Abstract
The melanocortin receptor (MCR) family consists of 5 G protein-coupled receptors (MC1R–MC5R) with diverse physiologic roles. MC2R is a critical component of the hypothalamic–pituitary–adrenal axis, whereas MC3R and MC4R have an essential role in energy homeostasis. Mutations in MC4R are the single most common cause of monogenic obesity. Investigating the way in which these receptors signal and traffic to the cell membrane is vital in understanding disease processes related to MCR dysfunction. MRAP is an MC2R accessory protein, responsible for adrenal MC2R trafficking and function. Here we identify MRAP2 as a unique homologue of MRAP, expressed in brain and the adrenal gland. We report that MRAP and MRAP2 can interact with all 5 MCRs. This interaction results in MC2R surface expression and signaling. In contrast, MRAP and MRAP2 can reduce MC1R, MC3R, MC4R, and MC5R responsiveness to [Nle4,D-Phe7]alpha-melanocyte-stimulating hormone (NDP-MSH). Collectively, our data identify MRAP and MRAP2 as unique bidirectional regulators of the MCR family.
Keywords: GPCR accessory proteins, receptor signalling, receptor trafficking
The melanocortin receptor (MCR) family is involved in a diverse range of physiologic and disease processes (1). MC1R is important in pigmentation, MC2R in steroidogenesis, and MC5R has an exocrine function especially in sebaceous gland secretion. MC3R and MC4R are both highly expressed in the brain and play key roles in energy homeostasis. Mutations in MC4R are the most common cause of monogenic obesity. More recently, fat mass, weight, risk of obesity, and insulin resistance were associated with common variants near the MC4R locus (2, 3). Both MC4R knockout mice and humans with MC4R mutations display early-onset obesity associated with hyperphagia (4, 5). MC3R knockout mice, however, develop a milder phenotype with later-onset obesity (6, 7).
We previously identified MRAP (melanocortin-2-receptor accessory protein), a small transmembrane protein, as an MC2R accessory protein, enabling the functional expression of MC2R in transfected cells. The identification of MRAP provides a molecular explanation for the difficulties encountered in the expression of the MC2R in nonadrenal cell lines (8). Furthermore, mutations in MRAP result in the autosomal recessive disorder familial glucocorticoid deficiency type 2 (9).
Here we report the identification and characterization of a unique MRAP homologue encoded by C6orf117 on human chromosome 6q14.3, which we have named MRAP2. We show that both MRAP and MRAP2 can modulate the signaling of all 5 MCRs. MRAP2 is primarily expressed in human brain and adrenal gland. In the brain, MRAP2 expression is seen in the hypothalamus (10), a site that also expresses a high level of MC3R and MC4R (6, 11). MRAP expression in the hypothalamus has also been demonstrated by in situ hybridization (12). These findings suggest that MRAP and MRAP2 may regulate MC3R and MC4R function in the central nervous system.
Results
MRAP2: A Unique Homologue of MRAP.
The human MRAP2 gene consists of 4 exons, and its protein product comprises 205 aa residues, with a predicted molecular mass of 23.5 kDa (Fig. 1A). MRAP2 is homologous to MRAP, with 39% amino acid identity to MRAP in the N-terminal and transmembrane domains (Fig. 1B). The protein is highly conserved through vertebrates (supporting information Fig. S1) and like MRAP has no predicted signal sequence.
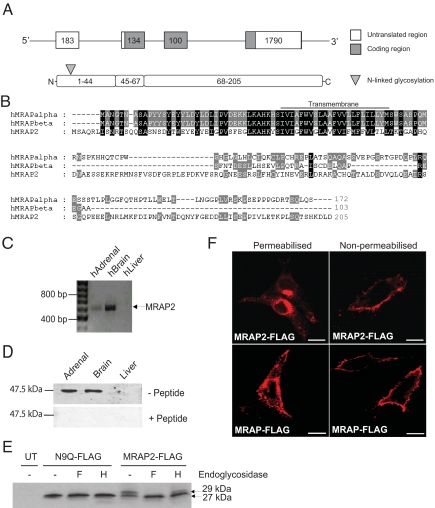
Fig. 1.
