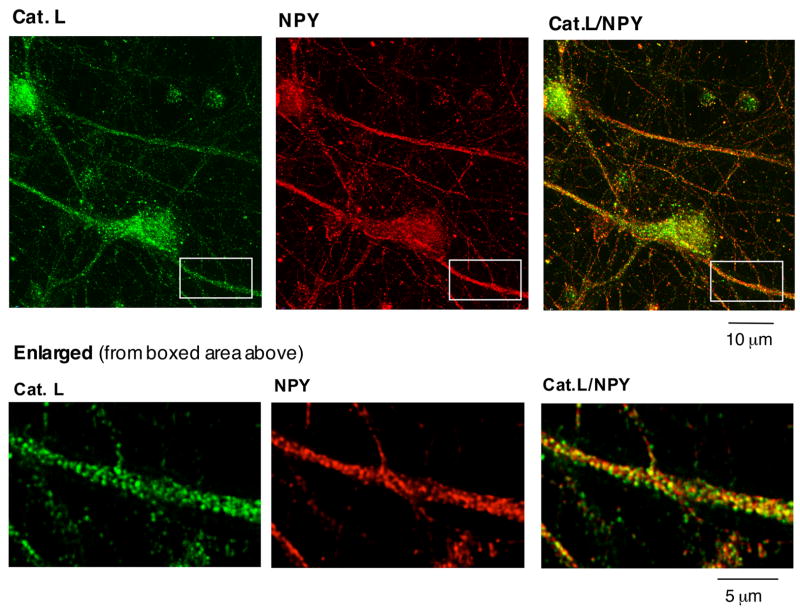
NPY production occurs in secretory vesicles where the precursor proNPY undergoes proteolytic processing to generate the smaller, biologically active NPY. Therefore, it was predicted that cathepsin L may be colocalized with NPY in secretory vesicles, for cathepsin L to be involved in production of NPY from proNPY. Colocalization of cathepsin L with NPY was examined in brain cortical neurons in primary culture by immunofluorescence confocal microscopy. Fluorescence imaging demonstrated a high degree of cathepsin L localization with NPY in secretory vesicles (fig. 2). The punctate pattern of cathepsin L and NPY localization in neurons was consistent with the known localization of NPY in secretory vesicles. Secretory vesicles containing NPY and cathepsin L were also present in neuritic extensions that represent neuronal axons (fig. 2, see enlarged view).
Figure 2. Brain cortical neurons: colocalization of cathepsin L with NPY in secretory vesicles illustrated by immunofluorescence confocal microscopy.
The colocalization of cathepsin L with NPY in brain cortical neurons in primary culture was demonstrated by immunofluorescence confocal microscopy. Cathepsin L immunoreactivity (green fluorescence) showed excellent overlapping colocalization with NPY (red fluorescence), shown by the yellow fluorescence of merged cathepsin L/NPY fluorescent immunostaining. The enlarged boxed area shows in more detail the punctate pattern of cathepsin L localization witih NPY in axonal extensions of neurons.
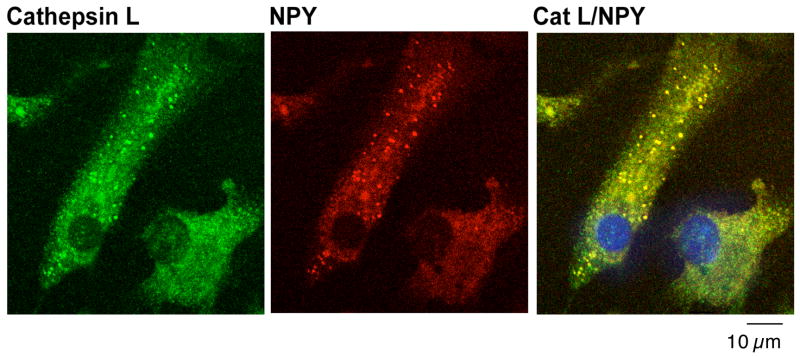
Further colocalization studies in chromaffin cells from adrenal medulla (in primary culture) showed excellent colocalization of cathepsin L with NPY in secretory vesicles (fig. 3). The distinct punctate pattern of NPY and cathepsin L colocalization represents the secretory vesicle localization of NPY. Examination by high resolution immunoelectron microscopy provided direct observation of the colocalization of cathepsin L with NPY in isolated secretory vesicles (fig. 4). Immunogold labeling demonstrated cathepsin L (shown by the 15 nm gold particles) present in chromaffin secretory vesicles that contain NPY (6 nm gold particles). These electron microscopy results confirm the colocalization of cathepsin L and NPY in secretory vesicles.
