Abstract
Pruning is a widely observed mechanism for developing nervous systems to refine their circuitry. During metamorphosis, certain Drosophila sensory neurons undergo large-scale dendrite pruning to remove their larval branches before regeneration of their adult dendrites. Dendrite pruning involves dendrite severing, followed with debris removal. Little is known about the molecular mechanisms underlying dendrite severing. Here, we show that both the Ik2 kinase and Katanin p60-like 1 (Kat-60L1) of the Katanin family of microtubule severing proteins are required for dendrite severing. Mutant neurons with disrupted Ik2 function have diminished ability in severing their larval dendrites in pupae. Conversely, premature activation of Ik2 triggers precocious dendrite severing in larvae, revealing a critical role of Ik2 in initiating dendrite severing. We found a role for Kat-60L1 in facilitating dendrite severing by breaking microtubule in proximal dendrites, where the dendrites subsequently separate from the soma. Our study thus implicates Ik2 and Kat-60L1 in dendrite severing that involves local microtubule disassembly.
Keywords: Ik2/IKK, microtubule severing
The precise wiring of neuronal circuitry is essential for proper function of the nervous system. Starting with the initial configuration of the developing nervous system, further remodeling is often necessary to achieve the mature connectivity. Neuronal pruning is one of the basic remodeling mechanisms at the level of individual neurons. Pruning, a tightly regulated process that selectively eliminates specific parts of neuronal processes without causing cell death, is widely observed in organisms ranging from the nematode C. elegans to vertebrates (1, 2). Besides ensuring the precise wiring during development, neuronal pruning allows adjustment of neuronal connections in response to injury or diseases. When axons are injured or transected, the wounded parts are disconnected from the cell body and undergo rapid degeneration, a process known as Wallerian degeneration. In neurodegenerative disorders, such as Huntington's and Alzheimer's diseases, there is a gradual loss of neuronal processes long before cell death (3, 4). Developmentally, programmed neuronal pruning and the pruning that ensues in neuronal injury and diseases likely share some of the machinery that executes the elimination of neuronal processes, such as the ubiquitin-proteasome system (5–8).
In Drosophila, extensive neuronal remodeling takes place during metamorphosis, as some larval neurons survive and prune their processes, while others die and new adult neurons are generated (9). Neuronal pruning thus allows the larval neuronal processes and connectivity to be eliminated before the formation of adult-specific branches and connection, in both the central nervous system (CNS) (10, 11) and the peripheral nervous system (12, 13). Most of the larval peripheral neurons die during metamorphosis, but a subset including some of the class IV dendritic arborization (da) neurons survive through metamorphosis and undergo dendrite pruning, starting with severing of the proximal dendrites and followed with blebbing, and degeneration of disconnected dendrites (12, 13). After removal of the severed dendrites by phagocytes (12), 2 of the pruned class IV da neurons regenerate their adult dendrites and 1 disappears presumably via apoptosis (13). Unlike the CNS mushroom body (MB) γ neurons that prune their larval dendrites and axons (10, 14), class IV da neurons prune all their larval dendrites but leave their axons intact (13).
Dendrite pruning in class IV da neurons is regulated by intrinsic and extrinsic factors. Ecdysone signaling gives class IV da neurons the competence to commence the whole pruning processes (12, 13), executed by the ubiquitin-proteasome system (13), and involving both the matrix metalloproteases and the caspase activity (13, 15, 16). However, little is known about the underlying molecular mechanisms by which the dendrites of class IV neurons are severed at the early step of dendrite pruning. In this study, we show that the Ik2 kinase plays an essential role to initiate dendrite pruning and sever the proximal dendrites of class IV neurons. Whereas microtubule disruption is one of the earliest events observed during neuronal pruning in many systems, including class IV da neurons (2, 5, 12), the molecular machinery that breaks microtubules is unknown. We found a gene, katanin p60-like 1 (kat-60L1) encoding a protein containing an AAA ATPase domain, essential for microtubule disruption in the proximal dendrites and efficient severing of the dendrites.
Results
Sequence of Events in the Dendrite Pruning of Drosophila Sensory Neurons.
During metamorphosis, all 3 class IV da neurons in each hemisegment undergo the same sequential events of dendrite pruning. The dorsal ddaC neuron begins pruning first, before the ventral-lateral and ventral ones initiate their pruning. We will describe the morphological changes of ddaC dendrites during dendrite pruning as the primary example in this study.
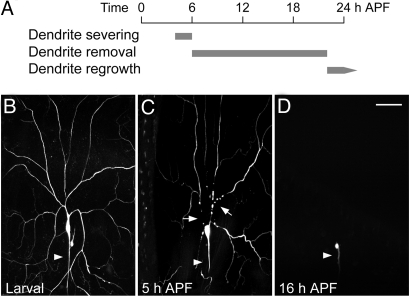
Having confirmed that the primary dendrites of ddaC neurons are separated from the soma at 10 h after puparium formation (APF) and then removed by 20 h APF (13) (Fig. 1A), we further examined ddaC neurons labeled via the class-specific ppk (pickpocket)-eGFP (17) by live imaging starting at the third instar larval stage (Fig. 1B). At 5 h APF, the cytosolic eGFP appeared discontinuous at the proximal primary dendrites as they underwent severing (Fig. 1C). Dendritic severing was also evident in ddaC cells labeled with membrane bound mCD8-GFP proteins at 5 h APF. By 16–18 h APF, the remnants of severed dendrites were eliminated completely and only the soma and axons of ddaC neurons remained intact (Fig. 1D) (13). Since morphological disconnection of dendrites from ddaC soma was detectable at 5 h APF, and the severed dendrites were eliminated by 16–18 h APF, the persistence of larval primary dendrites still attached to the soma in a mutant at 16–18 h APF would be evidence for a defect in dendrite severing.