MRAP2 encodes a glycosylated single transmembrane protein expressed in the brain and adrenal gland. (A) Schematic diagram of the human MRAP2 gene and protein. (B) Protein alignment of human MRAP isoforms and human MRAP2. The highest level of sequence identity is in the N-terminal and transmembrane domains. Transmembrane domain is underlined. (C) MRAP2 mRNA expression in human tissues was determined by RT-PCR analysis. Full-length MRAP2 mRNA (618 bp) was detected in human adrenal and brain. (D) Western blot analysis of mouse tissues using antiserum rMRAP2189–204. An immunoreactive band with a molecular mass of approximately 48 kDa was seen, which disappeared with peptide competition. The size of the bands seen by Western blot is consistent with an MRAP2 dimer, resistant to denaturing and reducing conditions. (E) MRAP2 undergoes N-linked glycosylation at the N terminus. Immunoblot analysis of MRAP2-FLAG transfected cells reveals 2 protein species, 27 and 29 kDa in size. Treatment with endoglycosidases PNGaseF (F) and EndoH (H) results in the removal of the 29-kDa band. The N9Q-FLAG construct formed by site-directed mutagenesis of the putative glycosylation site resulting in the substitution of the asparagine residue to a glutamine residue, was seen as a single band on Western blotting. UT, untransfected cells. (F) MRAP2-FLAG is seen at the cell surface and ER by confocal microscopy and shows similar localization to MRAP-FLAG. (Scale bars, 10 μm.)
MRAP2 Is Expressed in the Brain and Adrenal Gland.
MRAP2 gene expression was assessed using a panel of cDNAs derived from human tissues (13). MRAP2 expression was detected by RT-PCR in the adrenal gland and brain (Fig. 1C). No expression was seen in the other tissues analyzed (Fig. S2). PCR products were sequenced to confirm MRAP2 transcript. MRAP2 protein expression was detected in mouse brain and adrenal using the rMRAP2189–204 antiserum (Fig. 1D). On Western blotting a single large-molecular-mass band of approximately 48 kDa was detected. This molecular mass is consistent with a dimeric MRAP2 structure, resistant to denaturing and reducing conditions. Furthermore, these data are also consistent with the denaturation-resistant dimerization of endogenous MRAP, which is seen in mouse Y1 cells (14).
MRAP2 Is a Glycosylated Protein.
CHO cells (which have no endogenous expression of MRAP or MRAP2) were transfected with a MRAP2-FLAG construct, and Western blotting for FLAG revealed bands at approximately 27 and 29 kDa (Fig. 1E). A putative N-linked glycosylation site, with the consensus sequence NRTS, was identified at amino acid positions 9–12. Treatment with the endoglycosidases peptide N-glycosidase F (PNGaseF) and endoglycosidase H (EndoH) resulted in increased mobility of the higher-molecular-mass band, indicating that MRAP2 is a glycosylated protein. To confirm the putative glycosylation site, site-directed mutagenesis was undertaken to replace the asparagine at position 9 with a glutamine residue (N9Q-FLAG). A predicted single 27-kDa band was detected by Western blotting of cell lysates from CHO cells transfected with N9Q-FLAG, in agreement with the putative glycosylation site (Fig. 1E). To determine the subcellular localization of MRAP2, CHO cells transfected with MRAP2-FLAG were analyzed by immunocytochemistry using FLAG antibodies. Staining of transfected nonpermeabilized and permeabilized cells localized MRAP2 to the plasma membrane and endoplasmic reticulum (ER), respectively, similar to the distribution of MRAP (Fig. 1F).
MRAP2 Interacts with and Can Rescue the Expression and Function of MC2R in Nonadrenal Cell Lines.
Coimmunoprecipitation (Co-IP) was used to investigate protein–protein interaction between MRAP2 and MC2R. CHO cells were cotransfected with MYC-MRAP2 and HA-MC2R. Immunoprecipitation (IP) of MC2R using anti-HA resulted in the Co-IP of MYC-MRAP2 (Fig. 2A). Previous studies have shown MC2R retention in the ER in cells of nonadrenal origin (8). Coexpression with MRAP supports the expression of MC2R at the cell surface (9). To test the ability of MRAP2 to assist MC2R cell-surface expression, CHO cells were cotransfected with MRAP2-FLAG and HA-MC2R. Confocal imaging of cotransfected cells revealed cell-surface localization of HA-MC2R in the presence of MRAP2-FLAG (Fig. 2B). MC2R trafficking in the presence of MRAP2 was further assessed using a fluorescent cell-surface assay (Fig. 2C). The glycosylation mutant N9Q-FLAG also enabled MC2R trafficking to the cell surface, suggesting that glycosylation of this residue is not required to facilitate MC2R traffic to the plasma membrane.