Figure 3. Adrenal chromaffin cells: Colocalization of cathepsin L with NPY in secretory vesicles shown by immunofluorescence confocal microscopy.
Adrenal medullary chromaffin cells in primary culture were analyzed for colocalization of cathepsin L with NPY present in secretory vesicles assess by immunofluorescence confocal microscopy. Excellent colocalization of cathepsin L (green fluorescence) and NPY (red fluorescence) was observed in the merged image (yellow fluorescence), with the majority of cellular cathepsin L localized with NPY in secretory vesicles.
Figure 4. Immunoelectron microscopy demonstrates cathepsin L in NPY-containing secretory vesicles.
Secretory vesicles were isolated from chromaffin cells of bovine adrenal medulla and prepared for immunoelectron microscopy. NPY localization within these vesicles was visualized by 6 nm gold particles, and cathepsin L is visualized by 15 nm gold particles. NPY and cathepsin L were found to be colocalized within identical secretory vesicles.
Coexpression of cathepsin L and proNPY cDNAs in neuroendocrine PC12 cells results in production of NPY
The ability of cathepsin L to generate NPY was assessed by coexpression of cathepsin L and proNPY cDNAs in PC12 neuroendocrine cells. Expression of cathepsin L transfected in PC12 cells has been confirmed by western blots (Hwang et al. 2007). Cathepsin L coexpression with proNPY resulted in elevated production of cellular NPY by approximately 3.5-fold, compared to expression of proNPY alone (fig. 5). Since the radioimmunoassay (RIA) for NPY does not crossreact with proNPY, the results indicate production of NPY by cathepsin L.
Figure 5. Production of NPY by coexpression of cathepsin L with proNPY in neuroendocrine PC12 cells.
Elevation of cellular NPY in PC12 cells was observed after coexpression of cathepsin L (CL) and proNPY. The radioimmunoassay (RIA) for NPY measures processed NPY (as well as NPY-Gly) since the RIA does not crossreact with proNPY. Controls included cells transfected with vector alone (no insert, control), proNPY alone, and cathepsin L (Cat. L) alone. Experiments were conducted with triplicate cell samples for each group, with RIA assay of NPY conducted in replicate assays. NPY levels in cells (pg NPY/μg protein) are are expressed as x ± s.e.m. (mean ± standard error of the mean), with significance indicated by *p < 0.005 (student’s t-test).
Processing of proNPY by cathepsin L, in vitro
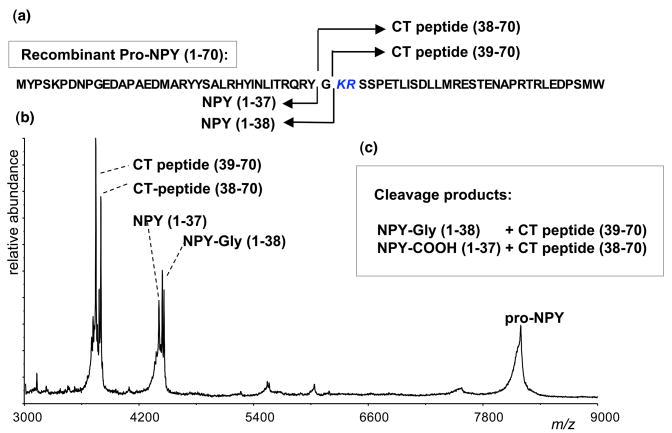
In vitro processing of proNPY by cathepsin L was assessed with recombinant proNPY, and proNPY cleavage products were assessed by MALDI-TOF mass spectrometry (fig. 6). Results indicated that proNPY was cleaved by cathepsin L at the Lys-Arg dibasic processing site, located at the junction of NPY and the COOH-terminal peptide within proNPY. Cathepsin L cleaved proNPY (residues 1–70) to generate NPY-Gly(1–38) and the COOH-terminal (CT) peptide (39–70), indicating cleavage at the NH2-terminal side of the ↓Lys-Arg (KR) processing site. NPY-Gly represents the substrate for α-amidation by peptidylglycine alpha-hydroxylating monooxygenase (Eipper et al. 1992), to generate NPY-amide. In addition, cathepsin L cleaved proNPY to generate NPY(1–37) and the CT-peptide(38–70); this finding suggests that NPY with a free COOH-terminus can be generated by cathepsin L. The in vitro proNPY processing data are consistent with the cathepsin L gene knockout and cathepsin L expression results of this study demonstrating a role for cathepsin L in the production of NPY.
Figure 6. In vitro processing of proNPY by cathepsin L.
