Abstract
The present article reviews recent studies of monkeys and, in some cases, humans that have been conducted to examine the role of kisspeptin-GPR54 signaling in the regulation of the hypothalamic-pituitary-gonadal axis in higher primates. This area of peptide biology was initiated in 2003 by the discovery that loss of function mutations of GPR54 in man were associated with hypogonadotropic hypogonadism and absent or delayed puberty. Puberty in the monkey, an experimental model commonly used to study this fundamental developmental stage, are first described. This is followed by a review of the role of kisspeptin in the regulation of the postnatal ontogeny of GnRH pulsatility. The roles of kisspeptin in GnRH pulse generation and in the feedback loops governing gonadotropin secretion in primates are then discussed. A brief section on kisspeptin-GPR54 signaling at the pituitary and gonadal levels is also included. The review concludes with a discussion of the phenomenon of GPR54 downregulation by continuous exposure to kisspeptin and its therapeutic implications.
Keywords: rhesus monkey, puberty, GnRH pulse generator, feedback loops, GPR54 downregulation
1. Introduction
That kisspeptins, which are encoded by KiSS1, and signal at the kisspeptin receptor (KiSS1R), a G-protein coupled receptor1, play a profound role in the control of the hypothalamic-pituitary-gonadal axis was completely unappreciated until 2003. In that year, two groups, one led by de Roux in Paris [1] and the other by Crowley in Boston [30], each almost simultaneously reported that several members of a large consanguineous family presenting with hypogonadotropic hypogonadism and absent puberty carried a loss of function mutation for KiSS1R. Moreover, it was further reported by the Boston group [30] that, in one such patient, pituitary responsiveness to pulsatile GnRH administration was not compromised, indicating a hypothalamic locus for the deficit associated with this genetic disorder. These signal observations immediately prompted my laboratory, in collaboration with the Boston group and that of Ojeda at the Oregon National Primate Research Center, to pursue the notion that kisspeptin signaling at KiSS1R is critical to the initiation of puberty. The animal employed was the rhesus monkey, a representative higher primate. The monkey is a particularly powerful paradigm for the human situation because puberty in this primate, as in man, is triggered by a resurgence in robust pulsatile release of GnRH2 after a prolonged period of relative inactivity throughout childhood (man only) and juvenile development [41]. This “up-down-up” pattern in postnatal GnRH release in primates differs markedly from that in rodents where the development of the pubertal GnRH signal unfolds progressively and without interruption following birth [4,13]. In this article, we review results of studies since 2003 of the role of kisspeptin in regulating the development and activity of the hypothalamic-pituitary-gonadal axis in the rhesus monkey and other higher primates. The molecular biology of kisspeptin synthesis, the nomenclature used to describe these peptides, and the signal transduction pathways activated by their interaction with KiSS1R will not be discussed as these aspects of kisspeptin biology will be described in detail by others contributing to this special edition of Peptides.
Puberty in the monkey
As in man, the onset of puberty in Old World monkeys3 is delayed for several years following birth: in the rhesus monkey menarche occurs at 2-3 years of age and sperm are first recognized in the seminiferous tubule at 4-5 years of age [21]. Neither gonad, pituitary, nor the hypothalamic network of GnRH neurons are limiting to the onset of primate puberty [18]. Instead, a central neural mechanism upstream of the GnRH neuronal network governs the pulsatile release of this decapeptide during postnatal development. The pattern of GnRH release during postnatal development in primates is characterized by robust pulsatility during infancy and puberty that is interrupted by a prolonged hiatus during juvenile development: a feature of primates exemplified by the agonadal rhesus monkey in which gonadotropin secretion is amplified because of the absence of testicular or ovarian feedback signals [21, Figure 1]. It is important to recognize that initiation of the resurgence in GnRH release at the transition from the juvenile to the pubertal phase of development represents one of two critical postnatal switches timing the onset of puberty in primates. The other is operational during the infant-juvenile transition and leads to the arrest/suppression of pulsatile GnRH release to guarantee the relative quiescence of the pituitary-gonadal axis during juvenile development. Disturbance in the operation of either of these neuroendocrine switches will have a profound impact on sexual development as is seen in GnRH dependent disorders of pubertal development in man [41].
Figure 1.
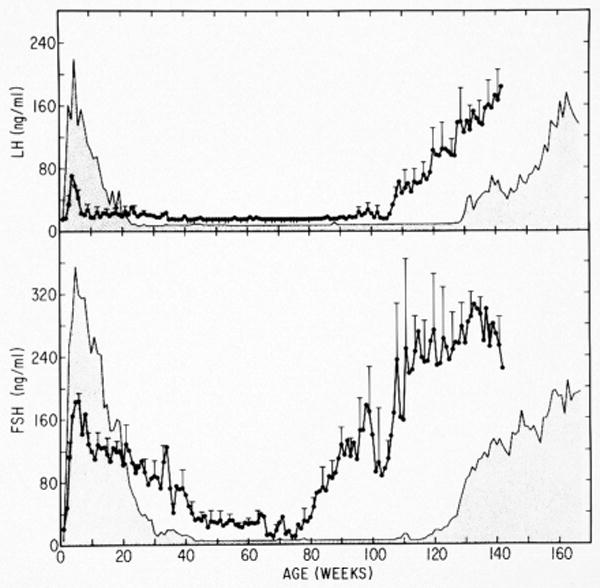
The up-down-up pattern of GnRH pulse generator activity during postnatal development in agonadal male (stippled area) and female (closed data points ± error bars) rhesus monkeys as reflected by circulating mean LH (top panel) and FSH (bottom panel) concentrations from birth to 142-166 weeks of age. Note, in females the intensity and duration of the prepubertal hiatus in the secretion of FSH, and LH to a lesser extent, is truncated in comparison to males. Redrawn with permission from [21].