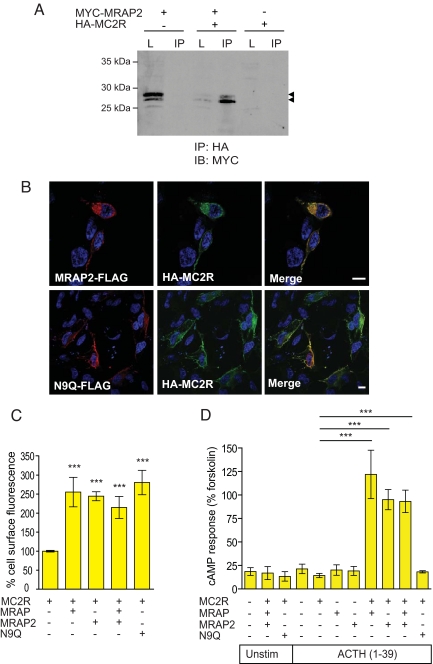
Fig. 2.
N-terminally glycosylated MRAP2 enables the functional expression of MC2R. (A) Co-IP showing interaction between HA-MC2R and MYC-MRAP2. L, lysate; IB, immunoblotting. Arrowheads indicate MRAP2 bands. (B) HA-MC2R colocalizes with MRAP2-FLAG at the cell surface, as seen by immunofluorescence. The deglycosylation MRAP2 mutant N9Q-FLAG also colocalizes with HA-MC2R at the cell surface. (Scale bars, 10 μm.) (C) MRAP2 allows trafficking of HA-MC2R to the cell surface. Using a fluorescent cell surface assay, HA-MC2R is detected at the cell surface when coexpressed with MRAP2, MRAP, MRAP+MRAP2, and N9Q. There is no significant difference between HA-MC2R trafficking with either MRAP or MRAP2. N9Q also enables HA-MC2R cell-surface trafficking. Error bars represent SEM. The x axis represents relative intensity normalized to MC2R alone (100%). (D) N-terminally glycosylated MRAP2 enables HA-MC2R responsiveness to ACTH using a cAMP accumulation assay. MC2R was expressed alone or with MRAP2, MRAP, MRAP+MRAP2, and N9Q in HEK293T cells. Cells were either unstimulated (Unstim) or stimulated with 10−6 M ACTH(1–39) or 10−5 M forskolin. MC2R is responsive to ACTH when coexpressed with MRAP2. There is no significant difference to response generated when MC2R is coexpressed with MRAP or both MRAP and MRAP2. With the MRAP2 glycosylation mutant, N9Q, MC2R shows no response to ACTH. ***, P < 0.0001.
To investigate whether MRAP2 could function in a similar manner to MRAP, HEK293T cells, which lack endogenous MCRs (15, 16), were transfected with MC2R in the presence and absence of MRAP2, MRAP, and N9Q. Cells transfected with MC2R alone did not respond to 10−6 M adrenocorticotropic hormone (ACTH)(1–39). The addition of MRAP2 resulted in the production of cAMP when stimulated with 10−6 M ACTH(1–39) (Fig. 2D), and cAMP generation was comparable to that seen with MRAP and MC2R. Coexpression of MRAP and MRAP2 with MC2R did not enhance ACTH-stimulated MC2R cAMP production over and above that observed with MRAP or MRAP2 alone. Interestingly, the glycosylation status of MRAP2 seems to be functionally important. Despite assisting MC2R to the cell surface, the addition of N9Q-FLAG failed to enable MC2R signaling. These data clearly suggest that MRAP2 may have 2 separate roles in MC2R regulation: first in trafficking of the receptor to the cell surface, and second in enabling the receptor to respond to ACTH.
Both MRAP and MRAP2 Interact with all Melanocortin Receptors.