Recombinant, purified proNPY was incubated with cathepsin L, and cleavage products were identified by MALDI-TOF mass spectrometry. The determined molecular masses indicated cleavage of proNPY at the dibasic Lys-Arg processing site of proNPY (panel a). Cathepsin L cleaves at the NH2-terminal side of the ↓Lys-Arg (KR) dibasic processing site to generate NPY-Gly(1–38) (4460 daltons) and the COOH-terminal (CT) peptide(39–70) (3747 daltons); NPY-Gly is the appropriate substrate for COOH-terminal amidation to produce NPY-amide (panel b). In addition, cathepsin L cleaved proNPY to generate NPY(1–37) (4403 daltons) and the CT peptide(38–70) (3804 daltons), indicating that NPY-COOH can be generated (panel b). These results demonstrate cathepsin L processing of proNPY.
Discussion
This study provides evidence that the cysteine protease cathepsin L plays a key role in the production of NPY in brain and adrenal medulla, demonstrated by protease gene knockout and expression experiments in this study. In cathepsin L gene knockout mice, NPY levels in brain were substantially reduced by 80%, and significantly reduced by approximately 90% in adrenal medulla. Cellular localization studies showed that cathepsin L possesses a high degree of colocalization with secretory vesicle NPY in brain cortical neurons and neuroendocrine chromaffin cells, indicating that cathepsin L is localized within the subcelllular organelle where proNPY processing occurs. Importantly, expression of the cathepsin L cDNA in PC12 cells resulted in the production of NPY from proNPY. Furthermore, cathepsin L cleaved recombinant proNPY in vitro at its paired basic processing site to yield NPY peptides. These findings indicate the key role of secretory vesicle cathepsin L for production of NPY from proNPY.
Notably, cathepsin L knockout mice displayed substantial reduction of NPY, but little change in NPY was observed in PC2 knockout mice (Miller et al. 2003b). In fact, PC2 knockout mice showed no change in NPY levels in brain and neuroendocrine tissues (Miller et al. 2003b). The field has not yet reported studies of NPY in PC1/3 knockout mice. Furthermore, in the cathepsin L knockout mice, no change in PC1/3 or PC2 levels were detected in brain (unpublished observations). It is noted that the same mouse strain (C57BL/6J) was used to develop the cathepsin L knockout (Roth et al. 2000), as well as the PC1/3 and PC2 knockout mice (Furuta et al. 1997; Zhu et al. 2002); therefore, the differences observed in these protease gene knockout mice are comparable in the same strain. It may be possible that an alternative means for the effects of cathepsin L on NPY production may involve cathepsin L mediated activation of another protease that then cleaves proNPY. However, the data from the cathepsin L knockout and expression studies of this study support the hypothesis for a role of cathepsin L for the production of NPY that functions as a brain peptide neurotransmitter and as a peptide hormone.
In addition to NPY, the production of other neuropeptides, enkephalin and POMC-derived peptides, has been shown to utilize cathepsin L as a proneuropeptide processing enzyme in several recent reports (Yasothornsrikul et al. 2003; Funkelstein et al. 2008). More specifically, the role of cathepsin L in the production of peptide neurotransmitters has been demonstrated for the biosynthesis of the active enkephalin opioid peptide neurotransmitter (Yasothornsrikul et al. 2003; Hwang et al. 2007). Enkephalin in brains of cathepsin L gene knockout mice is reduced by 50% compared to wild-type controls. Furthermore, coexpression of cathepsin L with proenkephalin results in conversion of proenkephalin to enkephalin. In addition, PC2 knockout mice show a 50% reduction in brain enkephalin (Miller et al. 2003). These results indicate roles for both cathepsin L and PC2 in enkephalin neurotransmitter production. However, no changes in enkephalin have been observed in brains of PC1/3 knockout mice (Hook et al., unpublished observations). These findings indicate that the dual cathepsin L and PC protease pathways participate in the proteolytic processing of proenkephalin.
Cathepsin L has recently been found to participate in the proteolytic processing of POMC into the peptide hormones ACTH, α-MSH, and β-endorphin (Funkelstein et al. 2008). These peptide hormones are substantially reduced in pituitaries of cathepsin L knockout mice. Moreover, cathepsin L is colocalized with POMC-derived peptide hormones present in pituitary secretory vesicles, the primary site of prohormone processing. While PC2 knockout mice show changes in POMC-derived peptide hormones (Miller et al. 2003a; Laurent et al. 2004; Allen et al. 2001; Pan et al. 2006), PC1 knockout mice show little change in these peptide hormones (Pan et al. 2005). These findings demonstrate a biological role for cathepsin L, together with PC2, in secretory vesicles for production of POMC-derived peptide hormones.