Fig. 1.
Sequence of events in dendrite pruning of Drosophila sensory neuron ddaC during metamorphosis. (A) Dendrite pruning in ddaC neurons begins with dendrite severing at 4–6 h APF, which is followed by dendrite removal by 16–18 h APF. The pruned ddaC neurons then start to regrow adult dendrites after 20–22 h APF. (B–D) The sequence of events has been delineated by monitoring ppk-eGFP labeled wild-typed ddaC neurons at 25 °C. (B) The ddaC neuron in a third instar larva, showing dendritic arbors and axon (arrowhead). (C) At 5 h APF, the proximal dendrites of ddaC neuron are severed (arrows), revealed by the discontinuous eGFP signals (77% show at least one dendrite severed, n = 120). (D) At 16 h APF, the severed dendrites have been removed completely (100%, n = 75), and only the soma and axon (arrowhead) are left intact. Dorsal is up and anterior is to the left. (Scale bar, 50 μm.)
The Ik2 Kinase Is Essential for Dendrite Severing in Pupae.
Neuronal pruning is a highly controlled process that bears some resemblance to apoptosis, although the outcomes of these 2 processes are obviously different. As previously reported, dendrite pruning of class IV da neurons is altered by several mutations affecting the apoptotic pathway, such as dronc (an initiator caspase) loss-of-function (LOF) and DIAP1 (an inhibitor of caspases) gain-of-function (GOF) mutations (15, 16). To test whether any upstream regulator of DIAP1 might also be involved in dendrite pruning, we examined the role of Ik2, a non-canonical member of the IκB kinase family that is closely related to the mammalian IKKε/IKKι and TANK binding kinase 1 (TBK1), and functions as an upstream negative regulator of DIAP1 by promoting DIAP1 protein degradation (18, 19).
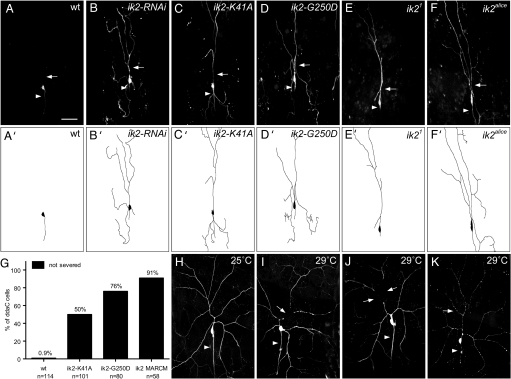
We found that Ik2 is required for dendrite severing in class IV da neurons based on experiments including RNA interference (RNAi), expression of dominant negative transgenes and MARCM (mosaic analysis with a repressive cell marker) analysis (20) of LOF mutants. First, we used 2 independent ik2 RNAi lines to reduce the endogenous Ik2 protein level, causing the proximal dendrites to remain intact for most ddaC neurons at 18 h APF (Fig. 2B). Besides wild-type control (Fig. 2A), we also examined ddaC neurons with lacZ RNAi and found no alteration in their dendrite severing. Second, we examined 2 transgenic lines expressing Ik2 proteins bearing dominant negative mutations, the Ik2-K41A and Ik2-G250D mutations that abolish the kinase activity (18, 19), and found the primary dendrites of most ddaC neurons remaining attached to soma (Fig. 2C, D, and G). Third, 2 independent ik2 EMS mutant alleles, ik21 and ik2alice (21), were used in MARCM analyses to examine the role of ik2 during neuronal development in larvae and during dendrite severing in pupae. In larvae, the dendritic morphology of all 4 classes da neurons in ik2 mutant MARCM clones was indistinguishable from that in the wild-type clones. However, dendrite severing was strongly inhibited in the ik2 mutant MARCM clones of ddaC neurons at 20 h APF (91%, n = 58) (Fig. 2 E and F). In fact, the dendrites of ik2 MARCM clones of ddaC cells were still detectable at 25 h APF. In contrast to this dendritic pruning defect, most of the peripheral sensory neurons underwent apoptosis normally at early pupal stages, a process independent of Ik2 activity, in the loss of ik2 function MARCM clones (90%, n = 39). This observation is consistent with the previous report that ik2 is not involved in the developmentally programmed cell death in flies (18).
Fig. 2.