MRAP2 and MRAP are more widely expressed than MC2R. MRAP2 is detected in both the adrenal gland and brain (Fig. 1C), whereas human MRAP is expressed in a number of tissues (9). We hypothesized that MRAP and MRAP2 may regulate the expression and signaling ability of the other MCRs. Experiments were performed using CHO cells coexpressing MRAP-FLAG or MYC-MRAP2 and HA-MCR. Co-IP with HA-conjugated agarose and immunoblotting with either anti-FLAG or anti-MYC showed that MRAP and MRAP2 interact with each of the MCRs (Fig. 3A). The multiple MRAP bands seed in this figure are similar to those we have observed previously in studies with human MRAP, although the principal full-length MRAP migrates at 24 kDa (Fig. S1) (17). The reciprocal Co-IP, using FLAG-conjugated agarose to precipitate MRAP2-FLAG or MRAP-FLAG, resulted in detection of HA-MCR by immunoblotting (data not shown). The possibility that this was a nonspecific interaction was investigated by attempting Co-IP of MRAP2 with the β2-adrenergic receptor (BAR), AT1 angiotensin receptor (AT1R), and calcitonin-like receptor (CLR) transfected into CHO cells. No Co-IP was identified with the AT1R or CLR, but Co-IP was detectable with the BAR (Fig. S3).
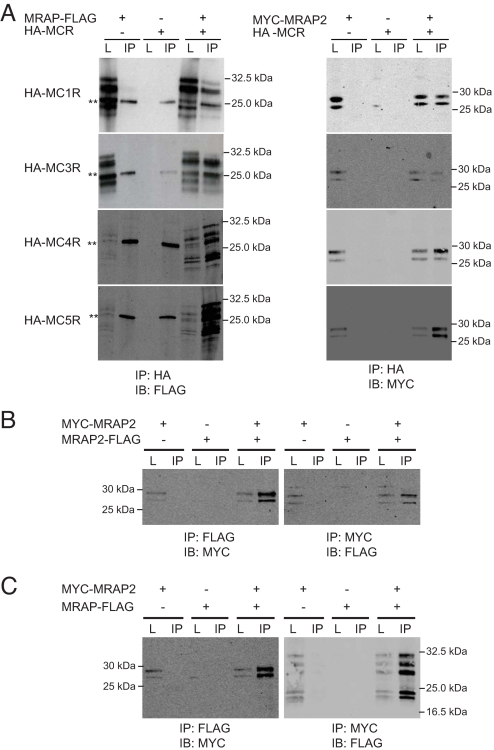
Fig. 3.
MRAP and MRAP2 interactions. (A) MRAP and MRAP2 interact with all 5 MCRs. Both MRAP-FLAG and MYC-MRAP2 interact with each of the HA-MCRs, as seen by immunoblotting with anti-FLAG and anti-MYC, respectively, after immunoprecipitation with HA. Double asterisks indicate light chain. (B) MRAP2 forms homodimers. MRAP2-FLAG and MYC-MRAP2 were coexpressed in CHO cells. Co-IP with anti-FLAG followed by immunoblotting with anti-MYC shows MRAP2 homodimerization. The reciprocal Co-IP is also shown. (C) MRAP and MRAP2 heterodimerize. MRAP-FLAG and MYC-MRAP2 were coexpressed in CHO cells. Co-IP with anti-FLAG followed by immunoblotting with anti-MYC shows MRAP/MRAP2 heterodimerization. The reciprocal Co-IP is shown. L, lysates; IB, immunoblotting.
MRAP2 Can Homodimerize and form Heterodimers with MRAP.
MRAP has been shown to form homodimers (14, 18). To demonstrate MRAP2 homodimerization, CHO cells were transfected with MYC-MRAP2 and MRAP2-FLAG. Co-IP showed that MRAP2 forms homodimers (Fig. 3B). Because both MRAP and MRAP2 are expressed in the adrenal gland and brain, we tested the possibility that MRAP and MRAP2 could form heterodimers. Co-IP was performed on CHO cells cotransfected with MRAP-FLAG and MYC-MRAP2 and showed that MRAP and MRAP2 can heterodimerize (Fig. 3C).
MRAP and MRAP2 Can Downregulate the Expression and Signaling of the MCRs, and the Effects of MRAP and MRAP2 Can Be Additive.
