Abstract
The objective of this study was to investigate the efficacy of first-line chemotherapy containing irinotecan and/or oxaliplatin in patients with advanced mucinous colorectal cancer. Prognostic factors associated with response rate and survival were identified using univariate and multivariate logistic and/or Cox proportional hazards analyses. The population included 255 patients, of whom 49 (19%) had mucinous and 206 (81%) had non-mucinous colorectal cancer. The overall response rates for mucinous and non-mucinous tumours were 18.4 (95% CI, 7.5–29.2%) and 49% (95% CI, 42.2–55.8%), respectively (P=0.0002). After a median follow-up of 45 months, median overall survival for the mucinous patients was 14.0 months compared with 23.4 months for the non-mucinous group (hazard ratio (HR), 1.74; CI 95%, 1.27–3.31; P=0.0034). After adjustment for significant features by multivariate Cox regression analysis, mucinous histology was associated with poor overall survival (HR, 1.593, 95% CI, 1.05–2.40; P=0.0267), together with performance status ECOG 2, number of metastatic sites ⩾2, and peritoneal metastases. This retrospective analysis shows that patients with mucinous colorectal cancer have poor responsiveness to oxaliplatin/irinotecan-based first-line combination chemotherapy and an unfavourable prognosis compared with non-mucinous colorectal cancer patients.
Keywords: mucinous, colorectal cancer, chemotherapy, irinotecan, oxaliplatin, 5-fluorouracil
Colorectal cancers are derived from the cells of the colonic epithelium, and they are mainly constituted by non-mucinous cancers. However, mucinous carcinomas make up 10–20% of all colorectal cancers (Symonds and Vickery, 1976; Minsky et al, 1987; Green et al, 1993), with different clinicopathological characteristics, distinct genetic profiles, and histogenic pathways (Parham, 1923; Hanski, 1995; Zhang et al, 1999; Kim et al, 2005; Song et al, 2005). It has also been hypothesised that a mucinous pathway of carcinogenesis leads to a mucinous phenotype (Hanski, 1995). Mucinous adenocarcinoma is characterised by abundant extracellular mucin produced by tumour cells. By definition, a 50% or greater mucinous component is required for the designation of mucinous colorectal carcinoma (Hamilton and Aaltonen, 2000). This subtype of tumour is to be differentiated from signet ring cell carcinoma that is constituted by single tumour cells with intracytoplasmic mucin displacing their nuclei aside with 50% or more of such components. The prognostic significance of mucinous carcinoma is controversial. In some studies, mucinous histology has been shown to be an independent negative prognostic factor (Connelly et al, 1991; Green et al, 1993; Secco et al, 1994), but not in others (Minsky et al, 1987; Green et al, 1993; Enriquez et al, 1998; Consorti et al, 2000). Both the American Joint Committee on Cancer and the College of American Pathologists consider that the mucinous subtype has not been proven as a statistically significant prognostic factor independent of histological grade (Compton et al, 2000a, 2000b).
For the treatment of advanced colorectal cancer, after decades in which 5-fluorouracil (5-FU) was the only drug approved, irinotecan (IRI) and oxaliplatin (OXA) have been added during the last decade to the armamentarium of agents with activity in colorectal cancer, setting a new benchmark of survival for patients with unresectable advanced colorectal at around 20 months (Grothey et al, 2004; Goldberg et al, 2007). Interestingly, with the more recent incorporation of biological therapies, such as bevacizumab and cetuximab, overall survival (OS) of advanced colorectal cancer patients has been further improved. Now, various treatment options combining cytotoxic and targeted therapies are currently available for these patients (Grothey and Marshall, 2007).
The effect of mucinous histology on advanced colorectal cancer patients treated with first-line chemotherapy has been only recently examined by Negri et al (2005). In this report, 45 patients with advanced mucinous colorectal cancer have been shown to have a poorer response to 5-FU-based first-line chemotherapy and to have reduced survival compared with 90 patients with non-mucinous colorectal cancer. The overall response rate and median survival for mucinous colorectal cancer were 22% and 11.8 months compared with 47% (P=0.0058) and 17.9 months (P=0.0372) for non-mucinous tumours (Negri et al, 2005). No data were available on the effect of mucinous histology over the treatment efficacy of first-line regimens containing IRI and OXA. The aim of this analysis was to investigate the response rate and the OS of fluoropyrimidines in combination with IRI and/or OXA as first-line chemotherapy in patients with mucinous colorectal carcinoma.
