Abstract
The underlying molecular mechanisms that promote bone marrow failure in Fanconi anemia are incompletely understood. Evidence suggests that enhanced apoptosis of hematopoietic precursors is a major contributing factor. Previously, enhanced apoptosis of Fanconi anemia type C–deficient (Fancc−/−) progenitors was shown to involve aberrant p38 MAPK activation. Given the importance of c-Jun N-terminal kinase (JNK) in the stress response, we tested whether enhanced apoptosis of Fancc−/− cells also involved altered JNK activation. In Fancc−/− murine embryonic fibroblasts, tumor necrosis factor α (TNF-α) induced elevated JNK activity. In addition, JNK inhibition protected Fancc−/− murine embryonic fibroblasts and c-kit+ bone marrow cells from TNF-α-induced apoptosis. Importantly, hematopoietic progenitor assays demonstrated that JNK inhibition enhanced Fancc−/− colony formation in the presence of TNF-α. Competitive repopulation assays showed that Fancc−/− donor cells cultured with the JNK inhibitor had equivalent levels of donor chimerism compared with Fancc−/− donor cells cultured with vehicle control. In contrast, culturing Fancc−/− cells with a p38 MAPK inhibitor significantly increased repopulating ability, supporting an integral role of p38 MAPK in maintaining Fancc−/− hematopoietic stem cell function. Taken together, these data suggest that p38 MAPK, but not JNK, has a critical role in maintaining the engraftment of Fancc−/−-reconstituting cells under conditions of stress.
Introduction
Persons with Fanconi anemia (FA) are at high risk for developing life-threatening hematologic diseases, most commonly bone marrow (BM) failure, myelodysplasia, and acute myeloid leukemia. Despite significant advances in identification of FA genes (13 to date) and in elucidating FA protein-protein interactions,1 few data are available investigating the molecular basis for the functional deficits detected in primary FA hematopoietic stem and progenitor cells. To understand FA disease pathogenesis with the ultimate goal of designing novel therapeutic targets, exploration of the molecular underpinnings of hematopoietic stem and progenitor cell dysfunction is required.
Enhanced apoptosis of hematopoietic stem and progenitor cells is a key cellular mechanism promoting evolution of the hematologic sequelae in FA. In particular, inflammatory cytokines such as tumor necrosis factor α (TNF-α) contribute to the loss of FA stem and progenitor cells via at least 2 distinct mechanisms. First, inflammatory cytokine overproduction including TNF-α is documented in human subjects2–4 and FA type C–deficient (Fancc−/−) mice challenged with lipopolysaccharide (LPS),5 a potent inflammatory stimulus. Second, Fancc−/− hematopoietic progenitors are exquisitely sensitive to low doses of TNF-α and other inflammatory mediators such as interferon γ and LPS with a concomitant increase in apoptosis and senescence.5–8 Interestingly, LPS sensitivity of Fancc−/− progenitors is abrogated by blocking TNF-α, emphasizing the importance of TNF-α in promoting hematopoietic deficits in FA. In addition, Fancc−/− progenitors are highly sensitive to oxidants.9–11 Indeed, enhanced sensitivity of Fancc−/− progenitors to inflammatory mediators requires the production of reactive oxygen species (ROS).5,6,9,12,13 These observations are disconcerting because hematopoietic stem and progenitor cells are continuously exposed to low levels of inflammatory cytokines and ROS under homeostatic conditions and to high levels during times of hematopoietic stress. Collectively, these data support a critical role for altered TNF-α signaling in the pathogenesis of BM failure associated with FA.
Previously, we demonstrated that enhanced oxidant and TNF-α–induced apoptosis in Fancc−/− murine embryonic fibroblasts (MEFs) and hematopoietic progenitors is dependent on apoptosis signal-regulating kinase 1 (ASK1).9,12 ASK1 activation signals apoptosis via downstream kinases such as p38 mitogen activated protein kinase (MAPK) and c-jun N-terminal kinase (JNK).14 In addition, p38 MAPK inhibition ameliorates the enhanced apoptotic response of Fancc−/− MEFs and hematopoietic progenitors to oxidants and TNF-α.12 Recently, aberrant p38 MAPK and JNK activation was shown to partially contribute to LPS-induced Fancc−/− hematopoietic suppression.5 However, the role of JNK in regulating TNF-α–induced apoptosis of Fancc−/− cells remains unknown. Furthermore, no studies have investigated whether aberrant JNK and/or p38 MAPK activation are molecular mechanisms that promote Fancc−/− hematopoietic stem cell (HSC) dysfunction. Therefore, the current studies investigate the contribution of aberrant JNK activation in signaling enhanced TNF-α–induced apoptosis of Fancc−/− MEFs and hematopoietic progenitors and examine whether JNK or p38 MAPK inhibition improves Fancc−/− HSC repopulating ability.