Ik2 is essential for dendrite severing during dendrite pruning. (A–D) ddaC neurons were visualized by expressing UAS-mCD8GFP driven by ppk-GAL4 at 18 h APF. (A) The dendrites of wild-type ddaC neuron were pruned, and only the soma and axon (arrowhead) remained intact. (B–D) The dendrite severing was suppressed in the proximal dendrites (arrows) of ddaC neurons by expressing either ik2-RNAi (B), or dominant negative ik2-K41A (C) and ik2-G250D (D). The ik21 (E) and ik2alice (F) mutant MARCM clones of ddaC neurons failed to severe their dendrites at 20 h APF. (G) Quantitative analysis of dendrite-severing phenotypes in ddaC neurons. The percentage of cells was determined by dividing the number of ddaC neurons, whose primary dendrites were connected to cell bodies at 16 h APF (for wild type and for expression of ik2-K41A and ik2-G250D by ppk-GAL4) or at 18–25 h APF (for the ik2 mutant MARCM clones) by the total number of ddaC neurons examined (n) for each genotype. (H–K) The transient increase of Ik2 activity in ddaC neurons in larvae was controlled by the temperature-sensitive GAL80. Each animal contained the following transgenes: tub-GAL80ts, ppk-eGFP, ppk-GAL4, and UAS-ik2. The dendrites of their larval ddaC neurons exhibited wild-type morphology at 25 °C (H), but were precociously severed at the proximal regions (arrows) in larvae at 29 °C (I and J). (K) Apoptosis also occurred in some ddaC cells at 29 °C, as indicated by the blebs of dendrites (arrow) and axons (arrowhead). (Scale bar, 50 μm.)
The distribution of Ik2 proteins in class IV da neurons was examined by staining with an available Ik2 antibody (19) in larvae and pupae. Consistent with prior report that ik2 mRNA and protein were detected ubiquitously in embryos (19), we only detected low level of signals present ubiquitously in larvae and pupae, including class IV neurons. Nonetheless, the defective dendrite severing in ik2 mutant ddaC neurons strongly argues that Ik2 plays a critical role in dendrite severing. Taken together, our study indicates that ik2 is neither required for the dendritic morphogenesis of da neurons in larvae, nor for the programmed cell death in pupae, but is essential for dendrite severing in class IV da neurons during dendrite pruning.
Ik2 Is Sufficient to Trigger Precocious Dendrite Severing in Larvae.
We reasoned that if Ik2 is a key regulator of dendrite severing, an increase of Ik2 activity in larval ddaC neurons might be sufficient to cause precocious dendrite severing. Consistent with previous report (18), we observed massive cell death in larval class IV neurons with Ik2 overexpression that hampered our study of dendrite pruning. To temporally control the extent of ik2 expression so as to avoid cell death, we used the ubiquitously expressed temperature-sensitive GAL80 (GAL80ts) to achieve precocious activation of Ik2 in larvae by elevating temperature. Under the permissive temperature 25 °C, ddaC neurons showed normal dendritic morphology (Fig. 2H). Upon shifting to non-permissive temperature to relieve the inhibition of GAL80 on GAL4, the abrupt increase of Ik2 activity in class IV da neurons triggered precocious dendrite severing in the proximal dendrites of some ddaC neurons in larvae (Fig. 2 I and J), reminiscent of dendrite severing in pupae. Although Ik2 expression was under the control of GAL80ts, it still caused cell death in some ddaC cells, characterized by the blebbing dendrites and axons (Fig. 2K) that resembles the appearance of apoptotic class IV da neurons induced by overexpressing a cell death gene reaper (rpr) or by DIAP1 RNAi. Taken together, these results indicate that transient up-regulation of Ik2 activity is sufficient to trigger precocious dendrite severing in larval ddaC neurons, further supporting the critical role of Ik2 in dendrite severing.
Localized Breakage of Microtubule and Actin Cytoskeletons in the Proximal Dendrites of ddaC Neurons During Severing.
Given that Ik2 functions as a negative regulator of DIAP1 (18) and both genes are involved in dendrite pruning, we wondered whether Ik2 relies entirely on DIAP1 to initiate dendrite severing in class IV da neurons. We found that all ddaC neurons severed their dendrites in DIAP1 GOF mutants at 16–18 h APF, whereas some ventral-lateral class IV neurons retained their primary dendrites, a phenotype consistent but weaker than previously reported (15), indicating that the dendrite severing in class IV neurons was delayed but not inhibited. The effect of ik2 LOF mutation is therefore much stronger than that of DIAP1 GOF mutation on suppressing dendrite severing, raising the possibility that Ik2 might regulate other cellular processes to mediate dendrite severing in class IV neurons. Since ik2 is known to have an effect on cytoskeletons in other systems (18, 19, 21), we next examined the microtubule and actin cytoskeletons in class IV da neurons at pupal stages.