The agonadal male monkey: a model for studying the postnatal ontogeny of GnRH release
Because the agonadal male monkey has been used extensively to examine the neurobiological mechanisms dictating the postnatal pattern of GnRH secretion (including that involving kisspeptin), the rationale for employing this animal model [16] is briefly reiterated here. The postnatal hypothalamus retains the capacity for both structural and molecular plasticity; neuronal processes that are profoundly modulated by steroids to which the hypothalamus is exposed. Thus, it is reasonable to propose that, in intact monkeys, structural and molecular remodeling occurs in response to the dramatic changes in steroid levels during infancy and puberty. In the intact situation, therefore, it becomes impossible to distinguish whether a hypothalamic correlate of GnRH pulsatility, for example a change in expression of a particular gene or the appearance or loss of a specific synaptic interaction, represents the primary event underlying the change in hypothalamic function, or is simply a secondary consequence due to a change in gonadal steroid that has been triggered by the fundamental neural event being sought. These confounding influences are eliminated in the agonadal situation. At the same time, the fundamental developmental changes in pulsatile GnRH release are preserved in the absence of the gonad (Figure 1). The male has been employed because the prepubertal hiatus in pulsatile GnRH release is more marked in the male than in the female (Figure 1), and it may therefore be anticipated that the molecular and structural concomitants of the up-down-up pattern in pulsatile GnRH release will be correspondingly more conspicuous in the male and thus easier to identify for the first time.
Role of kisspeptin in regulation of the postnatal ontogeny in GnRH pulsatility
a. Infant-juvenile transition
Little attention has been paid to the molecular and cellular mechanisms that are involved in suppressing GnRH pulsatility during infancy and maintaining the check on this neuroendocrine system during subsequent juvenile development. Interestingly, GnRH mRNA and peptide levels in the MBH of the infant and juvenile monkey are indistinguishable [3]. A possible insight into the neurobiology underlying the reduction in GnRH pulsatility during infancy is provided by the observation that circulating gonadotropin levels were undetectable in a two-month-old infantile boy bearing a loss of function mutation of KiSS1R [31]. This finding is consistent with the earlier observation of hypogonadotropism and hypogonadism in KiSS1R deficient patients of pubertal and postpubertal age [1,30], and supports the notion that kisspeptin input to the GnRH neuronal network in the hypothalamus of the infant is necessary for the robust GnRH pulsatility during this phase of development.
b. Juvenile-pubertal transition
Most attention regarding the role of kisspeptin in regulating the timing of puberty in primates has focused on the neurobiological mechanisms responsible for triggering the pubertal resurgence of pulsatile GnRH release at the termination of the juvenile phase of development. If an increase in kisspeptin signaling at KiSS1R is the proximate trigger for increased pulsatile GnRH release at this stage of development then the following conditions should be met: first, KiSS1 and KiSS1R (and the ligand and receptor) should be expressed in the monkey medial basal hypothalamus (MBH); second, increased KiSS1R signaling should occur in association with the pubertal resurgence in pulsatile GnRH release; and third, premature activation of KiSS1R in the hypothalamus of the juvenile should lead to a precocious pubertal-like pattern of robust GnRH release. In situ hybridization demonstrated that KiSS1 is expressed in the MBH and that, as in rodents [22], expression in this region of the MBH is restricted to neurons in the arcuate nucleus [32]. Similar findings have been reported for both female cynomolgus monkeys and women [28]. Expression of KiSS1R was also observed in the arcuate nucleus, although expression of the receptor was less discrete, extending into more lateral regions of the ventral medial hypothalamus [32]. The expression of KiSS1 increased nearly five-fold in association with the pubertal resurgence in GnRH release in both agonadal males and intact females, and an increase in KiSS1R expression during this developmental transition was observed in the female [32]. At this time, however, it should not be concluded that there is necessarily a fundamental gender difference in the peripubertal regulation of KiSS1R expression in primates because the males studied were agonadal (they were castrated between 16 and 21 months of age - see Figure 1) while the females were ovarian intact.