Patients and methods
The population consisted of 255 consecutive unselected patients who had undergone first-line chemotherapy for colorectal cancer at five oncology departments between September 2001 and December 2006. All patients had histologically confirmed diagnosis of colorectal adenocarcinoma, unidimensionally measurable disease, first-line chemotherapy containing fluoropyrimidines plus IRI and/or OXA, adjuvant/neoadjuvant treatment completed more than 6 months earlier, adequate hematological/clotting, hepatic, renal, and cardiac functions. Moreover, patients were excluded if they had received prior chemotherapy for metastatic colorectal cancer, had previous malignancy within 5 years (except for basal cell skin cancer or in situ carcinoma of the cervix), and were from families with familial adenomatous polyposis or hereditary non-polyposis colorectal with a highly penetrant genetic predisposition to colorectal cancer.
The pathologists from the five referral hospitals were asked to review tumour specimens and assessed the tumour type. To avoid evaluator variability in the patients, all the pathologists were not aware of the clinical results. By definition, tumours with mucinous histology had mucin constituting more than 50% of tumour volume. The colorectal adenocarcinomas without any mucinous or <50% of the mucinous component were designated as non-mucinous carcinoma (Hamilton and Aaltonen, 2000). Tumours with signet ring cells component and undifferentiated carcinoma were excluded from the analysis.
The following data were collected from the hospital records for each patient: sex, age, performance status (PS) evaluated according to the Eastern Cooperative Oncology Group (ECOG) criteria, primary tumour location, histology, earlier resection of the primary tumour, earlier tumour location, adjuvant therapy (chemotherapy and/or radiotherapy); baseline haemoglobin, CEA, and CA19-9 levels; number and sites of metastatic disease; regimen used as first-line treatment (containing fluoropyrimidines plus IRI, OXA, or both), and objective response to treatment. Patients receiving fluoropyrimidines alone or biologic agents (bevacizumab and cetuximab) as first-line chemotherapy were excluded from the analysis. Laboratory variables were initially recorded as continuous variables and later dichotomised according to the normal upper limit. Primary tumours were assigned to one of the two anatomical sites: right-sided colon (arising in the caecum, ascending colon, hepatic flexure, and transverse colon); left-sided colon (arising in the descending colon, sigmoid colon, rectosigmoid junction, and rectum).
Treatment protocols and evaluation of response
The following first-line regimens were used to treat this population: (i) FOLFOX: OXA 85 mg m−2 day 1, leucovorin 200 mg m−2 day 1–2, bolus 5-FU 400 mg m−2 day 1–2, 22 h continuous infusion 5-FU 600 mg m−2 day 1–2, every 2 weeks; (ii) XELOX: capecitabine 1000 mg m−2 b.i.d. day 1–14, OXA 100–130 mg m−2 day 1, every 3 weeks; (iii) FOLFIRI: IRI 180 mg m−2 day 1, leucovorin 200 mg m−2 day 1–2, bolus 5-FU 400 mg m−2 day 1–2, 22 h continuous infusion 5-FU 600 mg m−2 day 1–2, every 2 weeks; (i.v.) XELIRI: capecitabine 1,000 mg m−2 b.i.d. day 1–14, IRI 250 mg m−2 i.v. day 1, every 3 weeks; (v) FOLFOXIRI: IRI 165 mg m−2 followed by OXA 85 mg m−2 leucovorin 200 mg m−2, and 5-FU 3200 mg m−2 administered as a 48-h flat continuous infusion, every 2 weeks.
Response evaluation criteria in solid tumour (RECIST) guidelines were used to define all responses (Therasse et al, 2000). All radiology studies were reviewed for confirming the treatment outcomes.
Statistical analysis
The two groups of patients were compared using 2 × 2 tables for binary factors using the χ2-test, or the Fisher's exact test where appropriate. OS was calculated from the starting date of first-line chemotherapy until death of any cause, or censored at last follow-up visit. Time-to-progression (TTP) was calculated from the starting date of first-line chemotherapy to the date of progression (per investigator assessment), or death from any cause. Survival data were analysed using the Kaplan–Meier product-limit method. Comparison of survival curves was carried out using the log-rank test. The first part of the analysis consisted of the univariate comparison of survival functions for factors that could potentially affect the survival time using the log-rank test. Then, we performed a multivariate analysis using stepwise Cox proportional hazards regression modelling. P-values <0.05 were considered statistically significant, and all P-values correspond to two-sided significance tests. Approval of the study was obtained from local research and ethics committees.