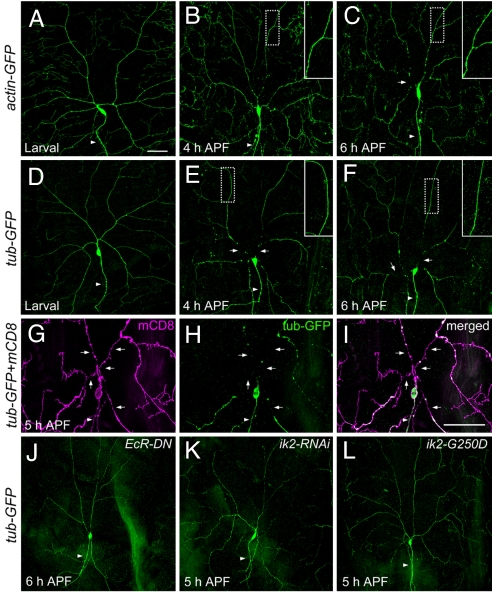
To visualize the cytoskeletons, we expressed actin-GFP (Fig. 3 A–C) and tubulin-GFP (Fig. 3 D–F) to specifically label the actin and microtubule cytoskeletons in class IV da neurons and used anti-GFP antibody staining to enhance the signals. In wild-type larvae, tubulin-GFP proteins were evenly incorporated into the microtubules in soma, axons, and dendrites of ddaC neurons, except for the terminal dendrites that are enriched with actin and devoid of microtubules (Fig. 3D) (22). Consistent with the report that the microtubule cytoskeletons in the dendrites of da neurons become destabilized and fragmented in pupae (12), we detected microtubule breakage localized to the dendrite severing sites, with microtubule disassembly initiated before dendrite severing. At 4 h APF, the disruption of microtubule cytoskeletons in ddaC neurons was detected in the proximal dendrites, whereas the microtubules in dendrites distal to the severing site remained intact (Fig. 3E). At 6 h APF, the gap between microtubules in ddaC soma and dendrites became bigger (Fig. 3F). These observations indicate that the microtubule breakage in ddaC neurons begins in proximal dendrites, and the microtubule disassembly then propagates from the breakage sites to the more distal portions of severed dendrites. These observations were further supported by live imaging of tubulin-GFP and fly Tau-GFP. In addition, marking the microtubules and membranes of ddaC neurons by antibody double staining at 5 h APF, we found that the dendritic membranes were still connected to the soma in some regions of proximal dendrites where the microtubules were already disrupted or absent (Fig. 3 G–I) (12). Thus, the microtubule breakage in the proximal dendrites appears to precede the physical separation of dendrite membrane from the cell body. Similarly, whereas actin-GFP proteins appeared uniformly distributed throughout the soma, axon, and dendrites of ddaC neurons in larvae (Fig. 3A), at 4 h APF the actin cytoskeletons became thinner and beaded in the proximal dendrites, but the actin cytoskeletons in the more distal portion of dendrites remained stable (Fig. 3B). At 6 h APF, the actin cytoskeletons were clearly disjointed between the proximal dendrites and the soma of ddaC neurons (Fig. 3C). Taken together, these observations indicate that the microtubule and actin cytoskeletons of ddaC neurons are first disrupted in the proximal dendrites before the disconnection of dendritic membrane, while cytoskeletons in the more distal parts of dendrites remain intact for hours, even after they are severed from the neuron.
Fig. 3.
The cytoskeletons of ddaC neurons in wild type and mutants during dendrite pruning. (A–C) The actin cytoskeletons were visualized by anti-GFP antibody staining in wild-type animals with UAS-actin-GFP driven by ppk-GAL4. (A) The actin-GFP proteins of ddaC cells in larva. (B) At 4 h APF, the actin cytoskeletons in the proximal dendrites of ddaC showed signs of disruption, becoming thinner and blebbing. (C) Breakage of actin cytoskeletons (arrow) was evident at 6 h APF. (D–F) The microtubules in wild-type ddaC neurons, which expressed UAS-tub-GFP (tubulin-GFP) driven by ppk-GAL4 and labeled by anti-GFP antibodies, were intact in larva (D), and showed breakage in proximal dendrites (arrows) at 4 h APF (E) and 6 h APF (F). Magnified views (Insets) of the more distal dendrites (dashed boxes, ≥120 μm from the soma) showed that the actin cytoskeletons (B and C) and the microtubules (E and F) were fairly intact. (G–I) Double staining was carried out to visualize the membrane by mCD8 signals (G) and the microtubules by GFP signals (H) in wild-type ddaC neurons at 5 h APF. The proximal regions of dendrites showed the gaps of microtubules (arrows in H) and the presence of continuous membrane (arrows in G). The merged image was shown in I. (J–L) The microtubules revealed by the expression of ppk-GAL4, UAS-tub-GFP, and anti-GFP antibody staining were intact in the pupae with the expression of dominant negative ecdysone receptor (EcRDN-W650A) (J), of ik2-RNAi (K), and of dominant negative ik2 (ik2-G250D) (L). Arrowheads indicate the axons. (Scale bars, 50 μm.)
We next examined whether the microtubule integrity of class IV neurons is affected in the mutants with defects in dendrite severing. Overexpression of dominant negative ecdysone receptor (EcR-DN) efficiently suppressed dendrite pruning in ddaC neurons (12, 13), and eliminated any sign of microtubule disruption in the dendrites at 6 h APF (Fig. 3J). Furthermore, in the mutants with ik2 RNAi or ik2-G250D overexpression, the microtubules remained intact in the dendrites of ddaC neurons at 5–6 h APF (Fig. 3 K and L). Thus, the inability to sever and prune ddaC dendrites in EcR-DN and ik2 mutants is likely a consequence of the failure to disrupt microtubules at the dendrite severing sites.