Methods
Mice
B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ, CD45.1+) mice were obtained from the Stem Cell Transplant Mouse Core in the Indiana University Cancer Center (Indianapolis, IN) for use as competitor cells and recipient mice in competitive repopulation experiments. Fancc+/− mice15 in a C57Bl/6J genetic background (CD45.2+) were bred to generate Fancc−/− and wild-type (WT) mice for hematopoietic assays and timed embryos for MEF cell lines as previously described.9,12 All studies using MEFs were conducted in at least 2 or 3 different cell lines per genotype, and only MEFs less than passage 5 were used. All of the studies were approved by the Indiana University School of Medicine Animal Care and Use Committee.
JNK in vitro kinase assay
WT and Fancc−/− MEFs were either untreated or treated with 50 ng/mL TNF-α for 30 minutes. MEFs were then washed twice with cold phosphate-buffered saline containing 1 mM sodium orthovanadate and lysed in nonionic lysis buffer. JNK immunoprecipitation was conducted using protein A Sepharose beads (GE Healthcare, Little Chalfont, United Kingdom) and anti-JNK antibody (Cell Signaling Technology, Danvers, MA). Kinase assays were conducted as previously described, except that Elk-1 (Cell Signaling Technology) was used as a substrate.9 Densitometry was conducted using National Institutes of Health Image software to quantitate arbitrary density units. Mean arbitrary densitometry units were calculated to demonstrate statistical significance.
Western blotting
Cell lysates were prepared as previously described.9 Whole-cell extracts were separated by electrophoresis and transferred to nitrocellulose membranes before immunodetection of JNK. JNK immunoblotting was conducted using a rabbit anti–JNK antibody (Cell Signaling Technology) at a 1:200 dilution before incubating with a secondary antibody anti–rabbit horseradish peroxidase (1:3000 dilution; GE Healthcare).
Small interfering RNA transfection
The JNK1 siGENOME SMARTpool (Dharmacon RNA Technologies, Lafayette, CO) was used in these studies. The JNK1 small interfering RNA (siRNA) pool contains 4 oligonucleotides that efficiently target JNK1 (Table 1). In addition, because JNK1 and JNK2 share a high degree of homology, JNK2 is also targeted. Thus, transfection of the JNK1 siRNA pool results in decreased expression of both JNK1 and JNK2. The JNK1 siRNA pool was used according to the manufacturer's recommendations. For every transfection experiment, either JNK1 pool siRNA or scrambled siRNA (control) oligonucleotides (Table 1) was used at a 200-nM concentration in Opti-MEM (Invitrogen, Carlsbad, CA). WT and Fancc−/− MEFs were cultured in a 6-well tissue culture dish (2 × 105 cells/well) at 30% to 50% confluence. Oligofectamine transfections were conducted exactly per the manufacturer's recommendations (Invitrogen) and as previously described.9 After the transfections, MEFs were incubated for 48 hours at 37°C before harvesting for apoptosis assays and JNK Western blotting. Three independent transfections were conducted with similar results.
Table 1.
JNK1 and control siRNA oligonucleotide sequences
| Pool | siRNA sequence |
|---|---|
| JNK1 | |
| 1 | 5′-UUGUAUAUCAGUUCUUUCCUU |
| 2 | 5′-UCUUUCAUGAUCUAACUCCUU |
| 3 | 5′-GAAUGUAGAAUCUCCAAUCUU |
| 4 | 5′-UACUGUUGGUUGUAGUUUCUU |
| Control siRNA | |
| 1 | 5′-AUGAACGUGAAUUGCUCAA |
| 2 | 5′-UAAGGCUAUGAAGAGAUAC |
| 3 | 5′-AUGUAUUGGCCUGUAUUAG |
| 4 | 5′-UAGCGACUAAACACAUCAA |
Apoptosis assay
MEFs were maintained as previously described,9 and c-kit+ cells were purified by fluorescence cytometry using a BD FACStar flow cytometer (BD Biosciences, San Jose, CA) as previously described.9 WT and Fancc−/− MEFs or c-kit+ cells were cultured with 10 μM JNK inhibitor (JNK III; Calbiochem, San Diego, CA) overnight before treating with TNF-α. Apoptosis was analyzed using a TdT-mediated dUTP nick-end labeling (TUNEL) assay (Roche Diagnostics, Indianapolis, IN) as previously described.9 For each experiment, at least 100 cells were evaluated (Hoechst+) to determine the percentage of apoptotic cells (TUNEL+) in each condition.