Results
The characteristics of 255 patients are presented in Table 1. Forty-nine patients (19%) had a histologically confirmed diagnosis of mucinous colorectal cancer. There were 153 male and 102 female patients, with a median age of 67 years (range, 43–89). Mucinous tumours were more frequently located into the right colon (55% compared with 29%, respectively; P=0.002). More patients in the mucinous group had ⩾2 metastatic sites compared with non-mucinous patients (47 and 33%, respectively; P=0.096). Liver, peritoneum, and lymph nodes were the most common metastatic sites in patients with mucinous cancers, whereas liver, lungs, and peritoneum were most common in patients with non-mucinous cancers. The peritoneum was more commonly noted in patients with mucinous colorectal cancer (39% compared with 12% of patients with non-mucinous tumours; P<0.001), whereas liver and lung metastases were found in 76 and 28% of the non-mucinous group compared with 53 and 12% of the mucinous group, respectively (P=0.002 and P=0.034, respectively). Only two patients had unresectable locally advanced disease.
Table 1. Patient characteristics.
| Characteristic | Non-mucinous (n=206) | Mucinous (n=49) | P-value | Overall (n=255) |
|---|---|---|---|---|
| Sex | ||||
| Male | 122 (59%) | 31 (63%) | 0.721 | 153 (60%) |
| Female | 84 (41%) | 18 (37%) | 102 (40%) | |
| Median age, years (range) | 67 (43–84) | 67 (45–89) | 0.927 | 67 (43–89) |
| Peformance status (ECOG) | ||||
| 0 | 102 (50%) | 21 (43%) | 0.263 | 123 (48%) |
| 1 | 91 (44%) | 22 (45%) | 113 (44%) | |
| 2 | 13 (6%) | 6 (12%) | 19 (8%) | |
| Primary tumour site | ||||
| Right-sided | 61 (29%) | 27 (55%) | 0.002 | 88 (34%) |
| Left-sided | 144 (70%) | 22 (45%) | 16 (65%) | |
| Synchronous primaries | 1 (1%) | 0 (0%) | 1 (1%) | |
| Grading | ||||
| 1 | 22 (11%) | 3 (6%) | 0.583 | 25 (10%) |
| 2 | 121 (59%) | 26 (53%) | 147 (57%) | |
| 3 | 52 (25%) | 14 (29%) | 66 (26%) | |
| Missing | 11 (5%) | 6 (12%) | 17 (7%) | |
| Resection of primary tumour | 144 (70%) | 31 (63%) | 0.466 | 175 (69%) |
| Earlier adjuvant chemotherapy | 74 (36%) | 19 (39%) | 0.835 | 93 (36%) |
| Number of metastatic sites | ||||
| 0–1 | 138 (67%) | 26 (53%) | 0.096 | 164 (64%) |
| ⩾2 | 68 (33%) | 23 (47%) | 91 (36%) | |
| Site of metastatic disease | ||||
| Liver | 157 (76%) | 26 (53%) | 0.002 | 183 (72%) |
| Peritoneum | 24 (12%) | 19 (39%) | <0.001 | 43 (17%) |
| Lymph node | 18 (9%) | 8 (16%) | 0.188 | 26 (10%) |
| Lung | 58 (28%) | 6 (12%) | 0.034 | 64 (25%) |
| CNS | 4 (2%) | 0 (%) | 4 (2%) | |
| Bone | 3 (1%) | 1 (2%) | 4 (2%) | |
| Other | 20 (10%) | 15 (30%) | 35 (14%) | |
| Haemoglobin level, g l−1 | 12.9 (8.2–17.4) | 12.2 (8.2–16.1) | 0.077 | 12.7 (8.2–17.4) |
| CEA, ng ml−1 | 18.4 (0–1084) | 14.4 (0.9–1151) | 0.851 | 17.0 (0–1151) |
| CA19-9, U ml−1 | 456 (0–20000) | 122 (3.2–48000) | 0.928 | 45.0 (0–48000) |
| First-line regimen | ||||
| IRI-based regimen | 56 (27%) | 9 (18%) | 0.730 | 65 (26%) |
| OXA-based regimen | 135 (66%) | 34 (70%) | 0.275 | 169 (66%) |
| IRI/OXA-based regimen | 15 (7%) | 6 (12%) | 21 (8%) | |
CEA=carcinoembryonic antigen; CNS=central nervous system; ECOG=Eastern Cooperative Oncology Group; IRI=irinotecan; OXA=oxaliplatin.
Chemotherapy regimens
Details of first-line regimens used for each patient are shown in Table 1. In all, 66% of patients were treated with OXA-based regimens, 26% of patients with IRI-based regimens, and 8% of patients with OXA/IRI-based chemotherapy. There was no significant difference between mucinous and non-mucinous groups in terms of treatment regimens. Moreover, no significant difference in the mean number of administered courses of first-line chemotherapy between mucinous and non-mucinous tumours (8 and 10 cycles, respectively) was encountered. A total of 143 (69%) patients with non-mucinous tumour (IRI-based=94 patients; OXA-based=33 patients; IRI/OXA-based=7 patients; and =9 patients) and 31 (63%) patients with mucinous tumours (IRI-based=22 patients; OXA-based=5 patients; IRI/OXA-based=2 patients; and other=2 patients) received second-line chemotherapy.
