Abstract
Among 242 Japanese pancreatic cancer patients, three patients (1.2%) encountered life-threatening toxicities, including myelosuppression, after gemcitabine-based chemotherapies. Two of them carried homozygous CDA*3 (CDA208G>A [Ala70Thr]), and showed extremely low plasma cytidine deaminase activity and gemcitabine clearance. Our results suggest that homozygous *3 is a major factor causing gemcitabine-mediated severe adverse reactions among the Japanese population.
Keywords: gemcitabine, toxicity, CDA208G>A, pancreatic cancer, pharmacogenomics, polymorphism
Gemcitabine (2′,2′-difluorodeoxycytidine) is a nucleoside anticancer drug for various solid tumours (Noble and Goa, 1997). Gemcitabine exerts its cytotoxic effect through phosphorylation by nucleotide kinases, including the deoxycytidine kinase (DCK), whereas most of the administered gemcitabine is rapidly degraded by cytidine deaminase (CDA) into its inactive metabolite, 2′,2′-difluorodeoxyuridine (Plunkett et al, 1995). Various genetic variations have recently been reported in human DCK and CDA genes (Ueno et al, 2007).
Our earlier prospective pharmacogenetic study using 256 Japanese cancer patients treated with gemcitabine-based chemotherapies revealed that one of the CDA single-nucleotide polymorphisms (SNPs), CDA*3 (CDA208G>A [Ala70Thr], rs60369023), showed significant associations with reduced CDA activity, reduced gemcitabine clearance, increased gemcitabine area under the concentration–time curve (AUC), and an increased incidence of severe neutropaenia (Sugiyama et al, 2007). Most notably, one patient who had developed life-threatening toxicities, including severe myelosuppression, was found to be homozygous for CDA*3 (CDA*3/*3), and excessive exposure to gemcitabine was considered responsible for the severe toxicities (Yonemori et al, 2005; Sugiyama et al, 2007).
Owing to a low allele frequency of CDA*3 (3.7% in the Japanese population), only one homozygous patient was found in the earlier study, necessitating further examination. For this purpose, we have carefully monitored toxicities in gemcitabine-treated patients in the National Cancer Center Hospital for 4.5 years. Three patients with life-threatening adverse reactions, including serious myelosuppression, were identified, and their CDA genotypes, plasma CDA activities, and pharmacokinetic parameters (when available) were determined and compared with those of the earlier cases.
Patients and methods
The ethics committees of the National Cancer Center and the National Institute of Health Sciences approved this study. Written informed consent was obtained from each participant. Of 176 and 66 pancreatic cancer patients who received gemcitabine monotherapy and gemcitabine-based combination chemotherapies, respectively, at the National Cancer Center Hospital between 1 September 2003 and 31 March 2008, three showed severe and prolonged myelosuppression with other complications. Characteristics of the three patients designated A, B, and C are summarised in Table 1. All these patients received a 30 min intravenous gemcitabine infusion at an initiation dose of 1000 mg m−2. Patient A initially received gemcitabine and S-1 combination therapy, whereas patients B and C were given gemcitabine alone.
Table 1. Patient characteristics at baseline.
| Patient A | Patient B | Patient C | |
|---|---|---|---|
| Sex | Female | Male | Female |
| Age (years) | 57 | 70 | 70 |
| Performance status | 1 | 2 | 1 |
| Stagea | IV | IV | IV |
| Previous treatment | Surgery | None | None |
| Body surface area (m2) | 1.3 | 1.9 | 1.1 |
| Laboratory data | |||
| Leukocyte (mm−3) | 6300 | 9700 | 4300 |
| Neutrophil (mm−3) | 4200 | 6400 | 2700 |
| Haemoglobin (g dl−1) | 12.4 | 16.0 | 10.8 |
| Platelet (mm−3) | 116 000 | 163 000 | 185 000 |
| Total bilirubin (mg dl−1) | 0.6 | 1.4 | 0.8 |
| ALT (IU l−1) | 26 | 35 | 24 |
| Creatinine (mg dl−1) | 0.6 | 1.2 | 0.4 |
| Initial regimen | Gemcitabine+S-1b | Gemcitabine alone | Gemcitabine alone |
ALT=alanine aminotransferase.