Hematopoietic progenitor assays
WT and Fancc−/− BM low-density mononuclear cells (MNCs) were prepared and plated in clonogenic hematopoietic progenitor assays as previously described.7,12,16 Briefly, low-density MNCs from the BM of WT and Fancc−/− mice were resuspended in Iscove modified Dulbecco medium (IMDM; Invitrogen) supplemented with 20% fetal calf serum (Lonza Walkersville, Walkersville, MD). Cells were plated in methylcellulose colony assays (3 × 104 cells/mL) in the presence or absence of TNF-α. All conditions were conducted in triplicate. For inhibitor studies, either 10 μM JNK III or vehicle control (dimethyl sulfoxide [DMSO]) was added directly to the methylcellulose cultures. Total colonies (including colony-forming unit–granulocyte, erythroid, monocyte, megakaryocyte; burst-forming unit–erythroid; and colony-forming unit–granulocyte macrophage) were scored 7 days after plating.
Transplantation experiments
Competitive repopulation experiments were conducted similar to previously described methods.16 Donor low-density MNCs were cultured in IMDM supplemented with 20% fetal calf serum, 200 U/mL of hIL-6 (PeproTech, Rocky Hill, NJ), 100 ng/mL mSCF (PeproTech), and either a DMSO vehicle control, or 10 μM JNK III, or 5 μM SB203580 (a p38 MAPK inhibitor, Calbiochem) for 4 days before harvesting cells for transplantation studies. Freshly isolated low-density MNCs from B6.BoyJ mice were used as competitor cells. A total of 2 × 106 cells were transplanted into lethally irradiated B6.BoyJ recipients. Each cell mixture was resuspended in 0.25 mL of IMDM 20% fetal calf serum before injecting into the tail vein of 6 to 8 recipients. CD45.1 and CD45.2 chimerism was analyzed by fluorescence cytometry as previously described.16,17 Two-color multilineage analysis was conducted using phycoerythrin-conjugated antibodies against B220, CD3, Gr1, and Mac1 (BD Biosciences Pharmingen, San Diego, CA).
Statistical analyses
For all data shown, an unpaired Student t test was conducted to evaluate for differences between treatment groups, and a P value less than or equal to .05 was considered significant. Data are presented as mean plus or minus SEM, unless otherwise stated.
Results
JNK contributes to the hypersensitivity of Fancc−/− MEFs and progenitors to TNF-α–mediated apoptosis
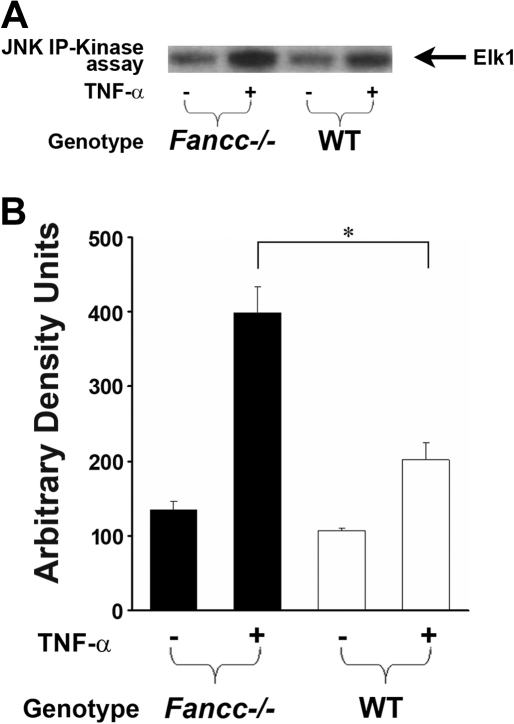
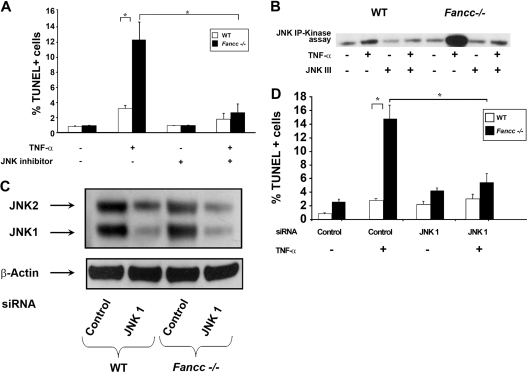
Previously, we showed that TNF-α–induced apoptosis of Fancc−/− MEFs and hematopoietic progenitors is dependent on ASK1 hyperactivation.12 Given that JNK is a downstream effector of ASK1 together with data demonstrating that prolonged JNK activation induces apoptosis,18 we questioned whether hyperactivation of JNK is involved in the proapoptotic phenotype observed in Fancc−/− cells. To test this hypothesis, JNK activity was examined