The Role of Kat-60L1 in Facilitating Dendrite Severing by Local Disruption of Microtubules in the Proximal Dendrites.
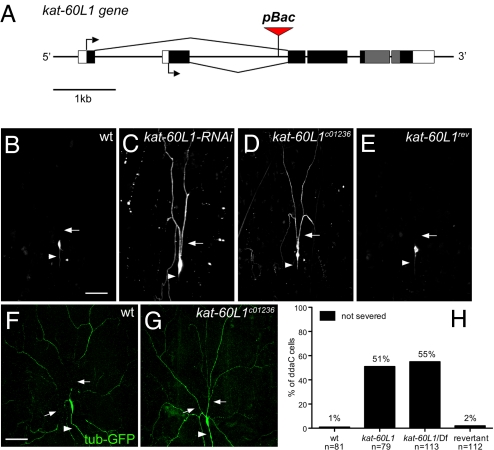
Next, to identify molecules that might be responsible for disrupting microtubules at the dendrite severing sites, we conducted an RNAi screen on candidate molecules with microtubule-severing or -destabilizing activities, including katanin-60, spastin, CG1193, stathmin, Klp10A (KIF2A), and Klp59C (KIF2B). By using ppk-GAL4 to drive RNAi that targets each of the candidate genes, we examined the integrity of primary dendrites in ddaC neurons at 18 h APF. We found that reducing the activity of CG1193 in class IV da neurons resulted in the most prominent dendrite-severing phenotype (Fig. 4C, compared to wild type in 4B). The CG1193 gene encodes a protein of the AAA ATPase family. According to an unrooted phylogenetic tree analysis, CG1193 is most related to Drosophila and human Katanin p60 (23). The C-terminal part of CG1193, which includes the AAA ATPase domain, shows 66% identity to Drosophila Katanin-60 (Kat-60). Thus, we named the CG1193 gene katanin p60-like 1 (kat-60L1). Drosophila Kat-60 and Kat-60L1 both belong to the meiotic subgroup of AAA ATPase (24, 25), including members involved in vesicle trafficking or regulation of microtubule dynamics. The biological function of this Kat-60L1 protein in Drosophila has not been characterized previously.
Fig. 4.
Kat-60L1 is involved in dendrite severing of ddaC neurons during dendrite pruning. (A) Schematic diagram of the kat-60L1 gene structure and the insertion site of 2 piggyBac lines, kat-60L1c01236 and kat-60L1f00564. The start sites of the ORF (in black and gray) for 2 splicing variants are indicated by arrows and the exons encoding the AAA ATPase domain are in gray. (B–E) The ddaC neurons were labeled by UAS-mCD8GFP and ppk-GAL4, and their morphology at 16 h APF was shown. Arrowheads mark the axons, and arrows indicate the proximal dendrites. Dendrite severing was prevented in pupa with kat-60L1-RNAi expression (C) and in homozygous kat-60L1c01236 mutants (D). (E) The ddaC neurons in a revertant line of kat-60L1c01236 showed normal dendrite pruning as in wild type (B). At 5 h APF, the microtubules marked by anti-GFP antibody staining showed breakage in the proximal dendrites (arrows) of wild-type ddaC neurons (F), but remained intact in those of kat-60L1c01236 mutants (G). (H) Quantitative analysis of dendrite-severing phenotypes in ddaC neurons. The percentage of cells was determined by dividing the number of ddaC neurons, whose primary dendrites were connected to soma at 16 h APF by the total number of cells examined for each genotype. The precise excision of piggyBac enables normal dendrite pruning in revertants. kat-60L1: piggyBac line kat-60L1c01236; Df: deficiency line Df(3R)Exel6145, which deletes the kat-60L1 gene. (Scale bars, 50 μm.)
To further verify the role of kat-60L1 in dendrite severing of class IV da neurons, we identified 2 piggyBac insertion lines, both with insertions in the first intron of kat-60L1 gene (Fig. 4A), and confirmed by genomic PCR and DNA sequencing. We found that the dendritic morphology of ddaC neurons were normal in larvae carrying the kat-60L1 piggyBac insertion on both chromosomes. To assess the role of kat-60L1 in dendrite severing, we examined the class IV neurons in the homozygous mutants of kat-60L1 piggyBac lines at 16 h APF, and found that dendrite severing was reduced (Fig. 4 D and H). Dendrite severing was also suppressed in kat-60L1 hemizygous mutant pupae, which carry the kat-60L1 piggyBac insertion on one chromosome and a deficiency that removes the entire kat-60L1 gene on the other chromosome (Fig. 4H). The similarity in the dendrite severing phenotypes between the homozygous and hemizygous kat-60L1 mutants indicates that the piggyBac insertion affects kat-60L1 function.