The question of whether premature activation of KiSS1R in the hypothalamus of the juvenile monkey leads to precocious GnRH release has been examined using pituitary LH secretion to indirectly monitor endogenous GnRH release in response to human (h) kisspeptin-10 (metastin 45-541) administration. In order to use this approach in the juvenile male monkey, in which pituitary responsiveness to GnRH is minimal, the gonadotrophs must first be “primed” with an intermittent iv infusion of synthetic GnRH [35]. This is accomplished with a dose and frequency of GnRH administration that produces a pattern of pulsatile LH release in the agonadal juvenile male monkey that is comparable to that observed in postpubertal castrate males [35]. Using this paradigm, initiation of an intermittent iv infusion of h-kisspeptin-10 (2 μg/monkey administered as brief 1 min pulses once every h) immediately following termination of the priming infusion of GnRH was able to sustain without interruption an adult pattern of pulsatile LH secretion in the agonadal juvenile male [20, Figure 2]. This LH response to intermittent kisspeptin stimulation was abolished when the experiment was repeated in the presence of a GnRH receptor antagonist [20, Figure 3], indicating that the action of kisspeptin to release LH was indirect and most likely mediated by the discharge of a train of GnRH pulses from the hypothalamus of the juvenile monkey. It is of interest to note here that a gain of function mutation (non-constitutive) of KiSS1R has recently been described in a seven-year-old girl with GnRH dependent precocious breast development [36]. Transfection studies indicated that the mutation resulted in prolonged activation of KiSS1R signal transduction pathways following simulation with kisspeptin [36]. To date, attempts to induce a sustained precocious pubertal pattern of GnRH release from the hypothalamus of the juvenile monkey has only been achieved when kisspeptin was administered as an intermittent infusion. Continuous iv infusion of the peptide over a wide range of doses in this experimental model stimulates GnRH secretion only transiently, and at high doses KiSS1R is downregulated [29, see below].
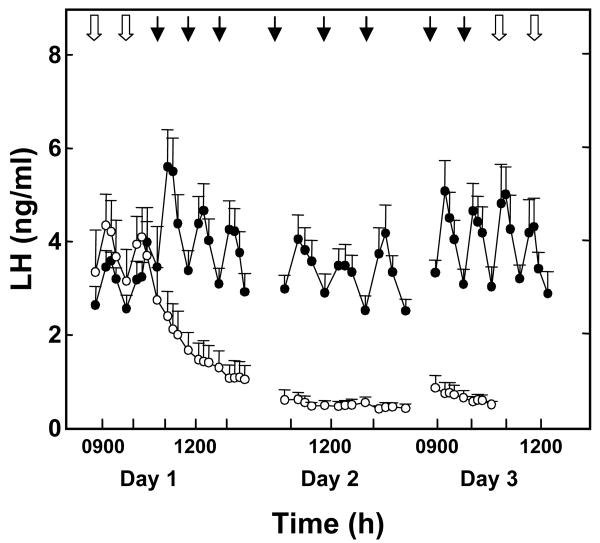
Figure 2.
LH discharges induced precociously in agonadal juvenile male monkeys (20-24 months of age) by an intermittent iv infusion of GnRH that mimics a castrate adult hypophysiotropic drive to the gonadotrophs (open arrows on Day 1) and by brief infusions of h-kisspeptin-10 every h (closed arrows) initiated on Day 1 immediately following termination of GnRH priming and maintained for 48 h in a crossover design (kisspeptin-10, closed data points; vehicle, open data points). LH responses are shown for the first three kisspeptin-10 or vehicle pulses on Day 1, three such pulses on Day 2 and the last two pulses on Day 3. GnRH priming (open arrows) was reinitiated after completion of kisspeptin-10 or vehicle administration on Day 3. Reprinted with permission from [20].
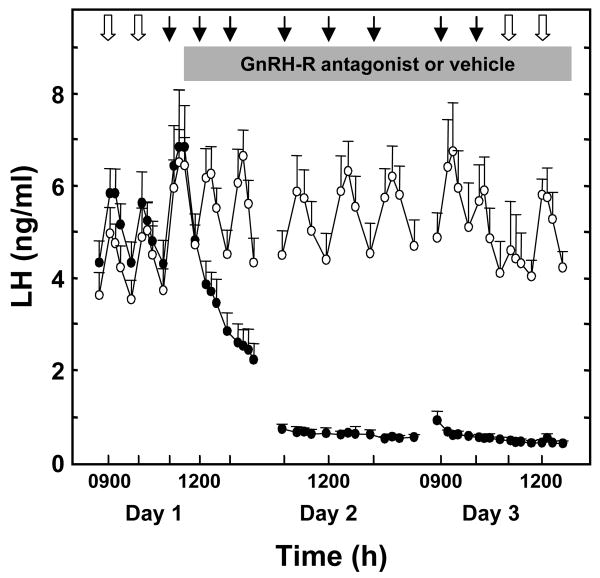
Figure 3.
Human kisspeptin-10 induced LH discharges induced in GnRH primed agonadal juvenile male monkeys by brief iv infusions every h for 48 h shown in Figure 1 were completely blocked by treatment with a GnRH receptor antagonist initiated immediately after the first pulse of kisspeptin-10 (black data points) at 1100 h on Day 1. The open data points show kisspeptin-10 induced LH pulses during injection of the antagonist vehicle. LH responses are shown for the first three kisspeptin-10 or vehicle pulses on Day 1, three such pulses on Day 2 and the last two pulses on Day 3. GnRH priming (open arrows) was reinitiated after completion of kisspeptin-10 or vehicle administration on Day 3. Reprinted with permission from [20].
Taken together, the foregoing findings in the monkey, and those in man, support the view that kisspeptin signaling is a key component of the hypothalamic mechanism that activates the pubertal resurgence in GnRH release, and thereby initiates the onset of puberty in primates. It should be recalled, however, that there is compelling evidence for the involvement of other neurotransmitters (GABA and glutamate) and neuropeptides (neuropeptide Y) in the neurobiological mechanism holding and/or releasing the check on GnRH pulsatility during juvenile development [18,21,38]. Thus, the role of kisspeptin in triggering the pubertal resurgence in pulsatile GnRH release must be integrated with that of other neural signals, and also inputs of glial origin [12], in order to provide a rational model for the neurobiological machinery that controls of the postnatal ontogeny of pulsatile GnRH release. Additionally, it is not known whether the kisspeptin neurons in the arcuate nucleus are a component of a pubertal clock or growth-tracking device [17], or simply serve as a link that relays information from such a puberty control center to the GnRH neuronal network. The resolution of these issues is a major challenge for the future.