in WT and Fancc−/− MEFs that were either untreated or treated with TNF-α using an in vitro kinase assay. Consistently, Fancc−/− MEFs treated with TNF-α exhibited an increase in JNK activity compared with WT (Figure 1). To examine whether JNK mediates enhanced TNF-α–induced apoptosis of Fancc−/− cells, MEFs were treated with a JNK inhibitor before exposing to TNF-α and quantitating apoptosis using a TUNEL assay. These studies demonstrate that JNK inhibition reduced TNF-α–induced apoptosis of Fancc−/− MEFs to WT levels (Figure 2A). In vitro kinase assays verified that JNK activity was diminished in the presence of the JNK inhibitor (Figure 2B). To confirm that JNK was involved in promoting apoptosis of Fancc−/− MEFs, a genetic approach to knockdown expression of JNK1 and JNK2 was conducted using a JNK1 siRNA pool that targets both JNK1 and JNK2. A pool of 4 JNK1 siRNAs or 4 control scrambled siRNAs (Table 1) were transfected into WT and Fancc−/− MEFs, similar to previous studies.9 Forty-eight hours after transfection, MEFs were treated with TNF-α and apoptosis examined. Figure 2C illustrates a representative Western blot demonstrating approximately 75% reduction in JNK1 and JNK2 expression. Fancc−/− MEFs with reduced JNK 1/JNK2 expression had levels of apoptosis comparable with WT MEFs (Figure 2D). Together, these data demonstrate that enhanced TNF-α–induced apoptosis of Fancc−/− MEFs requires JNK.
Figure 1.
TNF-α induces aberrant JNK activation in Fancc−/− MEFs. (A) JNK activity assays. WT and Fancc−/− MEFs were either untreated or treated with 50 ng/mL TNF-α for 30 minutes before conducting JNK in vitro kinase assays. Autoradiography of a representative JNK kinase assay is shown. (B) Mean densitometry data for JNK kinase experiments are shown; n = 4. *P < .05.
Figure 2.
JNK inhibition protects Fancc−/− MEFs from TNF-α-induced apoptosis. (A) JNK inhibitor and apoptosis assays. WT and Fancc−/− MEFs were either grown in normal conditions or pretreated with JNK III before 50 ng/mL TNF-α treatment. Apoptosis was analyzed by TUNEL; n = 3, *P < .003. (B) JNK inhibitor and activity assays. WT and Fancc−/− MEFs were either untreated or treated with 50 ng/mL TNF-α for 30 minutes in the presence or absence of JNK III before conducting JNK in vitro kinase assays. Autoradiography of a representative JNK kinase assay of 3 independent experiments is shown. (C) JNK siRNA Western blot. WT and Fancc−/− MEFs were transfected with either a pool of JNK1 or control siRNA oligonucleotides. Forty-eight hours after transfection, cells were used for Western blotting and for culture with 50 ng/mL TNF-α overnight. Representative JNK and β-actin Western blots are shown. (D) JNK siRNA apoptosis assays. Data shown are the mean of 3 independent transfection experiments with similar results. *P < .01.