Because all MCRs interact with MRAP and MRAP2 by Co-IP, we investigated the effect of these accessory proteins on MC1R, MC3R, MC4R, and MC5R cell-surface expression. CHO cells were cotransfected with HA-MCR alone or in the presence of either MRAP or MRAP2 or both. Cell-surface MCR expression was analyzed using a fluorescent cell-surface assay (Fig. 4A). The addition of MRAP or MRAP2 resulted in a small but significant reduction in the surface expression of MC4R and MC5R but did not alter the cell-surface expression of MC1R or MC3R.
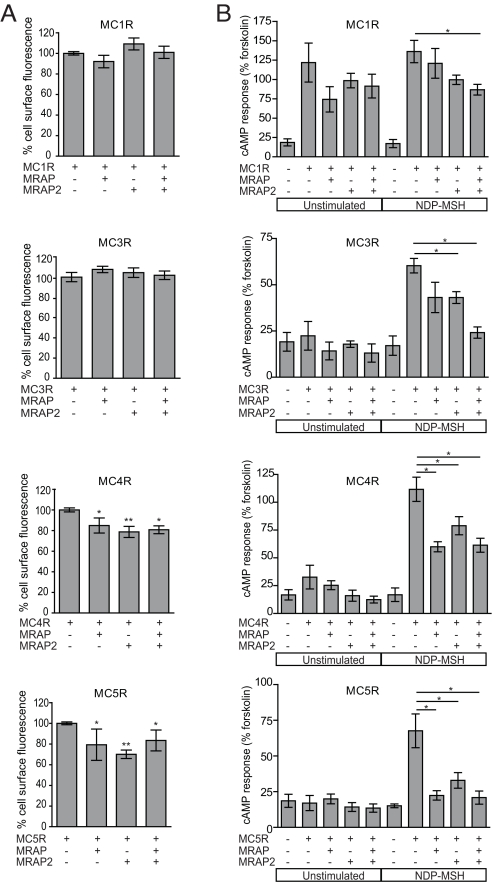
Fig. 4.
MRAP and MRAP2 can modify surface expression and signaling of the other MCRs. (A) MCR at the cell surface is quantified using a fluorescent cell-surface assay and normalized to MCR alone (100%). The addition of MRAP and MRAP2 either alone or in combination significantly reduces the expression of MC4R and MC5R at the cell surface in CHO cells. No change is seen in MC1R or MC3R cell-surface expression when coexpressed with MRAP or MRAP2. (B) MCR response to NDP-MSH is reduced in the presence of MRAP and MRAP2. Signaling of the MCRs was determined using the cAMP generation assay. In the presence of MRAP2, cAMP generation of MC3R, MC4R, and MC5R in response to NDP-MSH (10−9 M) is significantly reduced. With MRAP a significant decrease in NDP-MSH response is seen with MC4R and MC5R. When coexpressed with both MRAP and MRAP2, there is a significant decrease in NDP-MSH response with each of the receptors. Data are represented as mean ± SEM. *, P < 0.05; **, P < 0.001.
To determine how MRAP and MRAP2 affect MCR signaling, we used a cAMP generation assay and compared MCR responsiveness with [Nle4,D-Phe7]alpha-melanocyte-stimulating hormone (NDP-MSH) in the presence or absence of MRAP and/or MRAP2 (Fig. 4B). A significant reduction in MC4R- and MC5R-mediated cAMP generation in response to ligand (10−9 M NDP-MSH) was seen with the addition of MRAP and MRAP2. Interestingly, cAMP generation was significantly reduced for MC1R and MC3R when both MRAP and MRAP2 were coexpressed. This suggests a possible additive inhibitory effect on MC1R and MC3R signaling in the presence of both accessory proteins. MRAP2 alone also significantly reduced MC3R responsiveness to NDP-MSH. The specificity of this effect was investigated in view of the Co-IP of MRAP2 with the BAR (Fig. S3). However, neither accessory protein influenced cAMP generation in response to isoproterenol (Fig. S4). In an attempt to explore the possible mechanisms of action of MRAPs on the MCRs, the dose-responsiveness of the effect of MRAP2 on the MC5R was investigated and suggested that the accessory protein acts to reduce the maximal responsiveness of the receptor, in contrast to shifting the dose–response curve to the right (Fig. S5).
Discussion
We have previously identified MRAP as an accessory protein for MC2R, required for receptor trafficking to the cell surface and the formation of a functional MC2R. Here we have identified MRAP2 as a homologue of MRAP. Like MRAP, MRAP2 is able to support MC2R cell-surface expression, producing a functional ACTH-responsive receptor. In addition, we have shown a unique role for MRAP and MRAP2 in the modulation of the other MCRs. MRAP2, together with MRAP, therefore forms a new family of MCR-accessory proteins important in the trafficking and signaling of the MCRs.