Treatment response
All the patients had measurable disease and were evaluated for response (Table 2A and B). Ten patients discontinued the treatment prematurately because of early disease progression, seven (3%) patients with non-mucinous, and three (6%) with mucinous cancer (P=NS). Fourteen patients achieved complete response and 96 patients achieved partial remission,; thus, the overall response rate was 43.1% (95% CI, 37.1–49.2). In the mucinous colorectal cancer group, nine patients reported a partial response for an overall response rate of 18.4% (95% CI, 7.5–29.2%). In the non-mucinous group, 14 patients achieved complete response and 87 achieved partial remission for an overall response rate of 49.0% (95% CI, 42.2–55.8%). The difference of response rate between the two groups was statistically significant (P=0.0002). On multivariate analysis (Table 3), patients with PS 0–1 (risk ratio, 6.06; 95% CI, 1.32–27.7; P=0.02), non-mucinous histology (risk ratio, 3.41; 95% CI, 1.53–7.61; P=0.002), and without peritoneal metastases (risk ratio, 2.70; 95% CI, 1.14–6.37; P=0.026) had a significantly increased probability of tumour response to chemotherapy.
Table 2. Response rate according to histology (A) and regimen of first-line chemotherapy (B).
|
(2A)
| |||
|---|---|---|---|
| Response | Mucinous (n=49) | Non-mucinous (n=206) | |
| Complete response | 0 | 14 (6.8%) | |
| Partial response | 9 (18.4%) | 87 (42.2%) | |
| Overall response rate, % (95% CI) | 18.4 (7.5–29.2) | 49.0 (42.2–55.8) | |
| Stable disease | 18 (36.7%) | 57 (27.7%) | |
| Progressive disease | 22 (44.9%) | 48 (23.3%) | |
|
(2B)
| |||
| Chemotherapy regimen | N | Mucinous responders a | Non-mucinous responders a |
| OXA-based | 169 | 6/34 (17.6%) | 57/135 (42.2%) |
| IRI-based | 65 | 1/9 (11.1%) | 32/56 (57.1%) |
| OXA/IRI-based | 21 | 2/6 (33.3%) | 12/15 (80.0%) |
CI=confidence interval; IRI=iriotecan; OXA=oxaliplatin.
Responders=complete plus partial responses according to the RECIST.
Table 3. Multivariate logistic regression model for tumour response to 1st line chemotherapy (n=255).
| Variable | Risk ratio (95% CI) | P-value |
|---|---|---|
| Performance status (ECOG) | ||
| 0–1 | 6.06 (1.32–27.7) | 0.020 |
| Histology | ||
| Nonmucinous | 3.41 (1.53–7.61) | 0.002 |
| No peritoneal metastases | 2.70 (1.14–6.37) | 0.026 |
ECOG=Eastern Cooperative Oncology Group; CI=confidence interval.
Survival
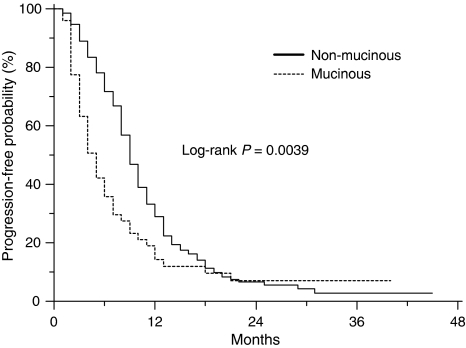
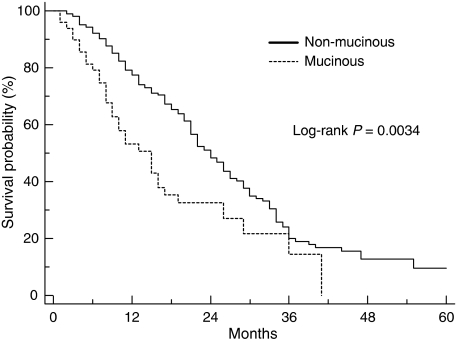
After a median follow-up of 45 months, the median TTP for mucinous colorectal cancer patients was 4.1 months compared with 8.6 months for the non-mucinous group (P=0.0039) (Figure 1). The HR for risk of progression for patients with mucinous colorectal cancer compared with non-mucinous tumours was 1.57 (95% CI, 1.21–2.74). The median OS for the mucinous colorectal cancer patients was 14.0 months compared with 23.4 months in the non-mucinous colorectal cancer group (HR=1.74; 95% CI, 1.27–3.31; P=0.0034; Figure 2). The 1-year OS was 53.1% (95% CI, 45.5–60.7%) for the mucinous group compared with 77.4% (95% CI, 74.4–80.4%) for the non-mucinous group.