Cathepsin L possesses specificity for processing dibasic residue processing sites of prohormones and proneuropeptides. Cleavage site studies with peptide-MCA substrates containing dibasic residues indicated that cathepsin L prefers to cleave at the N-terminal side of dibasic residues, as well as between the dibasic residues (Azaryan and Hook 1994). In contrast, similar evaluation with dipeptide-MCA substrates showed that PC1/3 and PC2 prefer to cleave at the C-terminal side of dibasic residue processing sites (Azaryan et al 1995). These data indicate that following cathepsin L cleavage, removal of remaining basic residue extensions at the N-termini of peptide products can be accomplished by Arg/Lys aminopeptidase activity. Arg/Lys aminopeptidase activity is present in pituitary secretory vesicles (Gainer et al. 1984), and this activity has been identified as aminopeptidase B in neuropeptide-containing secretory vesicles of neuroendocrine chromaffin cells (Hwang et al. 2007). However, processing by PC2 or PC1/3 results in peptide intermediates with basic residue extensions at their C-termini, which are removed by the neuroendocrine-specific carboxypeptidase E enzyme (Fricker 1988). Thus, based on differences in cleavage specificity, different exopeptidase steps follow processing by cathepsin L or the PC enzymes for production of peptide neurotransmitters and hormones.
Active cathepsin L resides within secretory vesicles of chromaffin cells, demonstrated by its labeling with the activity-based proteomic probe for cysteine proteases, DCG04 (Yasothornsrikul et al., 2003). Furthermore, isolation of the DCG04-labeled enzyme and peptide sequencing by mass spectrometry revealed the identity of the active cysteine protease as cathepsin L. The presence of soluble cathepsin L with neuropeptides in regulated secretory vesicles of chromaffin cells suggested that cathepsin L would be cosecreted with such neuropeptides. Indeed, active cathepsin L (27 kDa) undergoes cosecretion with the neuropeptide (Met)enkephalin during stimulation of the regulated secretory pathway of chromaffin cells (Yasothornsrikul et al., 2003). Furthermore, expression of cathepsin L in PC12 cells results in its expression and localization to secretory vesicles (Hwang et al., 2007). These findings demonstrate that active cathepsin L is present in secretory vesicles, consistent with the hypothesis for cathepsin L-mediated production of neuropeptides from their proneuropeptide precursors.
The secretory vesicle function of cathepsin L for neuropeptide production is distinct compared to its well known role in lysosomes for protein degradation (Collette et al. 2004; Ishidoh and Kominami 2002). The findings from this study demonstrate that cellular cathepsin L functions in the production of biologically active peptides in secretory vesicles, rather than solely as a degradative protease for elimination of proteins and peptides in lysosomes. It is apparent from cellular localization studies that in neuroendocrine cells, a large portion of cathepsin L is present in secretory vesicles for neuropeptide biosynthesis. Examination of chromaffin cells for comparative localization of cathepsin L and lamp-1, a marker for lysosomes, showed that cathepsin L was partially colocalized with lamp-1 (unpublished observations). It is noted that visualization of the smaller lysosome organelles in chromaffin cells required higher magnification than that utilized to observe large dense core secretory vesicle localization of NPY with cathepsin L (fig. 3). Furthermore, in pituitary, cathepsin L also shows a high degree of colocalization with secretory vesicles compared to lysosomes (Funkelstein et al., 2008). These data support the proposal that in neuroendocrine cells, cathepsin L resides in secretory vesicles for extralysosomal functions for neuropeptide production.
In summary, this study demonstrates that cathepsin L in secretory vesicles functions as a key processing protease for the production of NPY in brain neurons and adrenal medullary chromaffin cells. These findings indicate the unique function of cathepsin L in secretory vesicles for production of active peptide hormones. Results from this study demonstrate cathepsin L as a novel cysteine protease pathway for production of NPY in secretory vesicles.
Acknowledgments
Support from the National Institutes of Health (to VH) is appreciated. The authors thank Brendan Brinkman (Facility Manager) and Dr. Joseph Gleeson (Director) of the UCSD Neuroscience Microscopy Shared Facility for confocal microscopy resources (supported by a grant from NIH). The technical assistance of Charles Mosier is appreciated.