In this study we show that MRAP2, like MRAP, is a single-pass transmembrane domain protein that is localized to the cell surface. Furthermore, MRAP2 undergoes posttranslational modification such that it is glycosylated at the N terminus. Functionally for the MC2R, MRAP2 is comparable to MRAP, with the ability to facilitate trafficking of the MC2R to the plasma membrane and to enable the receptor to signal in response to its unique ligand, ACTH. Western blotting using an antibody to the C-terminal region of MRAP2 revealed an approximately 48-kDa band in adrenal gland and brain tissue, consistent with a MRAP2 dimeric structure in vivo that is resistant to the action of SDS, reducing agents and heat, as described previously for MRAP (14). Recent work suggests that MRAP adopts a dual-topology homodimeric structure (18). N-terminal glycosylation and a C-terminal out orientation as shown by immunofluorescent staining of live cells suggest that MRAP2 also has dual topology.
Our study reveals a unique observation: disruption of the single MRAP2 glycosylation site enables normal MC2R trafficking to the cell surface, but the receptor fails to generate cAMP in response to ACTH. Importantly, this suggests that MRAP2 has 2 separate MC2R accessory roles: first in trafficking of the receptor, and second in enabling receptor responsiveness to ACTH.
MRAP2 is expressed in the adrenal gland, and in situ data localizes expression to all 3 zones of the adrenal cortex (zona glomerulosa, zona fasciculata, and zona reticularis) (data not shown). The possibility of MRAP2 heterodimerization with MRAP is interesting and may imply the requirement of both MRAP and MRAP2 interaction with MC2R for expression and signaling of the receptor. However, the temporal expression pattern of MRAP and MRAP2 in development has yet to be established. This would enable dissection of MRAP and MRAP2 roles in MC2R adrenal physiology.
The tissue expression of both MRAP and MRAP2 extends beyond that of MC2R, suggestive of wider physiologic roles of the MRAP proteins. Here, we show that MRAP2 is expressed in human brain. Mouse Mrap2 (XM_147017) was examined as part of the genome-wide atlas of gene expression in the adult mouse brain (10). In situ hybridization is suggestive of expression in the paraventricular hypothalamus (PVH). MC4R is highly expressed in the PVH and MC3R in the ventromedial nucleus of the hypothalamus (6, 11). MRAP expression has been reported in a number of tissues, including both human and mouse brain and mouse hypothalamus (12). Our data support a role of MRAP and MRAP2 in the regulation of the central melanocortin system. MRAP and MRAP2 can reduce the surface expression of MC4R and also the signaling of this receptor. This seems to be specific for this receptor family in that although Co-IP with the BAR was identified, this had no influence on cAMP signal generation by this receptor. MRAP2 and MRAP can also regulate the signaling of the MC3R, but interestingly without changing the surface expression of the receptor. These data support the concept of a dual functional role for the MRAP proteins, first in receptor trafficking and second in regulating receptor responsiveness to ligand.
In addition, we observed a significant decrease in the cell-surface expression of MC4R and MC5R in the presence of MRAP and MRAP2. It is interesting that MRAP and MRAP2 have opposite effects in the modulation of different MCR family members. The MC2R seems unique among MCRs in that it requires an accessory protein for expression and function. In contrast, MRAP and MRAP2 separately and together can reduce the signaling and in some cases plasma membrane expression of the other members of the MCR family. This is not the only difference between the MC2R and other MCRs; for example, the unique ligand specificity of the MC2R differentiates this receptor from the other MCRs. Further studies may reveal the structural and mechanistic basis of these differences. The reason for differential control of the MC2R and MC4R is unclear; however, one may envisage a physiologic advantage of switching off appetite in situations in which maximal MC2R responsiveness is required, such as acute stress. The effect of MRAP2 on the dose–response curve in which the maximum responsiveness of the MC5R is reduced is consistent with the hypothesis that interaction with an MRAP converts the receptor into one that preferentially binds ACTH over MSH; this concept will be the subject of future work.