Kisspeptin and GnRH pulse generation
In 1978, Knobil's laboratory reported that in ovariectomized adult rhesus monkeys selective destruction of the posterior two-thirds of the arcuate nucleus with radiofrequency current resulted in a profound suppression of gonadotropin secretion in the absence of hyperprolactinemia [19, Figure 4]. These and related findings led Knobil to the conclusion that the hypothalamic GnRH pulse generator in the primate resided within the arcuate nucleus [10]. It is, therefore, very interesting to now note that the region of the arcuate nucleus lesioned in the early studies of the female monkey overlaps precisely with the location of KiSS1 expressing neurons in this nucleus in women [27; Figure 5]. Moreover, using a polyclonal antibody raised against h-kisspeptin-54 (GQ2) and kindly provided by Professor Stephen Bloom, London, UK [2], we have recently demonstrated [23] that location of kisspeptin containing perikarya in the MBH of the adult male monkey is entirely consistent with the earlier hybridization histochemical studies of KiSS1 expression, i.e. in the posterior two-thirds of the arcuate nucleus [28, Figure 6]. Because the medial location of kisspeptin perikarya in the arcuate nucleus is distinct from the lateral location of the majority of neuroendocrine GnRH neurons in the ventral hypothalamic tract (VHT) (Figure 6), the earlier arcuate lesions would have preserved the GnRH neuronal network but destroyed the vast majority of the kisspeptin perikarya. Thus, it seems reasonable to propose that kisspeptin neurons in the arcuate nucleus may represent the substrate of the GnRH pulse generator. In this regard, intermittent kisspeptin release at an approximate hourly frequency, has very recently been reported in the stalk-median eminence of the female rhesus monkey [8].
Figure 4.
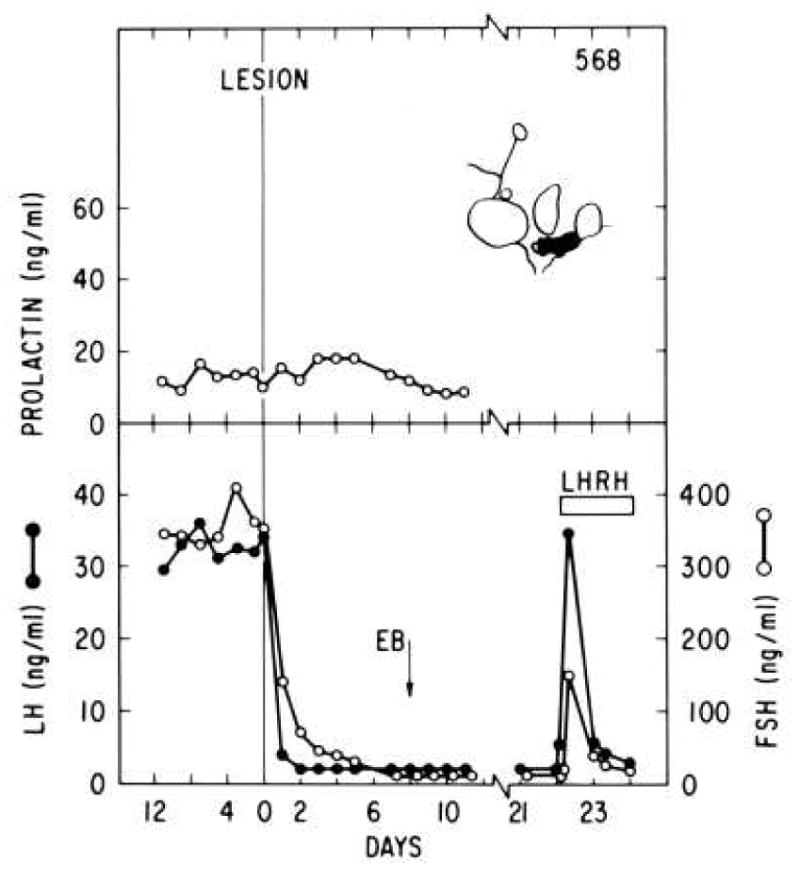
The top panel shows a parasagittal schematic of the region of the arcuate nucleus destroyed by a radiofrequency lesion placed on day 0 in an ovariectomized rhesus monkey. While this lesion preserved circulating prolactin concentrations (top panel) and pituitary response to a continuous infusion of GnRH (LHRH) on day 22, spontaneous and estradiol induced LH and FSH secretion were abolished. Reprinted with permission from [19].
Figure 5.