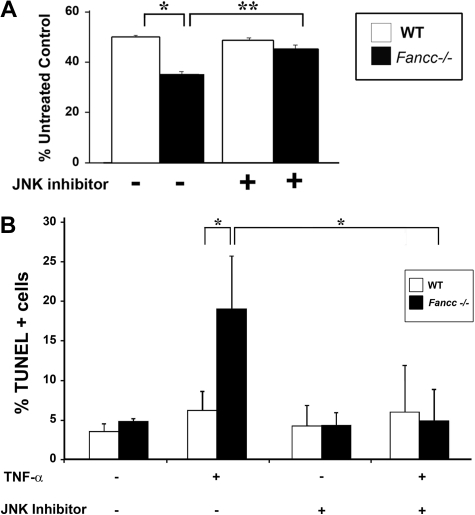
To extend these observations in the hematopoietic system, we investigated whether TNF-α hypersensitivity of primary Fancc−/− hematopoietic progenitors is mediated through JNK. Clonogenic progenitor assays were conducted in the presence of TNF-α and either vehicle control or the JNK inhibitor. In these studies, the JNK inhibitor enhanced Fancc−/− progenitor colony formation to WT levels (Figure 3A), similar to data in MEFs. To investigate whether the mechanism responsible for enhanced colony formation was the result of improved survival, apoptosis was assessed in c-kit+ cells. Similar to Fancc−/− MEFs, inhibition of JNK in Fancc−/− c-kit+ cells reduced TNF-α–induced apoptosis to WT levels (Figure 3B). Collectively, these data suggest that enhanced TNF-α sensitivity of Fancc−/− hematopoietic progenitors involves altered JNK activity. Furthermore, these data support the idea that aberrant JNK activation has a role in the pathogenesis of BM failure in FA.
Figure 3.
Inhibition of JNK enhances survival of Fancc−/− progenitors treated with TNF-α. (A) JNK inhibitor and progenitor assays. WT and Fancc−/− low-density MNCs were cultured in colony assays with either JNK III or a DMSO control in the presence or absence of 10 ng/mL TNF-α. Data are shown as a percentage of untreated control conditions where no TNF-α was added; n = 3 mice/genotype. *P < .01; **P < .002. (B) TNF-α–induced apoptosis in c-kit+ cells. WT and Fancc−/− c-kit+ cells were either pretreated with JNK III or a DMSO control before a 24-hour exposure to 10 ng/mL TNF-α. Cytospins of cells from each experimental group were made before analyzing apoptosis using a TUNEL assay; n = 3. *P < .03.
Inhibition of p38 MAPK, but not JNK, improves multilineage repopulating ability of Fancc−/− HSCs
Given the important role that HSC dysfunction has in the pathogenesis of BM failure in FA, we next sought to determine whether Fancc−/− HSC function could be enhanced by JNK inhibition. We took advantage of previous data from our group showing that Fancc−/− HSCs undergo enhanced apoptosis during a short ex vivo culture system, resulting in reduced repopulating ability.16,19 If Fancc−/− HSC apoptosis is JNK dependent, we predicted that adding the JNK inhibitor to the culture system would enhance survival of Fancc−/− HSCs and increase repopulating ability. To test this hypothesis, competitive repopulation transplantations were conducted using WT and Fancc−/− donor cells cultured with either a vehicle control or the JNK inhibitor. Surprisingly, Fancc−/− donor cells cultured with the JNK inhibitor had levels of donor chimerism that were equivalent to Fancc−/− donor cells cultured with the vehicle control 6 months after transplantation (64.9% ± 2.7% compared with 62.3% ± 4.0%, n = 8 recipients/transplantation group). These data suggest that Fancc−/− HSC activity is not enhanced by JNK inhibition, even though JNK inhibition clearly protects Fancc−/− MEFs and hematopoietic progenitors in vitro.