MC3R and MC4R are essential for the control of energy homeostasis. We anticipate that MRAP and MRAP2 will prove to be important endogenous regulators of MC3R and MC4R. Future studies using transgenic mouse models are now required to dissect the differential and synergistic functions of the 2 melanocortin accessory proteins MRAP and MRAP2.
Experimental Procedures
Tissue Distribution.
Expression of MRAP2 and GAPDH was performed on a panel of cDNA derived from adult human tissues (adrenal, brain, liver, pancreas, lung, esophagus, fallopian tube, ileum, rectum, breast, lymph node, buccal mucosa, myocardium, gallbladder, atrium, kidney, fundus, ovary, prostate, thyroid, and placenta) (13). The reaction mixture and PCR program used were previously described (9), using MRAP2 intron-spanning primers (Table S1).
Expression Constructs and Site-Directed Mutagenesis.
MRAP2-FLAG (C-terminal tag) and MYC-MRAP2 (N-terminal tag) constructs were prepared using MRAP2 cDNA derived from human brain, which was directly cloned into the mammalian expression vector pcDNA3.1(+) (Invitrogen). MRAP-FLAG was constructed by directional cloning into p3xFLAG-CMV-14 expression vector (Sigma) after PCR amplification of human MRAPα from human adrenal cDNA. N9Q-FLAG mutant construct was made by site-direct mutagenesis (Stratagene QuikChange site-directed mutagenesis kit) using the MRAP2-FLAG construct as a template. All constructs were confirmed by sequencing. cDNA clones for human MCRs (MC1R–MC5R) triple HA tagged at the N terminus (HA-MCR) were obtained from the Missouri S&T cDNA Resource Center.
Cell Culture and Transfections.
CHO cells were maintained in 1:1 DMEM/Ham's F12 media (Sigma) supplemented with 10% FCS and 1% penicillin/streptomycin mixture (Sigma). HEK293T cells were maintained in DMEM with 10% FCS and 1% penicillin/streptomycin. Transient transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Total plasmid DNA amounts were kept constant with pcDNA3.1(+).
Western Blotting and Co-IP.
Whole-cell lysates were prepared 24 h after transfection. Cells were washed 3 times with PBS and lysates generated using lysis buffer (PBS/1% n-dodecyl β-D-maltoside with protease inhibitors) and incubated for 30 min on ice. Samples were then spun for 30 min at 19,000 × g at 4 °C. The supernatant was passed through a 0.22-μm filter (Millipore) before use for either Western blotting or Co-IP. For Co-IP, supernatant was incubated overnight at 4 °C with anti-FLAG, anti-HA, or anti-MYC agarose (Sigma). After incubation, agarose was washed 5 times in lysis buffer, supernatant removed, and SDS loading buffer added. After boiling for 5 min, Western blotting was performed as previously described (9). Anti-HA (Sigma), anti-FLAG (Sigma), or anti-MYC (Abcam) antibodies were used at a dilution of 1:2,000 for immunoblotting.
rMRAP2189–204 Antibody Production.
Antisera to rat MRAP2 (rMRAP2189–204) were produced in rabbits by Eurogentec. Rabbits were immunized with a conjugate of thyroglobulin and a peptide (KLLENKPVSQTARTDLD) comprising residues 189–204 of rat MRAP2 (underlined) and an N-terminal lysine residue to provide a reactive site for coupling to thyroglobulin. Conjugation of the peptide to thyroglobulin was performed using glutaraldehyde as a coupling reagent (19). The rMRAP2189–204 antisera were used for Western blot analysis of MRAP2 expression in tissues at a dilution of 1:1,000. Antibody specificity in Western blots was assessed by preabsorption of antisera with the KLLENKPVSQTARTDLD peptide antigen (20 μM for 1 h at room temperature). Tissue lysates were prepared in radioimmunoprecipitation assay buffer. Protein concentration of the supernatant was determined using a protein assay (Bio-Rad).
Deglycosylation.
Deglycosylation was performed using EndoH and PNGaseF, as previously described (20). Typically 15 μg protein was digested with PNGaseF (1,500 U) or EndoH (1,500 U) at 37 °C for 2 h and analyzed by Western blotting.
Immunofluorescence.