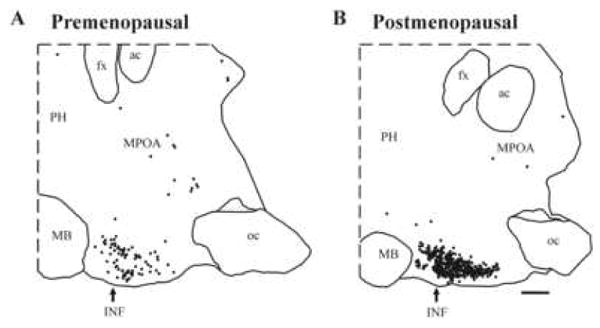
The distribution of KiSS1 mRNA expressing neurons as revealed by in situ hybridization in a representative sagittal hypothalamic section from a premenopausal (left) and postmenopausal (right) woman. KiSS1 expressing perikarya are concentrated only in the infundibular nucleus (INF), also known as the arcuate nucleus (see Figure 4) of the MBH. Only the occasional KiSS1 mRNA expressing neuron was observed in the medial preoptic area (MPOA). KiSS1 was upregulated in the postmenopausal women. Each small black dot represents one KiSS1 expressing neuron. ac, anterior commissure; fx, fornix; INF, infundibular nucleus; MB, mammillary body; oc, optic chiasm; PH, posterior hypothalamus. Scale bar, 2 mm. Reprinted with permission from [28].
Figure 6.
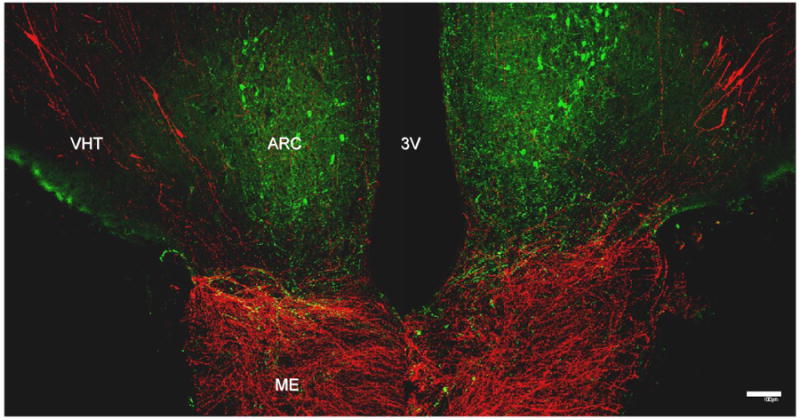
A confocal double fluorescence projection showing the relationship between kisspeptin containing perikarya (green Alexa Flor 488) in the arcuate nucleus (ARC) and GnRH cell bodies (red, Cy3) in the ventral hypothalamic tract (VHT) in a coronal hypothalamic section taken at the level of the median eminence (ME) of a male rhesus monkey. At this level, the median eminence is richly innervated with GnRH axons, while kisspeptin axons are found predominately at the boundary of the arcuate nucleus and median eminence. 3V, third ventricle. Scale bar, 100 μm. The primary antibody for kisspeptin was GQ2 (2), kindly provided by Dr Stephen Bloom and used at a dilution of 1:120,000. The primary antibody for GnRH was LR1 kindly provided by Dr. Robert Benoit (Montreal General Hospital, Canada) and previously validated [6]. It was used at a dilution of 1:100,000. Reprinted with permission from [24].
The foregoing argument, however, must be reconciled with the intriguing clinical observations that patients with loss of function mutations of KiSS1R exhibit low amplitude intermittent LH release at an approximately normal frequency [30,37], suggesting that kisspeptin functions to amplify the activity of the GnRH pulse generator, rather than to generate pulsatility. This view is consistent with the finding that, in rat, iv administration of kisspeptin-10 elicits a robust discharge of LH without influencing arcuate nucleus/median eminence multiunit electrical activity [8], which provides a robust electrophysiological correlate of GnRH pulse generator activity in several species including the monkey [11]. Also, only moderate fluctuations in portal blood kisspeptin concentrations, unrelated to LH discharges, have been reported in the ovariectomized ewe [34]. If the action of kisspeptin to enhance pulsatile GnRH release is the sole result of amplifying the activity of the GnRH pulse generator, then it might be predicted that continuous infusion of kisspeptin at doses that lead to downregulation of KiSS1R [29, see below] would reduce LH pulse amplitude but not effect LH pulse frequency. In a study of the adult male rhesus monkey, this was not the case [24]: both LH pulse amplitude and LH pulse frequency were reduced. Because reduction in LH pulse amplitude may result in LH increments falling below threshold for detection as an LH pulse, it remains to be confirmed whether the reduction in LH pulse frequency, which generally reflects GnRH pulse frequency [15], was due to a kisspeptin induced retardation of the GnRH pulse generator. Clearly, significant further study is required to elucidate the role of kisspeptin in GnRH pulse generation.
Role of kisspeptin neurons in the feedback regulation of gonadotropin secretion
Gonadotropin secretion in the male is regulated by negative gonadal feedback loops that involve inhibitory actions of testicular hormones (primarily testosterone and inhibin-B) [14,15] on the hypothalamic-pituitary unit. The regulation of LH and FSH secretion in the female is more complex, comprising both negative and positive gonadal feedback loops that involve inhibitory and stimulatory actions of ovarian hormones (primarily estradiol and progesterone) on hypothalamic and pituitary sites [42]. A negative feedback loop governs gonadotropin secretion throughout the luteal phase and most of the follicular phase of the menstrual cycle, while a positive feedback loop is responsible for triggering the preovulatory gonadotropin surge at the end of the follicular phase. The feedback actions of gonadal hormones may be exerted either at the level of the hypothalamus to indirectly regulate gonadotropin secretion by controlling GnRH release, the pituitary to directly regulate the activity of the gonadotroph, or by a combination of these mechanisms. Gonadal feedback at the level of the hypothalamus may be exerted either directly on GnRH neurons or indirectly on afferent inputs (neuronal and or glial) to this neuronal network. In the latter case, there is increasing evidence from studies of non-primate species that kisspeptin neurons are intimately involved in mediating the feedback actions of both ovarian and testicular hormones [22].