To rule out the possibility that other unknown mutations present in the kat-60L1 piggyBac line could have contributed to the dendrite severing phenotype, we generated revertants by removing the piggyBac insertion element and examined the dendrites of ddaC neurons in 3 independent revertant lines. In nearly all cases, the dendrites were properly pruned and eliminated at 16 h APF (Fig. 4 E and H). Thus, the dendrite severing phenotype in kat-60L1 piggyBac line is caused by the piggyBac insertion that disrupts the normal function of kat-60L1 gene. We next examined the microtubules in the dendrites of ddaC neurons of homozygous kat-60L1 piggyBac line, and found that microtubules remained intact in the mutant dendrites at 5–6 h APF (Fig. 4G, compared to wild type in 4F). These observations indicate that Kat-60L1 is required for microtubule breakage in the proximal dendrites of ddaC neurons. Despite the severe deficit in dendritic pruning at 16 h APF, the majority of primary dendrites of ddaC neurons in the homozygous kat-60L1 piggyBac lines became detached from the soma after 18 h APF. Thus, the function of Kat-60L1 is required for efficient severing of the primary dendrites of class IV neurons at an early stage of dendrite pruning. The delayed dendrite severing in kat-60L1 mutants suggests that either the kat-60L1 mutants are not null or other, yet unidentified, molecules may sever the dendrites albeit less efficiently.
Ik2 and Kat-60L1 Are Not Required for Axon Pruning in MB γ Neurons.
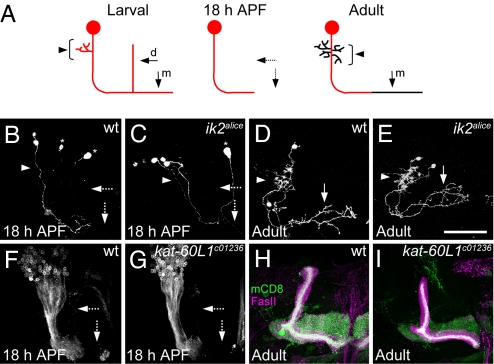
Given that dendrite and axon pruning are similar phenomena of neuronal process elimination (5, 13), we asked whether ik2 and kat-60L1 are involved in axon pruning of MB γ neurons. At larval stages, the axons of MB γ neuron bifurcate to form medial and dorsal branches (Fig. 5A) (10). At early pupal stages, MB γ neurons prune not only their larval dendrites but both their axonal branches; however, only the medial axonal branches regenerate to make their adult connections at later pupal stages. Both the axons and dendrites of ik2 MARCM clones of MB γ neurons showed normal pruning at 18 h APF (Fig. 5C, compared to wild type in 5B), and then regrew their dendrites and extended their medial axonal branches toward the midline (Fig. 5E). Therefore, ik2 is neither required for the pruning of MB γ neuron axon and dendrites, nor for the subsequent regeneration of adult axon and dendrites. Since the kat-60L1 piggyBac lines are homozygous viable, we used 201Y-GAL4 (5) driving mCD8-GFP expression to label γ neurons. We found normal axon pruning in γ neurons of homozygous kat-60L1 mutants at 18 h APF (Fig. 5G) and in adult (Fig. 5I). Thus, both ik2 and kat-60L1 are specifically required for dendrite pruning of class IV da neurons, but not for axon and dendrite pruning of MB γ neurons during metamorphosis.
Fig. 5.
Ik2 and Kat-60L1 are not required for the axon and dendrite pruning in MB γ neurons. (A) Schematic diagram of MB γ neurons at larval, pupal and adult stages. The MB γ neurons contains dendrites (arrowheads) and dorsal (d) and medial (m) axonal branches (solid arrows) in larvae. At 18 h APF, both axonal branches were pruned (dashed arrows). Only the medial axon (solid arrow) re-extend to the midline in adults. At 18 h APF, both axon branches (dashed arrows) and dendrites (arrowhead) of γ neurons pruned in the wild-type MARCM clones (B), and in the ik2 MARCM clones (C). Nearby non-γ neurons were indicated by asterisks. 2-cell wild type (D) and ik2 (E) MARCM clones of γ neuron regrew their dendrites (arrowhead) and medial axons (solid arrow) in adult. MB γ neurons marked by201Y-GAL4 and UAS-mCD8GFP at 18 h APF (F and G) and also by anti-Fas II antibody staining in adults (H and I), showed normal axon pruning in kat-60L1c01236 mutants (G and I), compared to wild type (F and H). n ≥ 8 clones (B–E), and n ≥ 10 brains (F–I). (Scale bar, 50 μm.)
Discussion
Ik2 Signaling in Dendrite Pruning.
Although the requirement of Ik2 signaling in dendrite severing may involve DIAP1 downregulation, inactivation of Ik2 function caused a much stronger dendrite severing phenotype than DIAP1 GOF mutation did, suggesting that Ik2 signals might be mediated by pathways other than or in addition to those involving DIAP1 and caspases to trigger dendrite severing in class IV da neurons during dendrite pruning. The function of ik2 is also required in several other developmental processes in Drosophila, including the regulation of F actin dynamics during trachea and bristle morphogenesis, and the organization of cytoskeletons for proper mRNA localization during oogenesis (18, 19, 21). One common feature in all these developmental processes is the dynamic organization of cytoskeletons. Our study identifies 1 of the potential regulators of cytoskeletal rearrangement concomitant with dendrite pruning.
The Dynamics of Cytoskeletons and the Function of Kat-60L1 During Dendrite Pruning.