Cells were grown on 8-well glass chamber slides (Labtec). Twenty-four hours after transfection, cells were washed (PBS; 3 × 5 min) and then fixed (3.7% formaldehyde; 10 min). After fixation, cells were either permeabilized with 0.1% Triton X-100 in PBS or placed in PBS for 10 min. After washes (PBS; 3 × 10 min), blocking was performed for 45 min [3% BSA (Sigma) and 10% donkey serum in PBS]. Primary antibody incubation (anti-FLAG or anti-HA; Sigma) was used at a titer of 1:200 for 2 h. After further washes (PBS; 3 × 10 min), a secondary Cy2 or Cy3 antibody was used at a concentration of 1:50 (Jackson Immunoresearch). Cells were washed (PBS; 3 × 10 min) and nuclei stained using 2 μg/mL DAPI in PBS for 1 min. A final wash with PBS was performed for 2 min. Fluorescent mounting media was added and a coverslip applied. Cells were examined by a Zeiss LSM 510 confocal microscope.
cAMP Competitive Protein Binding Assay.
This was performed as described previously (9, 21). Protein concentration was determined with the Bio-Rad protein assay. All results were expressed as a percentage of forskolin response.
Fluorescent Cell-Surface Immunoassay.
CHO cells were grown in 24-well plates and transfected with expression constructs as indicated. Transfections were performed in triplicate on duplicate plates. Twenty-four hours after transfection the cells were washed with PBS and fixed for 10 min with 3.7% paraformaldehyde. Cells from 1 plate were then subjected to permeabilization with PBS/0.1% Triton X-100. Both plates were then washed in PBS and blocked in Odyssey Blocking Buffer (LI-COR) for 1 h. Primary anti-HA antibodies were used at a concentration of 1:1,000 (Sigma), incubating for 1 h. This was followed by 3 washes in PBS and secondary antibody incubation (IRDye 800CW goat antimouse IgG; LI-COR) at a concentration of 1:750 for 1 h. After 3 further washes the permeabilized and nonpermeabilized plates were scanned using the Odyssey Infrared Imaging System (LI-COR). Data are presented as cell-surface protein over total protein, normalized to receptor alone.
Statistics.
Statistical analysis to determine significance was performed using a Student's t test. Each experiment was performed in triplicate and represents at least 3 experiments. Error bars represent the standard error of the mean of the independent experiments. Statistical significance was taken as P < 0.05.
Supplementary Material
Acknowledgments.
L.F.C. and T.T.C. are supported by Clinical Research Training Fellowships from the Medical Research Council. T.R.W. and L.A.M. are supported by the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809918106/DCSupplemental.
References
- 1.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 2.Loos RJ, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers JC, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi IS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 5.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen AS, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 7.Butler AA, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 8.Noon LA, et al. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: Evidence in support of a requirement for a specific adrenal accessory factor. J Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- 9.Metherell LA, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 10.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 11.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner K, Slavov D, Bechtel L, Davisson M. Annotation of human chromosome 21 for relevance to Down syndrome: Gene structure and expression analysis. Genomics. 2002;79:833–843. doi: 10.1006/geno.2002.6782. [DOI] [PubMed] [Google Scholar]
- 13.Gnanapavan S, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 14.Cooray SN, et al. The melanocortin 2 receptor accessory protein exists as a homodimer and is essential for the function of the melanocortin 2 receptor in the mouse y1 cell line. Endocrinology. 2008;149:1935–1941. doi: 10.1210/en.2007-1463. [DOI] [PubMed] [Google Scholar]
- 15.Rached M, et al. Expression of the human melanocortin-2 receptor in different eukaryotic cells. Peptides. 2005;26:1842–1847. doi: 10.1016/j.peptides.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Blondet A, et al. Characterization of cell lines stably expressing human normal or mutated EGFP-tagged MC4R. J Biochem (Tokyo) 2004;135:541–546. doi: 10.1093/jb/mvh064. [DOI] [PubMed] [Google Scholar]
- 17.Webb TR, et al. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology. 2009;150:720–726. doi: 10.1210/en.2008-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skowsky WR, Fisher DA. The use of thyroglobulin to induce antigenicity to small molecules. J Lab Clin Med. 1972;80:134–144. [PubMed] [Google Scholar]
- 20.Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: Quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 21.Brown BL, Albano JD, Ekins RP, Sgherzi AM. A simple and sensitive saturation assay method for the measurement of adenosine 3′:5′-cyclic monophosphate. Biochem J. 1971;121:561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.