In the male monkey, the testicular regulation of LH secretion is mediated by a negative feedback action of testosterone that is exerted at the level of the hypothalamus to decelerate pulsatile GnRH release [15]. As in rodents [22], testosterone suppression of LH secretion is associated with a reduction in KiSS1 mRNA levels in the MBH of the monkey [33]. Since KiSS1 and kisspeptin expressing neurons are found only in the arcuate nucleus of the monkey, it seems reasonable to conclude that, as in rodents [22], kisspeptin neurons in this region of the MBH represent an important neurobiological component in the negative feedback loop that regulates LH secretion in the male. It should be recalled, however, that opiatergic and GABergic neurons also respond to castration and testosterone replacement and that these neurotransmitters/peptides exert inhibitory actions on GnRH release [3]. Thus, an understanding of the integrative neurobiology of the negative feedback control of LH secretion in the male remains to be defined: a state of affairs reminiscent of that underlying the pubertal resurgence in pulsatile GnRH release at the end of juvenile development.
As discussed above, KiSS1 expressing neurons in the hypothalamus of women and female cynomolgus monkeys are also localized almost exclusively in the arcuate nucleus (referred to as the infundibular nucleus in man). As in the male, gonadal steroids suppress expression of KiSS1 in the hypothalamus of both the human and monkey female. In an elegant study by Rometo et al. [28], the number of KiSS1 mRNA expressing neurons was significantly higher in postmenopausal women and ovariectomized monkeys compared to premenopausal women and intact monkeys, respectively (results from women, Figure 5). Moreover, treatment of ovariectomized monkeys with estradiol and progesterone resulted in a profound decrease in KiSS1 expression [28]. Thus, in the monkey, as in rodents, it would appear that a component of the negative feedback action of estradiol and/or progesterone is mediated by kisspeptin neurons in the arcuate nucleus.
The role of hypothalamic kisspeptin signaling in mediating the positive feedback action of estradiol to elicit the preovulatory gonadotropin surge in primates has not yet been investigated. Here it is to be noted, however, that in the rodent the kisspeptin neurons that are activated by the positive feedback action of estradiol and thereby contribute to the generation of the preovulatory GnRH surge, an obligatory component of the preovulatory gonadotropin surge in these species [5], are located in rostral areas of the hypothalamus, notably the anteroperiventricular nucleus in the medial preoptic area [40,21]. This species difference in the distribution of kisspeptin neurons in the hypothalamus is consistent with marked differences in the neuroendocrine mechanisms that regulate ovulation in rodents and primates [5,42].
Kisspeptin signaling at the pituitary and gonadal levels
While several studies in non-primate species have demonstrated that kisspeptin signaling in the pituitary and gonads may potentially play a role in the regulation of ovarian and testicular function [26,27], these aspects of kisspeptin biology have received little attention in the primates. An initial immunocytochemical study of kisspeptin in the monkey pituitary indicates that this peptide is expressed by the majority of cells in pars intermedia and by occasional cells of unknown phenotype in the pars anterior [23]. At the testicular level, it was noted that in adult male rhesus monkeys a particular concentration of blood LH was generally associated with higher levels of testosterone during the continuous iv administration of h-kisspeptin-10 indicating the possibility of a local action of kisspeptin to regulate the responsivity of the Leydig cell to LH stimulation [25]. This view is consistent with the recent finding that kisspeptin enhances hCG induced testicular testosterone release in the adult monkey [7].