UICC sixth edition.
An oral product containing tegafur, gimeracil, and oteracil potassium.
Measurement of plasma CDA activity towards gemcitabine and genotyping of CDA and DCK were carried out in the three patients as reported earlier (Sugiyama et al, 2007; Kim et al, 2008). After recovery from severe adverse reactions, chemotherapy was resumed in two patients: patient A received gemcitabine monotherapy instead of gemcitabine plus S-1, whereas the gemcitabine dose was reduced in patient C. Gemcitabine was not resumed in patient B because of disease progression. Pharmacokinetics were carried out when 1000 and 450 mg m−2 of gemcitabine were administered to patients A and C, respectively. Blood sampling schedule and the measurement method of gemcitabine in plasma were reported earlier (Sugiyama et al, 2007). Pharmacokinetic parameters were estimated using WinNonlin ver 4.01 (Pharsight Corporation, Mountain View, CA, USA).
Results
Although 30 patients (about 12.4%) among the 242 developed grade 4 neutropaenia, the toxicity was transient and required no supportive treatment in most patients except in patients A, B, and C. Pretreatment organ functions, including bone marrow, renal, and hepatic functions, were preserved in the three patients (Table 1). Observed toxicities in the patients are summarised in Table 2. The serious haematotoxicities requiring intensive supportive treatments during hospitalisation were recognised in these patients: patient A was treated with antibiotics because of febrile neutropaenia, patient B received a platelet transfusion, and patient C received a red blood cell transfusion, a platelet transfusion, and a granulocyte colony-stimulating factor. Both the neutrophil and platelet nadir appeared at approximately day 15 of the first course of treatment in patients A and B, whereas in patient C, the nadir occurred on day 15 of the second course of treatment that was resumed after reducing the dose of gemcitabine. The symptomatic non-haematologic toxicities shown in Table 2 appeared before severe myelosuppression.
Table 2. Toxicities, treatment, genotype, and PK analysis.
|
Patient A
|
Patient B
|
Patient C
|
||||
|---|---|---|---|---|---|---|
| Grade | Value | Grade | Value | Grade | Value | |
| Haematologic toxicities a | ||||||
| Leukocyte (mm−3) | 4 | 800 | 3 | 1100 | 3 | 1000 |
| Neutrophil (mm−3) | 4 | 200 | 4 | 300 | 4 | 100 |
| Haemoglobin (g dl−1) | 2 | 8.1 | 1 | 13.2 | 4 | 6.3 |
| Platelet (mm−3) | 3 | 26 000 | 4 | 10 000 | 3 | 28 000 |
| Non-haematologic toxicity a | ||||||
| Fatigue | 2 | 3 | 2 | |||
| Anorexia | 3 | 3 | 2 | |||
| Diarrhoea | 3 | 0 | 1 | |||
| Stomatitis | 3 | 0 | 0 | |||
| Rash | 2 | 0 | 2 | |||
| Febrile neutropaenia | 3 | 0 | 0 | |||
| Treatment | ||||||
| Resumption of chemotherapy | Yes | No | Yes | |||
| Total number of gemcitabine doses | 10 | 2 | 10 | |||
| Final dose (mg m−2) | 600 | 1000 | 270 | |||
| Genotype | ||||||
| CDA haplotypeb | *1a/*1j | *3a/*3a | *3a/*3a | |||
| DCK haplotypec | *1a/*1a | *1a/*1a | *1a/*1a | |||
| PK analysis | ||||||
| Regimen at PK study | Gemcitabine alone | Gemcitabine alone | ||||
| Dose of gemcitabine (mg m−2) | 1000 | 450 | ||||
| Cmax (mg l−1) | 29.0 | Not available | 22.5 (49.7d) | |||
| AUC (mg h l−1) | 16.2 | 26.8 (59.0d) | ||||
| Clearance (l h−1 m−2) | 61.8 | 16.6 | ||||
AUC=area under the concentration–time curve; Cmax=maximum plasma concentration; CDA=cytidine deaminase; DCK=deoxycytidine kinase; PK=pharmacokinetics.
Toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
CDA haplotype was reported earlier in Sugiyama et al (2007).
DCA haplotype was reported earlier in Kim et al (2008).
On the basis of the assumption that patient C received 1000 mg m−2 of gemcitabine.
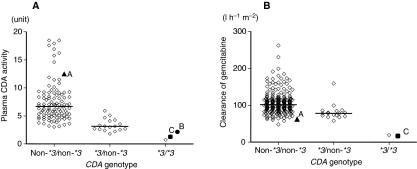
Patients B and C were found to be CDA*3/*3, whereas patient A did not have CDA*3 (Table 2). No SNPs of DCK, including DCK364C>T (Pro122Ser), which were reported to have reduced enzymatic activity (Lamba et al, 2007), were identified in our three patients. Plasma CDA activities of patients A, B, and C were compared with those of 121 patients in our earlier study (Sugiyama et al, 2007) (Figure 1A). Patient A without CDA*3 showed relatively high plasma CDA activity, whereas plasma CDA activities in the CDA*3/*3 patients (patients B and C) were comparably low to those in the earlier reported CDA*3/*3 patient.
Figure 1.
Effects of CDA*3 on (A) plasma CDA activity towards gemcitabine and (B) gemcitabine clearance. The data obtained earlier (Sugiyama et al, 2007) are expressed as open diamonds, and those obtained in this study are as closed symbols. A, B, and C represent patients A, B, and C, respectively. Lines represent median values for non-*3/non-*3 patients and *3/non-*3 patients.
Pharmacokinetic parameters of patients A and C were also shown in Table 2, and their gemcitabine clearances were compared with those of the earlier reported 250 patients (Sugiyama et al, 2007) (Figure 1B). Although the gemcitabine dose for patient C was low (450 mg m−2), her gemcitabine AUC was higher than the average value in the CDA*3-negative patients who were administered 1000 mg m−2 gemcitabine. When it was assumed that patient C received 1000 mg m−2 of gemcitabine, Cmax and AUC values similar to those observed in the earlier reported CDA*3/*3 patient were obtained. The value of gemcitabine clearance in patient C (CDA*3/*3) was less than one-fifth of the median value obtained earlier from CDA*3-negative patients. The clearance observed in patient A, who did not have CDA*3, was within the range obtained earlier from patients without CDA*3 (Figures 1B).
Discussion
We found two (patients B and C) of the three patients who experienced life-threatening adverse reactions to be homozygous for CDA*3 and to have an extremely low CDA activity. Taken together with our earlier observations (Yonemori et al, 2005; Sugiyama et al, 2007), these life-threatening adverse reactions appear to have been caused by reduced deamination activity of CDA because of CDA*3/*3 homozygosity. Sustained plasma gemcitabine elevations are most likely responsible for these severe adverse reactions. To date, we have had three CDA*3/*3 patients, including one reported earlier (Yonemori et al, 2005; Sugiyama et al, 2007), and all experienced life-threatening severe adverse reactions. As genotyping of CDA was not carried out in the remaining 239 patients, we may have overlooked patients with CDA*3/*3 who did not develop severe toxicities in this study. However, as the frequency of homozygous CDA*3 in the Japanese population was estimated to be 0.14% in an earlier study (Sugiyama et al, 2007), the possibility of missing any such patients would be very low. Thus, CDA*3/*3 is a potentially important biomarker for Japanese patients and for at least one African ethnic group (Fukunaga et al, 2004) for predicting severe gemcitabine-mediated adverse reactions including myelotoxicities.
We do not have sufficient pharmacokinetic data on CDA*3/*3 to determine the optimal dose for this fraction of the patient population. However, the clearance and AUC values for the earlier reported patient (Sugiyama et al, 2007) and for patient C (dose adjusted), and the final dose for patient C (270 mg m−2) suggest that a 75% reduction in the gemcitabine dose, at treatment initiation, may be appropriate for CDA*3/*3 patients.