Microtubule disassembly is a cellular feature typically observed in developmental axon pruning, Wallerian degeneration, and some neurodegenerative diseases (2, 5, 6). During axon pruning of MB γ neurons in Drosophila, the disruption of microtubule cytoskeletons precedes the disruption of other cellular makers in axons (5). We found that targeted microtubule breakage in the proximal dendrites of class IV da neurons also takes place before the membrane separation from the soma. Moreover, the breakage of microtubule cytoskeletons in the proximal dendrites was suppressed in mutants with impaired ecdysone signaling, and in ik2 and kat-60L1 mutants.
In our RNAi screen for molecules that are responsible for local microtubule disruption in the proximal dendrites, we tested 3 candidate genes encoding microtubule-severing proteins, kat-60, kat-60L1, and spastin, and found prominent dendrite severing phenotypes only in kat-60L1 RNAi mutants. The class IV da neurons in spastin LOF mutants (23) did not show noticeable dendrite severing phenotypes. Owing to the lethality caused by kat-60 mutations and the proximity of kat-60 gene to the centromere prohibiting MARCM analyses, it has been difficult to examine the dendrite severing phenotype of class IV neurons in kat-60 mutants besides the RNAi mutants. The in situ hybridization signals of kat-60L1 mRNA in fly embryos reported by BDGP (Berkeley Drosophila Genome Project) indicate that kat-60L1 is expressed in the embryonic nervous system, including brain and ventral nerve cord. Our genetic study indicates that one of the Kat-60L1 functions in the nervous system is to efficiently sever the proximal dendrites of class IV neurons in pupae. Whether Kat-60 also contributes to dendrite severing remains to be determined.
The protein sequence of Kat-60L1 shows high homology to Drosophila Katanin-60. Katanin, a microtubule-severing protein, is a heterodimer composed of 2 subunits, p60 that possesses the microtubule-severing activity, and p80 that regulates the enzymatic activity and targeting of p60 (26, 27). Drosophila Kat-60 can interact with Drosophila Kat-80 in yeast 2-hybrid screen (28). The N-terminal 29 residues of human Katanin p60 required for interaction with human Katanin p80 (27) is conserved in Drosophila Kat-60, but absent in Kat-60L1, indicating that Kat-60L1 is unlikely to be regulated by Kat-80 through similar direct interaction. It has been shown that phosphorylation could also regulate the microtubule-severing activity of Katanin during mitosis (29). Similar mechanisms could regulate the activity of Kat-60L1 to sever microtubules. Since both Ik2 kinase and Kat-60L1 are involved in dendrite severing of class IV da neurons, it will be of interest to test whether Ik2 kinase might regulate Kat-60L1 activity.
The Restriction of Severing Activity in Dendrites But Not in Axons.
One intriguing question about the pruning in class IV da neurons concerns the mechanisms by which neurons restrict the pruning activity only to their dendrites. It is possible that some microtubule-associated proteins (MAP) may preferentially bind to the microtubules in dendrites or those in axons and thus provide differential protection against the microtubule-severing activity of Kat-60L1. For example, in cultured rat hippocampal neurons, the microtubules in dendrites are more susceptible to excess activity of overexpressed Katanin p60 than those in axons (30), and the axonal Tau proteins protect axonal microtubules from severing by Katanin (31). The differential binding preference for microtubules in dendrites versus axons could also arise from regional posttranslational modification of MAPs. For example, the phosphorylated MAP4, MAP2, and Tau display lower affinity to microtubules than their non-phosphorylated counterparts (32, 33). It is also likely that the posttranslational modification of microtubule in dendrites is different from that in axons, since katanin has been suggested to display differential preference to microtubules with different modification in tetrahymena and in C. elegans (34, 35). In future studies, it would be interesting to test these possibilities to delineate the mechanisms of dendrite-specific pruning.
Materials and Methods
Fly Strains and Genetics.
The fly strains used in this study: 2 ik2 EMS mutant alleles, ik21 (G250D), and ik2alice (F297I), both with mutations in kinase domain (21); 2 piggyBac lines, kat-60L1C01236, and kat-60L1f00564 (Bloomington and Exelixis/Harvard); ppk-eGFP (17); ppk-GAL4; 201Y-GAL4, and OK107-GAL4 for MB γ neuron study; 2 ik2 RNAi transgenic lines, UAS-ik2-RNAi (VDRC), and UAS-DmIKKεIR (18); 2 UAS-kat-60L1-RNAi lines (VDRC); UAS-ik2 (UAS-DmIKKε) (18); UAS-ik2-G250D (18); UAS-ik2-K41A (19); UAS-LacZ IR (36); UAS-mCD8 (37). The following stocks are from the Blooming stock center: Df(3R)Exel6145; FRT40A, tubP-GAL80; tub-GAL80ts; UAS-tub-GFP; UAS-actin-GFP; UAS-mCD8GFP. Flies were raised at 25 °C. For the experiments with tub-GAL80ts, flies were transferred and kept at 29 °C for 40–42 h before analysis and imaging. The MARCM analysis for da neurons was done as described (22). The MARCM analysis for MB γ neurons was done as reported (10). For making the revertants of piggyBac line kat-60L1C01236, it was done according to the previous report (38) and verified by genomic PCR and DNA sequencing.