Downregulation of KiSS1R by continuous exposure to kisspeptin
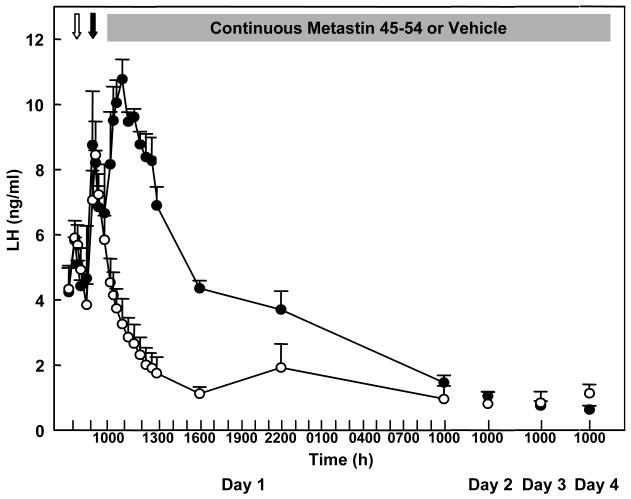
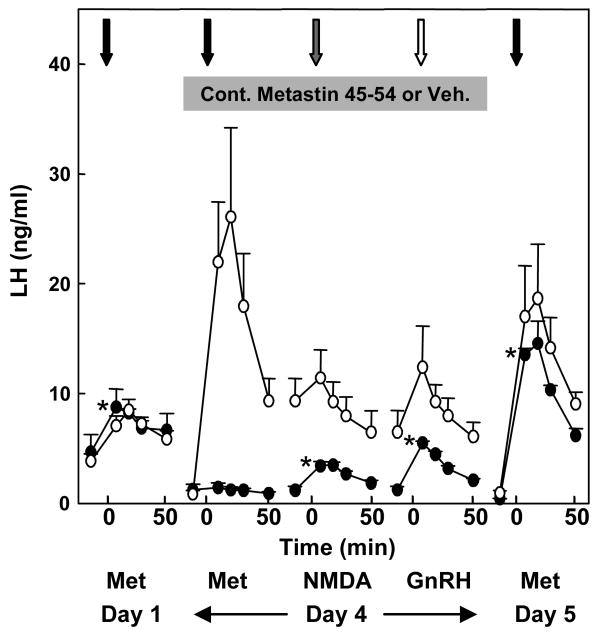
Using pituitary LH secretion from the GnRH primed juvenile male monkey pituitary to indirectly monitor endogenous GnRH release as earlier described to track discharges of this hypophysiotropic factor in response to intermittent kisspeptin administration, continuous iv infusion of h-kisspeptin-10 for four days was recently shown to lead to desensitization of KiSS1R [29]. Although continuous administration of the KiSS1R agonist elicited a brisk LH response for approximately three hours, this rise was followed by a precipitous drop in LH despite continuous exposure of KiSS1R to kisspeptin (Figure 7). During the last three hours of the continuous kisspeptin infusion, the integrity of the GnRH neuron-pituitary gonadotroph axis was interrogated by the administration of single boluses of h-kisspeptin-10 (10 μg), N-methyl-DL-aspartic acid (10 mg/kg, NMDA), and GnRH (0.3 μg). The h-kisspeptin-10 dose was approximately five fold higher than that employed in the experiment shown in Figure 2, while doses for the NMDA and GnRH were comparable to those previously established to elicit a physiological discharge of GnRH and LH, respectively [18,33]. Although boluses of NMDA and GnRH elicited an LH pulse, that of kisspeptin-10 did not (Figure 8), indicating functional integrity of the GnRH neuronal network (NMDA) and GnRH receptors (NMDA and GnRH) but desensitization of KiSS1R. Similar results have been obtained following continuous h-kisspeptin-10 administration to normal adult monkeys, in which the hypothalamic GnRH pulse generator was spontaneously active [25], and desensitization of KiSS1R has also been described in experiments with the male rat [39]. This phenomenon has therapeutic implications for a variety of conditions currently being treated by GnRH and its analogs, including restoration of fertility in patients requiring ovulation induction, and selective reversible suppression of the pituitary-gonadal axis to achieve suppression of gonadal steroids.
Figure 7.
Effect of continuous administration of h-kisspeptin-10 (closed data points) or vehicle (open data points) initiated at 1000h on day 1 and maintained for 98 h (shaded horizontal bar) on LH release in GnRH primed agonadal juvenile male rhesus monkeys. White arrow indicates iv injection of the last GnRH priming pulse at 0800 on day1 and the black arrow indicates iv injection of a bolus of kisspeptin-10 1 h later. Reprinted with permission for [29].
Figure 8.
Interrogation of the GnRH neuronal network-gonadotroph axis in GnRH primed agonadal juvenile male rhesus monkeys, during the last 3 h of the 48 h continuous infusion (shaded horizontal bar) of h-kisspeptin-10 (closed data points) or vehicle (open data points) shown in Figure 7, with single sequential boluses of kisspeptin-10 (black arrow), NMDA (gray arrow) and GnRH (white arrow). * Infusion of kisspeptin-10 significantly different (P<0.05) from preinjection mean. Reprinted with permission for [29].
Summary
In primates, kisspeptin is a critically important component of the neurobiological machinery that triggers the pubertal resurgence of robust pulsatile release, and also that which provides the drive to the GnRH neuronal network during infancy. How kisspeptin neuron activity is integrated with that of other neuropeptide/neurotransmitter systems to achieve the characteristic up-down-up pattern in GnRH pulse generator activity throughout the infant-juvenile-pubertal transitions remains to be established. Similarly, the physiological control system that dictates the timing of these developmental transitions, including the pubertal resurgence in pulsatile GnRH release that triggers puberty, remains a fundamental mystery. The precise role of kisspeptin signaling in the GnRH pulse generator remains to be clarified. Although kisspeptin neurons are likely to be involved in the feedback loops regulating gonadotropin secretion in primates, this area requires more study. Kisspeptin signaling may also be extant in the pituitary and gonad, but the full physiological significance of these extra-hypothalamic sites remains to be determined.
Acknowledgments
Work reported from the author's laboratory was supported by the National Institutes of Health (Grants HD 13254 and HD 08610). The authors are grateful to Dr. Stephen R. Bloom, Imperial College London, London, UK, for the generous gift of GQ2, an excellent polyclonal antibody to h-kisspeptin-54, and to Ms. Kathryn Guerriero, who played an important role in validating the immunocytochemical procedures for visualizing kisspeptin in the monkey hypothalamus.
Footnotes
KiSS1R was initially called G protein coupled receptor 54 (GPR54): the nomenclature used in the present review is that suggested by Gottsch et al. in this special edition of Peptides.
The decapeptide, GnRH (gonadotropin releasing hormone), which is synonymous with LRF and LHRH, is used throughout this review to describe mammalian GnRH (GnRH-1).