Patient A was given combined chemotherapy with oral S-1 (Table 1). As she could not tolerate gemcitabine monotherapy at the standard dose as shown in Table 2, gemcitabine itself appears to have been responsible for the life-threatening toxicities in this patient. Her gemcitabine clearance was within the range observed in patients without CDA*3 (Sugiyama et al, 2007). Therefore, we concluded that there was no involvement of altered CDA activity in the severe neutropaenia experienced by patient A. Further investigation of gemcitabine pathway genotypes is needed to clarify factors contributing to this patient's adverse reactions.
In conclusion, in the Japanese population, CDA*3/*3 is a major cause of gemcitabine-mediated life-threatening adverse reactions including myelosuppression. A substantial gemcitabine dose reduction is necessary for patients who are homozygous for CDA*3.
Acknowledgments
We thank Ms Emi Toshiro and Ms Tomoko Chujo for their assistance in sample collection and management. This study was supported in part by the Program for the Promotion of Fundamental Studies in Health Sciences from the National Institute of Biomedical Innovation (NiBio), and the Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare.
Footnotes
Conflict of interest
Dr Saijo reported receiving honoraria from Eli Lilly. None of the other authors reported financial interest.
References
- Fukunaga AK, Marsh S, Murry DJ, Hurley TD, McLeod HL (2004) Identification and analysis of single-nucleotide polymorphisms in the gemcitabine pharmacologic pathway. Pharmacogenomics J 4: 307–314 [DOI] [PubMed] [Google Scholar]
- Kim SR, Saito Y, Maekawa K, Sugiyama E, Kaniwa N, Ueno H, Okusaka T, Ikeda M, Morizane C, Yamamoto N, Yoshida T, Kamatani N, Furuse J, Ishii H, Saijo N, Ozawa S, Sawada J (2008) Twenty novel genetic variations and haplotype structures of the DCK gene encoding human deoxycytidine kinase (dCK). Drug Metab Pharmacokinet 23: 379–384 [DOI] [PubMed] [Google Scholar]
- Lamba JK, Crews K, Pounds S, Schuetz EG, Gresham J, Gandhi V, Plunkett W, Rubnitz J, Ribeiro R (2007) Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther 323: 935–945 [DOI] [PubMed] [Google Scholar]
- Noble S, Goa KL (1997) Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 54: 447–472 [DOI] [PubMed] [Google Scholar]
- Plunkett W, Huang P, Gandhi V (1995) Preclinical characteristics of gemcitabine. Anticancer Drugs 6(Suppl 6): 7–13 [DOI] [PubMed] [Google Scholar]
- Sugiyama E, Kaniwa N, Kim SR, Kikura-Hanajiri R, Hasegawa R, Maekawa K, Saito Y, Ozawa S, Sawada J, Kamatani N, Furuse J, Ishii H, Yoshida T, Ueno H, Okusaka T, Saijo N (2007) Pharmacokinetics of gemcitabine in Japanese cancer patients: the impact of a cytidine deaminase polymorphism. J Clin Oncol 25: 32–42 [DOI] [PubMed] [Google Scholar]
- Ueno H, Kiyosawa K, Kaniwa N (2007) Pharmacogenomics of gemcitabine: can genetic studies lead to tailor-made therapy? Br J Cancer 97: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori K, Ueno H, Okusaka T, Yamamoto N, Ikeda M, Saijo N, Yoshida T, Ishii H, Furuse J, Sugiyama E, Kim SR, Kikura-Hanajiri R, Hasegawa R, Saito Y, Ozawa S, Kaniwa N, Sawada J (2005) Severe drug toxicity associated with a single-nucleotide polymorphism of the cytidine deaminase gene in a Japanese cancer patient treated with gemcitabine plus cisplatin. Clin Cancer Res 11: 2620–2624 [DOI] [PubMed] [Google Scholar]