References
- Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, Lundblad JR, Washburn CL, Pintar JE. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. J Neurosci. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaryan AV, Hook VYH. Unique cleavage specificity of ‘prohormone thiol protease’ related to proenkephalin processing. FEBS Lett. 1994;341:197–202. doi: 10.1016/0014-5793(94)80456-7. [DOI] [PubMed] [Google Scholar]
- Azaryan AV, Krieger TJ, Hook VYH. Purification and characteristics of the candidate prohormone processing proteases PC2 and PC1/3 from bovine adrenal medulla chromaffin granules. J Biol Chem. 1995;270:8201–8208. doi: 10.1074/jbc.270.14.8201. [DOI] [PubMed] [Google Scholar]
- Brakch N, Rist B, Beck-Sickinger AG, Goenaga J, Wittek R, Burger E, Brunner HR, Grouzmann E. Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations. Biochemistry. 1997;36:16309–16320. doi: 10.1021/bi9714767. [DOI] [PubMed] [Google Scholar]
- Collette J, Bocock JP, Ahn K, Chapman RL, Godbold G, Yeyeodu S, Erickson AH. Biosynthesis and alternate targeting of the lysosomal cysteine protease cathepsin L. Int Rev Cytol. 2004;241:1–51. doi: 10.1016/S0074-7696(04)41001-8. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang S-R, Reinheckel T, Peters C, Hook V. Major role of cathepsin L for producing ACTH, β-endorphin, and α-MSH peptide hormones derived from POMC, illustrated by gene knockout and expression. 2007 doi: 10.1074/jbc.M709010200. submittted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Host J, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner D. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from beta-lipotropin 60–65. FEBS Lett. 1984;175:135–139. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yang HY, Sabol SL. Rat neuropeptide Y precursor gene expression, mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988;262:6288–6295. [PubMed] [Google Scholar]
- Hook VY, Schiller MR, Azaryan AV. The processing proteases prohormone thiol protease, PC1/3 and PC2, and 70-kDa aspartic proteinase show preferences among proenkephalin, proneuropeptide Y, and proopiomelanocortin substrates. Arch Biochem Biophys. 1996;328:107–114. doi: 10.1006/abbi.1996.0149. [DOI] [PubMed] [Google Scholar]
- Hwang S-R, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- Hwang SR, O’Neill A, Bark S, Foulon T, Hook V. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Shen GH, Ziegler MG. Neuropeptide Y-mediated pressor responses following high-frequency stimulation of the rat sympathetic nervous system. J Pharmacol Exp Therap. 1997;281:291–296. [PubMed] [Google Scholar]
- Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology. 2004;145:519–528. doi: 10.1210/en.2003-0829. [DOI] [PubMed] [Google Scholar]
- Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VYH. Obliteration of α-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J Neurochem. 2003a;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Miller R, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VY. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003b;37:140–148. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Mahata S, Mahata M, Hook V, Taupenot L. Primary culture of bovine chromaffin cells. Nature Protocols. 2007;2:1248–1253. doi: 10.1038/nprot.2007.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Che FY, Peng B, Steiner DF, Pintar JE, Fricker LD. The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. J Neurochem. 2006;98:1763–1777. doi: 10.1111/j.1471-4159.2006.04067.x. [DOI] [PubMed] [Google Scholar]
- Pan H, Nanno D, Che FY, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry. 2005;44:4939–4948. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- Paquet L, Massie B, Mains RE. Proneuropeptide Y processing in large dense-core vesicles: manipulatin of prohormone convertase expression in sympathetic neurons using adenoviruses. J Neurosci. 1996;16:964–973. doi: 10.1523/JNEUROSCI.16-03-00964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheckel T, Deussing JRoth W, Peters C. Towards specific functions of lysosomal cysteine peptidases. Biol Chem. 2001;382:735–741. doi: 10.1515/BC.2001.089. [DOI] [PubMed] [Google Scholar]
- Roth W, Deussing J, Botchkarev VA, Pauley-Evers M, Saftig P, Hafner A, Schmidt P, Schmahi W, Scherer J, Anton-Lamprecht I, Von Figura K, Paus R, Peters C. Cathepsin L deficiency as a molecular defect of furless: hyperproliferation of keratinocytes and perturbation of hair follicle cycling. FASEB J. 2000;14:2075–2086. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- Walker P, Grouzmann E, Burnier M, Waeber B. The role of neuropeptide Y in cardiovascular regulation. TIPS. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Hamilton BS, Krist B, Doods HN. The role of NPY in metabolic homeostasis: implications for obesity therapy. Expert Opin Investig Drugs. 2000;9:1327–1346. doi: 10.1517/13543784.9.6.1327. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Aaron W, Toneff T, Hook VYH. Evidence for the proenkephalin processing enzyme prohormone thiol protease (PTP) as a multicatalytic cysteine protease complex: activation by glutathione localized to secretory vesicles. Biochemistry. 1999;38:7421–7430. doi: 10.1021/bi990239w. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S, Toneff T, Hwang SR, Hook VYH. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst J, Steiner D. Disruption of PC/13 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]