Higher primates are comprised of 3 families: Old World monkeys, apes and humans.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–76. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 3.El Majdoubi M, Sahu A, Plant TM. Changes in hypothalamic gene expression associated with the arrest of pulsatile gonadotropin-releasing hormone release during infancy in the agonadal male rhesus monkey (Macaca mulatta) Endocrinology. 2000;141:3273–77. doi: 10.1210/endo.141.9.7687. [DOI] [PubMed] [Google Scholar]
- 4.Fraser MO, Plant TM. Further studies on the role of the gonads in determining the ontogeny of gonadotropin secretion in the guinea pig (Cavia porcelus) Endocrinology. 1989;125:906–11. doi: 10.1210/endo-125-2-906. [DOI] [PubMed] [Google Scholar]
- 5.Freeman MC. Neuroendocrine control of the ovarian cycle of the rat. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. Third. Chapter 43. Vol. 2. San Diego: Elsevier; 2006. pp. 2327–88. [Google Scholar]
- 6.Goldsmith PC, Thind KK, Perera AD, Plant TM. Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology. 1994;134:858–68. doi: 10.1210/endo.134.2.7905410. [DOI] [PubMed] [Google Scholar]
- 7.Irfan S, Anees M, Wahab F, Zaman W, Plant T, Shahab M. Evidence for a direct intratesticular action of kisspeptin in the adult rhesus monkey (Macaca mulatta). To be presented at the 90th Annual Meeting of The Endocrine Society; San Francisco. June 2008. [Google Scholar]
- 8.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in LHRH-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008 May 1; doi: 10.1210/en.2008-0231. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsey-Jones JS, Li XF, Luckman SM, O'Byrne KT. Effects of Kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149:1004–08. doi: 10.1210/en.2007-1505. [DOI] [PubMed] [Google Scholar]
- 10.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 11.O'Byrne KT, Knobil E. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod. 1993;8 2:37–40. doi: 10.1093/humrep/8.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 12.Ojeda SR, Prevot V, Heger S, Lomniczi A, Dziedzic B, Mungenast A. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Ann Med. 2003;35:244–55. doi: 10.1080/07853890310005164. [DOI] [PubMed] [Google Scholar]
- 13.Ojeda SR, Skinner MK. Puberty in the rat. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. Third. Chapter 38. Vol. 2. San Diego: Elsevier; 2006. pp. 2061–2126. [Google Scholar]
- 14.Plant TM. FSH secretion in the adult male rhesus monkey is controlled in a manner consistent with that proposed by the inhibin hypothesis. In: Burger HG, de Kretser D, Findlay J, Petraglia F, Robertson D, editors. Frontiers in Endocrinology, Volume 3: Inhibin and Inhibin-Related Proteins. Rome: Ares-Serono Symposia Publications; 1994. pp. 135–43. [Google Scholar]
- 15.Plant TM. Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocrine Rev. 1986;7:75–88. doi: 10.1210/edrv-7-1-75. [DOI] [PubMed] [Google Scholar]
- 16.Plant TM. Neurobiological bases underlying the control of the onset of puberty in the rhesus monkey: a representative higher primate. Frontiers Neuroendocrinol. 2001;22:107–39. doi: 10.1006/frne.2001.0211. [DOI] [PubMed] [Google Scholar]
- 17.Plant TM, Fraser MO, Medhamurthy R, Gay VL. Somatogenic control of GnRH neuronal synchronization during development in primates: A speculation. In: Delemarre van de Waal HA, Plant TM, van Rees GP, Schoemaker J, editors. Control of the Onset of Puberty, III. Amsterdam: Elsevier Science Publishers BV; 1989. pp. 111–121. [Google Scholar]
- 18.Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci USA. 1989;86:2506–10. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 20.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–13. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 21.Plant TM, Witchel S. Puberty in non-human primates and humans. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. Third. Chapter 40. Vol. 2. San Diego: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- 22.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–38. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Gibbs RB, Plant TM. Localization of kisspeptin cells and axonal fibers in the pituitary of the male rhesus monkey (Macaca mulatta). 41st Annual Meeting of the Society for the Study of Reproduction; Hawaii. May 2008; Abstract #9. [Google Scholar]
- 24.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008 May 29; doi: 10.1210/en.2008-0438. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous iv administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–70. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 26.Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–93. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 27.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–50. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 29.Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45-54 infusion desensitizes GPR54-induced GnRH release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): A finding with therapeutic implications. Endocrinology. 2006;147:2122–26. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 30.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 31.Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, et al. Two novel missense mutations in GPR54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 32.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate LH secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–38. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: Evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- 35.Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta) Endocrinology. 1998;139:2774–83. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 36.Teles MG, Bianco SDC, Brito VH, Trarbach EB, Kuohung W, Xu S, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Eng J Med. 2008;358:709–15. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–44. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- 38.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocrine Rev. 2001;22:111–51. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 39.Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–82. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- 40.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witchel SF, Plant TM. Puberty: Gonadarche and Adrenarche. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology. Fifth. Chapter 17. Philadelphia: Elsevier Inc; 2004. pp. 493–535. [Google Scholar]
- 42.Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Challis JRG, de Kretser DM, Neill JD, Pfaff DW, Plant TM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. Third. Chapter 45. Vol. 2. San Diego: Elsevier; 2006. pp. 2449–2510. [Google Scholar]