Figure 1.
Time-to-progression for patients with mucinous and non-mucinous colorectal cancer (n=255).
Figure 2.
Overall survival for patients with mucinous and non-mucinous colorectal cancer (n=255).
The univariate analysis (Table 4) showed the other four variables to be significantly associated with poor survival: PS ECOG 2, number of metastatic sites ⩾2, peritoneal metastasis, and haemoglobin ⩽12 g l−1. Two variables, CEA and CA19-9, had missing data, and they were not included in the multivariate analysis. After correcting for significant prognostic factors by multivariate Cox regression analysis, mucinous histology was confirmed as poor prognostic factor (HR, 1.593; 95% CI, 1.05–2.40; P=0.0267; Table 5). Multivariate regression analysis (Table 5) also found PS ECOG 2, number of metastatic sites ⩾2, and peritoneal metastases to be negative independent prognostic factors.
Table 4. Factors associated with overall survival in the univariate analysis.
| Variable | n | MST (months) | 1-year-survival (%) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 153 | 21.2 | 74.3 | |
| Female | 102 | 22.6 | 70.9 | 0.4040 |
| Age | ||||
| ⩽65 years | 111 | 21.7 | 76.9 | |
| >65 years | 144 | 21.5 | 69.8 | 0.0828 |
| Performance status (ECOG) | ||||
| 0–1 | 236 | 23.0 | 76.9 | |
| 2 | 19 | 6.2 | 22.6 | <0.0001 |
| Primary tumor site | ||||
| Right-sided | 88 | 21.0 | 75.6 | |
| Left-sided | 166 | 21.5 | 71.6 | 0.5222 |
| Histology | ||||
| Nonmucinous | 206 | 23.4 | 77.4 | |
| Mucinous | 49 | 14.0 | 53.1 | 0.0034 |
| Previous adjuvant treatment | ||||
| Yes | 93 | 27 | 75,3 | |
| No | 162 | 20,7 | 71,6 | 0.0619 |
| Number of metastatic sites | ||||
| 0–1 | 164 | 25.2 | 80.0 | |
| ⩾2 | 91 | 14.2 | 59.9 | 0.0003 |
| Liver metastasis | ||||
| Yes | 183 | 21.8 | 75.6 | |
| No | 72 | 20.5 | 66.4 | 0.3537 |
| Peritoneal metastasis | ||||
| Yes | 43 | 11.3 | 47.9 | |
| No | 212 | 23.7 | 78.0 | <0.0001 |
| Nodal metastasis | ||||
| Yes | 26 | 14.6 | 61.7 | |
| No | 229 | 22.6 | 74.1 | 0.0581 |
| Lung metastasis | ||||
| Yes | 64 | 26.0 | 83.6 | |
| No | 191 | 20.6 | 69.5 | 0.190 |
| Haemoglobin level | ||||
| ⩽12 g l−1 | 90 | 18.0 | 61.2 | |
| >12 g l−1 | 165 | 22.4 | 79.0 | 0.0489 |
| CEA | ||||
| ⩽5 ng ml−1 | 79 | 24.0 | 79.8 | |
| >5 ng ml−1 | 153 | 20.8 | 69.7 | 0.0214 |
| CA19-9 | ||||
| ⩽30 U ml−1 | 117 | 26.3 | 80.9 | |
| >30 U ml−1 | 114 | 17.2 | 65.2 | 0.0004 |
ECOG=Eastern Cooperative Oncology Group; CEA=carcinoembryonic antigen; MST=median survival time.
Table 5. Factors associated with a poor overall survival in mutivariate analysis (n=255).
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Performance status (ECOG) | ||
| 2 | 3.526 (2.07–5.99) | <0.0001 |
| Histology | ||
| Mucinous | 1.593 (1.05–2.40) | 0.0267 |
| Number of metastatic sites | ||
| ⩾2 | 1.472 (1.04–2.08) | 0.0300 |
| Peritoneal metastasis | ||
| Yes | 1.588 (1.01–2.49) | 0.0461 |
| Haemoglobin level | ||
| ⩽12 g l−1 | 1.123 (0.81–1.55) | 0.4870 |
ECOG=Eastern Cooperative Oncology Group; CI=confidence interval.