Immunohistochemistry and Imaging.
The larvae and pupae were dissected in cold PBS and fixed with 4% formaldehyde at room temperature for 40 min, washed with PBTX (PBS with 0.3% Triton X-100), and blocked in 5% normal donkey serum in PBTX. Primary antibodies were used at a concentration of 1:200 for rat anti-mCD8 (Caltag, CA), 1:50 for mouse anti-Fas II mAb 1D4 (DHSB, U. Iowa), 1:2,500 for chicken anti-GFP, 1:150 for mouse anti-Ik2 mAb (19) incubated overnight at 4 °C. Secondary antibodies were Cy2-conjugated donkey anti-rat Abs (1:200, Jackson Lab), Cy2-conjugated goat anti-chicken Abs (1:200, Jackson Lab), Rhodamine Red X-conjugated donkey anti-mouse Abs (1:200, Jackson Lab). Images were obtained by confocal microscopy on Leica TCS SP2 or SP5. Live images were acquired on a Bio-Rad MRC-600 confocal microscope. The images were processed using Image J and Adobe Photoshop.
Acknowledgments.
We thank K. Anderson (Sloan–Kettering, New York), M. Miura (University of Tokyo, Tokyo), S. Hayashi (Riken, Japan), R. Carthew (Northwestern University, IL), T. Lee (University of Massachusetts, Worcester), S. Zhu, W. Song, and the Bloomington Stock Center for fly lines; C. Kuo, S. Zhu, and Q. Yuan for technical advice; Hsu-Chen Cheng, R. Yang, and S. Zhu for comments on the manuscript. This work was supported by National Institutes of Health Grants R01NS40929 and R01NS47200 (to Y.-N.J.). L.Y.J., and Y.-N.J. are Howard Hughes Medical Institute Investigators.
Footnotes
The authors declare no conflict of interest.
References
- 1.Kage E, et al. MBR-1, a novel helix-turn-helix transcription factor, is required for pruning excessive neurites in Caenorhabditis elegans. Curr Biol. 2005;15:1554–1559. doi: 10.1016/j.cub.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 2.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Li SH, Yu ZX, Shelbourne P, Li XJ. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington's disease mice. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 5.Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: Evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Q, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 7.Hoopfer ED, et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: Sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 9.Truman JW. Metamorphosis of the central nervous system of Drosophila. J Neurobiol. 1990;21:1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 10.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 11.Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–737. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- 12.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: Insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132:3631–3642. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci USA. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T, Marticke S, Sung C, Robinow S, Luo L. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000;28:807–818. doi: 10.1016/s0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51:283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 17.Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 18.Kuranaga E, et al. Drosophila IKK-related kinase regulates nonapoptotic function of caspases via degradation of IAPs. Cell. 2006;126:583–596. doi: 10.1016/j.cell.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Oshima K, et al. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16:1531–1537. doi: 10.1016/j.cub.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro RS, Anderson KV. Drosophila Ik2, a member of the I kappa B kinase family, is required for mRNA localization during oogenesis. Development. 2006;133:1467–1475. doi: 10.1242/dev.02318. [DOI] [PubMed] [Google Scholar]
- 22.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004;2:e429. doi: 10.1371/journal.pbio.0020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frohlich KU. An AAA family tree. J Cell Sci. 2001;114:1601–1602. doi: 10.1242/jcs.114.9.1601. [DOI] [PubMed] [Google Scholar]
- 25.Frickey T, Lupas AN. Phylogenetic analysis of AAA proteins. J Struct Biol. 2004;146:2–10. doi: 10.1016/j.jsb.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Hartman JJ, et al. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. doi: 10.1016/s0092-8674(00)81578-0. [DOI] [PubMed] [Google Scholar]
- 27.McNally KP, Bazirgan OA, McNally FJ. Two domains of p80 katanin regulate microtubule severing and spindle pole targeting by p60 katanin. J Cell Sci. 2000;113:1623–1633. doi: 10.1242/jcs.113.9.1623. [DOI] [PubMed] [Google Scholar]
- 28.Giot L, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 29.McNally KP, Buster D, McNally FJ. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil Cytoskeleton. 2002;53:337–349. doi: 10.1002/cm.10080. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, et al. Regulation of microtubule severing by katanin subunits during neuronal development. J Neurosci. 2005;25:5573–5583. doi: 10.1523/JNEUROSCI.0834-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. J Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illenberger S, et al. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J Biol Chem. 1996;271:10834–10843. doi: 10.1074/jbc.271.18.10834. [DOI] [PubMed] [Google Scholar]
- 33.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 34.Sharma N, et al. Katanin regulates dynamics of microtubules and biogenesis of motile cilia. J Cell Biol. 2007;178:1065–1079. doi: 10.1083/jcb.200704021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu C, Srayko M, Mains PE. The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol Biol Cell. 2004;15:142–150. doi: 10.1091/mbc.E03-06-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennerdell JR, Carthew RW. Heritable gene silencing in Drosophila using double-stranded RNA. Nat Biotechnol. 2000;18:896–898. doi: 10.1038/78531. [DOI] [PubMed] [Google Scholar]
- 37.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 38.Thibault ST, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]