Discussion
It is generally recognised that mucinous tumours of the colon and rectum have a worse prognosis than non-mucinous tumours (Umpleby et al, 1985; Green et al, 1993; Consorti et al, 2000), and they occur more frequently in the proximal colon (Umpleby et al, 1985; Green et al, 1993; Sarli et al, 2008), metastasise with high frequency to lymph nodes and to the peritoneum (Umpleby et al, 1985; Minsky et al, 1987), are more prone to local recurrence (Umpleby et al, 1985), and are typically diagnosed at an advanced stage (Green et al, 1993; Younes et al, 1993).
Published literature presents conflicting results on the association between worse prognosis and mucinous histology of colorectal cancer. However, the interpretation of results may be arduous for many reasons. Data are mostly derived from series that assessed the prognosis of patients treated with curative surgery alone, at different stages of disease, and did not evaluate closely a role for chemotherapy in this subset of patients (Green et al, 1993; Younes et al, 1993; Consorti et al, 2000; Kanemitsu et al, 2003). Moreover, the geographical variations in the epidemiology of mucinous colorectal cancer may likely account for the conflicting results (Sarli et al, 2008).
Our analysis showed a highly statistically significant poor survival for patients with mucinous tumours compared those with non-mucinous tumours (14 months vs 23.4 months, respectively). All the patients included in the present analysis had advanced colorectal cancer and were treated with first-line chemotherapy containing IRI and/or OXA in addition to fluoropyrimidines, considered as standard drugs for this disease at that time. Characteristics of patients were well balanced according to the different clinicopathological variables, except for a higher proportion of patients with mucinous colorectal cancer who had peritoneal metastases and were right-sided. Conversely, more patients with non-mucinous tumours had liver and lung metastases. These findings were also found in earlier studies (Umpleby et al, 1985; Minsky et al, 1987; Negri et al, 2005), and, to our opinion, these imbalances cannot justify the poor prognosis of patients with mucinous carcinomas. Moreover, the multivariate analysis confirmed the independent poor prognostic role of mucinous histology (HR 1.593, 95% CI 1.05–2.40; P=0.0267), together with PS, number of metastatic sites, and peritoneal metastasis.
In advanced colorectal cancer, the less responsiveness to first-line chemotherapy of mucinous tumours compared with non-mucinous tumours has been only recently reported in a case–control study by the group of the Royal Marsden Hospital (Negri et al, 2005). Our aim was to confirm the same results in a similar subset of patients, but receiving IRI and OXA in addition to fluoropyrimidines as first-line chemotherapy. Mucinous colorectal cancers had a response rate to IRI and/or OXA-based chemotherapy of 18.4% compared with 49% for non-mucinous tumours (P=0.0002). Logistic regression analysis revealed that histology, namely, mucinous, together with bad PS, and peritoneal metastasis were independent predictive factors for poor response.
The mechanisms that lead to this significant difference in IRI, OXA, and fluoropyrimidine sensitivity of mucinous tumours compared with non-mucinous tumours are unknown. Mucinous tumours have been characterised by a number of genetic and biological features, which may explain in part the different behaviour compared with that of non-mucinous tumours. Mucinous colorectal carcinomas have higher incidence of high degree of microsatellite instability (MSI-H) (Kakar et al, 2004; Song et al, 2005; Ogino et al, 2006; Tanaka et al, 2006; Sarli et al, 2008), K-ras mutation (Zhang et al, 1999; Bazan et al, 2002; Ogino et al, 2006), BRAF mutation (Song et al, 2005; Li et al, 2006; Ogino et al, 2006; Tanaka et al, 2006), and less expression of p53 (Zhang et al, 1999; Ogino et al, 2006) than do non-mucinous colorectal cancer. Given the unclear significance and heterogeneity of mucinous colorectal cancers, it seems difficult to find possible explanations for the relatively chemoresistance of such tumours. It is recognised that defective DNA mismatch repair (MMR) leads to MSI and results in resistance to many antineoplastic drugs, such as antimetabolites, alkylating, and platinum agents, and inhibitors of topoisomerases. There are some evidences in vitro that suggest a correlation between response to 5-FU, OXA, and IRI and MSI (Magrini et al, 2002; Arnold et al, 2003; Warusavitarne and Schnitzler, 2007). Our analysis plan did not include an MSI analysis. First, we focused on the clinical role of mucinous histology without performing molecular analysis, as the simple knowledge on the histopathological mucinous feature may be per se a relevant information. Second, two recently published studies (Braun et al, 2008; Müller et al, 2008) have shown low MSI in colorectal carcinomas (about 4%), and no significant association with treatment (OXA- and IRI-based) outcomes in terms of response rate and OS; therefore, the use of this marker may be of limited value. The discrepancy between such a low percentage of MSI and the higher percentage of MSI reported in earlier studies might be the result of many reasons, such as a variable definition of MSI-H, the use of different markers, or a consequence of the selection of patients (Popat et al, 2005; Müller et al, 2008).
Recently, Glasgow et al (2005) analysed some molecular markers for response to chemotherapy in mucinous and non-mucinous Dukes C colorectal cancer. The authors found an overexpression of TS and GSTP1 (glutathione S-transferase pi) genes in mucinous tumours. As GSTP1 is a major rout of detoxification of platinum agents, one could expect that the overexpression of TS and GSTP1 genes in mucinous tumours may be responsible for decreased clinical response to treatment with 5-FU and OXA.
Several markers, oncogenes and suppressor genes, multidrug-resistance-related proteins, and genomic polymorphisms that influence DNA metabolism, DNA damage, programmed cell death, and angiogenesis may be responsible of colorectal cancer patient's variation in response to chemotherapy. It is important to stress that before clinical application, any biomarker need to be independently validated. Moreover, for mucinous carcinomas, many of the above reported biomarkers may differently be expressed in each tumour, and this could make very difficult to potentially predict response to chemotherapy for this heterogeneous tumours.
Another key point of discussion is the integration of conventional cytotoxic agents with novel biologic agents. Targeted agents enhance the efficacy of conventional cytotoxic agents (Goldberg et al, 2007). We actually lack data investigating the outcome of patients with mucinous colorectal cancer treated with novel agents and traditional drugs (fluoropyrimidines, IRI, and OXA).
Despite the possible limitations of this retrospective analysis, the observations of this study are consistent with those of the group of the Royal Marsden Hospital (Negri et al, 2005), which showed that mucinous advanced colorectal cancer patients treated with first-line fluoropyirimidine-based chemotherapy have similar response rate and OS. However, these retrospective analyses were based on a rather small number of patients. It would be very worthwhile to try to confirm these findings in an analysis of large, prospective, randomised trials, such as N9741, NO16966, and so on. The informations derived on mucinous histology may help researchers and practitioners in designing future studies (e.g., stratification of patients according to the mucinous histology) and in making clinical decisions that will improve the outcome of patients with such histological type. Trying to identify subsets of patients who are likely to derive more benefit from a particular treatment not only helps to derive greater efficacy, but also spares many patients from unnecessary toxicity.
In conclusion, this retrospective analysis on advanced colorectal cancer showed poor responsiveness and prognosis for patients with mucinous colorectal cancer treated with first-line chemotherapy containing 5-FU, IRI, and OXA. Further investigations should be carried out to better characterise the genetic profile and the pharmacological markers, which could explain the unfavourable responsiveness and prognosis of mucinous colorectal tumours. Assessment of clinical outcome of mucinous colorectal cancer treated with cytotoxic drugs and novel agents is highly warranted.
References
- Arnold CN, Goel A, Boland CR (2003) Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer 106: 66–73 [DOI] [PubMed] [Google Scholar]
- Bazan V, Migliavacca M, Zanna I, Tubiolo C, Grassi N, Latteri MA, La Farina M, Albanese I, Dardanoni G, Salerno S, Tomasino RM, Labianca R, Gebbia N, Russo A (2002) Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol 13: 1438–1446 [DOI] [PubMed] [Google Scholar]
- Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, Parmar MKB, Seymour MT (2008) Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol 26: 2690–2698 [DOI] [PubMed] [Google Scholar]
- Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP (2000a) American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 88: 1739–1757 [DOI] [PubMed] [Google Scholar]
- Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C (2000b) Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124: 979–994 [DOI] [PubMed] [Google Scholar]
- Connelly JH, Robey-Cafferty SS, Cleary KR (1991) Mucinous carcinomas of the colon and rectum. An analysis of 62 stage B and C lesions. Arch Pathol Lab Med 115: 1022–1025 [PubMed] [Google Scholar]
- Consorti F, Lorenzotti A, Midiri G, Di Paola M (2000) Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case–control study. J Surg Oncol 73: 70–74 [DOI] [PubMed] [Google Scholar]
- Enriquez JM, Diez M, Tobaruela E, Lozano O, Domínguez P, González A, Mugüerza JM, Ratia T (1998) Clinical, histopathological, cytogenetic and prognostic differences between mucinous and nonmucinous colorectal adenocarcinomas. Rev Esp Enferm Dig 90: 563–572 [PubMed] [Google Scholar]
- Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL (2005) Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer 92: 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RM, Rothenberg ML, Van Cutsem E, Benson III AB, Blanke CD, Diasio RB, Grothey A, Lenz HJ, Meropol NJ, Ramanathan RK, Becerra CH, Wickham R, Armstrong D, Viele C (2007) The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist 12: 38–50 [DOI] [PubMed] [Google Scholar]
- Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright Jr JB, Ray JE (1993) Mucinous carcinoma—just another colon cancer? Dis Colon Rectum 36: 49–54 [DOI] [PubMed] [Google Scholar]
- Grothey A, Marshall JL (2007) Optimizing palliative treatment of metastatic colorectal cancer in the era of biologic therapy. Oncology 21: 553–564 [PubMed] [Google Scholar]
- Grothey A, Sargent D, Goldberg RM, Schmoll HJ (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22: 1209–1214 [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Aaltonen LA (2000) Pathology and Genetics, Tumours of the Digestive System. World Health Organization Classification of Tumours, 3rd edn. IARC: Lyon [Google Scholar]
- Hanski C (1995) Is mucinous carcinoma of the colorectum a distinct genetic entity? Br J Cancer 72: 1350–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar S, Aksoy S, Burgart L, Smyrk T (2004) Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol 17: 696–700 [DOI] [PubMed] [Google Scholar]
- Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, Kodera Y, Yamamura Y (2003) Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum 46: 160–167 [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim JW, Cho JH, Baek SH, Kakar S, Kim GE, Sleisenger MH, Kim YS (2005) Expression of mucin core proteins, trefoil factors, APC and p21 in subsets of colorectal polyps and cancers suggests a distinct pathway of pathogenesis of mucinous carcinoma of the colorectum. Int J Oncol 27: 957–964 [PubMed] [Google Scholar]
- Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B (2006) BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrini R, Bhonde MR, Hanski ML, Notter M, Scherübl H, Boland CR, Zeitz M, Hanski C (2002) Cellular effects of CPT-11 on colon carcinoma cells: dependence on p53 and hMLH1 status. Int J Cancer 101: 23–31 [DOI] [PubMed] [Google Scholar]
- Minsky BD, Mies C, Rich TA, Recht A, Chaffey JT (1987) Colloid carcinoma of the colon and rectum. Cancer 60: 3103–3112 [DOI] [PubMed] [Google Scholar]
- Müller CI, Schulmann K, Reinacher-Schick A, Andre N, Arnold D, Tannapfel A, Arkenau H, Hahn SA, Schmoll SH, Porschen R, Schmiegel W, Graeven U (2008) Predictive and prognostic value of microsatellite instability in patients with advanced colorectal cancer treated with a fluoropyrimidine and oxaliplatin containing first-line chemotherapy. A report of the AIO Colorectal Study Group. Int J Colorectal Dis 23: 1033–1039 [DOI] [PubMed] [Google Scholar]
- Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ (2005) Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 16: 1305–1310 [DOI] [PubMed] [Google Scholar]
- Ogino S, Brahmandam M, Cantor M, Clark JW, Ryan DP, Kulke MH, Enzinger PC, Wolpin BM, Loda M, Fuchs CS (2006) Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol 19: 59–68 [DOI] [PubMed] [Google Scholar]
- Parham D (1923) Colloid carcinoma. Ann Surg 77: 90–105 [PMC free article] [PubMed] [Google Scholar]
- Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23: 609–618 [DOI] [PubMed] [Google Scholar]
- Sarli L, Bottarelli L, Azzoni C, Di Cola G, Barilli AL, Costi R, Mazzeo A, Salvemini C, Porrini C, Cecchini S, Taglia M, Roncoroni L, Bordi C (2008) Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol 15: 1429–1439 [DOI] [PubMed] [Google Scholar]
- Secco GB, Fardelli R, Campora E, Lapertosa G, Gentile R, Zoli S, Prior C (1994) Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology 51: 30–34 [DOI] [PubMed] [Google Scholar]
- Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS (2005) Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol 26: 745–750 [PubMed] [Google Scholar]
- Symonds DA, Vickery AL (1976) Mucinous carcinoma of the colon and rectum. Cancer 37: 1891–1900 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, Sleisenger MH, Kim YS (2006) BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer 118: 2765–2771 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Umpleby HC, Ranson DL, Williamson RC (1985) Peculiarities of mucinous colorectal carcinoma. Br J Surg 72: 715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warusavitarne J, Schnitzler M (2007) The role of chemotherapy in microsatellite unstable (MSI-H) colorectal cancer. Int J Colorectal Dis 22: 739–748 [DOI] [PubMed] [Google Scholar]
- Younes M, Katikaneni PR, Lechago J (1993) The value of the preoperative mucosal biopsy in the diagnosis of colorectal mucinous adenocarcinoma. Cancer 72: 3588–3592 [DOI] [PubMed] [Google Scholar]
- Zhang H, Evertsson S, Sun X (1999) Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol 14: 1057–1061 [DOI] [PubMed] [Google Scholar]