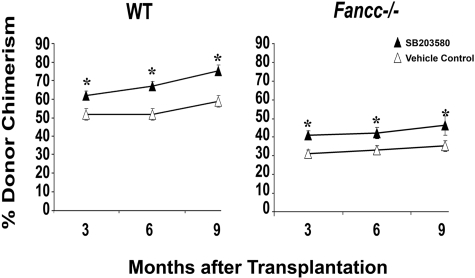
Previously, we identified p38 MAPK as a potential molecular target to enhance survival of Fancc−/− MEFs and progenitors.12 Given the current studies showing that Fancc−/− progenitors are protected by a JNK inhibitor whereas Fancc−/− HSCs are not, it was imperative to rigorously examine whether p38 MAPK is a viable molecular target for FA. Using the same experimental design described for JNK inhibitor studies, Fancc−/− cells were cultured with either a vehicle control or SB203580 (a p38 MAPK inhibitor) before transplanting as donor cells in competitive repopulation assays. In contrast to studies with the JNK inhibitor, a significant increase in donor chimerism was observed for Fancc−/− cells cultured with SB203580 compared with Fancc−/− cells cultured with the vehicle control (Figure 4). Equivalent increases in donor cell reconstitution of myeloid and lymphoid cells were observed (data not shown), supporting an increase in HSC function. Interestingly, an increase in donor chimerism was also observed in WT cells cultured with SB203580 compared with WT cells cultured with the vehicle control, consistent with previous studies showing that p38 MAPK regulates normal HSC function.20 At the time of death, all primary recipient mice had normal peripheral blood counts and BM and spleen histology (data not shown). Collectively, these data demonstrate that inhibition of p38 MAPK, but not JNK, may be a potential molecular target to enhance Fancc−/− HSC function.
Figure 4.
Inhibition of p38 MAPK enhances long-term repopulating ability of WT and Fancc−/− HSCs. WT and Fancc−/− low-density MNCs were cultured with either a vehicle control or SB203580 for 4 days. Cultured cells were enumerated and used as donor cells in competitive repopulation studies. Two separate transplantation experiments were conducted with 6 or 7 recipients transplanted for each of 4 experimental groups: WT plus vehicle control, WT plus SB203580, Fancc−/− plus vehicle control, and Fancc−/− plus SB203580. Peripheral blood donor chimerism from individual transplant recipients from both experiments were combined (a total of 53 transplanted mice). Data shown illustrate mean donor chimerism of 12 to 14 recipients from each of the 4 experimental groups at 3, 6, and 9 months after transplantation. *P < .05, comparing SB203580 to vehicle control within each genotype.
Discussion
Targeting molecular pathways that are aberrantly activated in diseased cells is a growing area of investigation, based on the success of imatinib mesylate (Gleevec; Novartis, East Hanover, NJ) for the treatment of chronic myelogenous leukemia.21,22 These seminal observations provide new promise for the management of other incurable or chronic hematologic diseases, including FA. Despite transplantation centers around the world reporting improved survival of persons with FA after HSC transplantation, a minority of FA patients find optimal donors, and FA persons continue to be at increased risk for transplantation-related mortality and morbidity.23,24 Important complications that impact successful hematopoietic reconstitution include graft failure, end organ toxicity, infections, graft-versus-host disease, and secondary malignancies. Given that HSC transplantation is currently the only therapy to restore long-term hematopoiesis in FA, improved treatment strategies are desperately needed. Identification of key molecular pathways that promote hematopoietic failure in FA may lead to novel targeted therapies.
Enhanced apoptosis of hematopoietic stem and progenitor cells has a central role in the pathogenesis of BM failure and leukemogenesis in FA. Multiple stimuli encountered in vivo promote apoptosis of Fancc−/− stem and progenitor cells, including TNF-α. Therefore, by dissecting aberrant activation of proteins involved in the proapoptotic phenotype of Fancc−/− cells, a rational selection of molecules to target may be elucidated with the ultimate goal of enhancing survival of Fancc−/− hematopoietic stem and progenitor cells. Using this approach, we and others have identified JNK as a potential molecular candidate. Our data examining the role of JNK in TNF-α sensitivity of Fancc−/− cells are consistent with recent studies showing prolonged JNK activation in Fancc−/− BM cells after LPS treatment.5 In addition, JNK inhibition partially protected Fancc−/− progenitors from LPS-induced growth suppression. Collectively, these observations suggest that altered JNK activity promotes hematopoietic dysfunction in FA.
In addition to JNK hyperactivation, previous studies from our group and others show that altered p38 MAPK activation in Fancc−/− and Fanca−/− cells contribute to hematopoietic dysfunction,5,12 at least in the progenitor compartment. Furthermore, p38 MAPK inhibition restores progenitor colony formation in BM samples from human patients with aplastic anemia and myelodysplastic syndrome,25,26 suggesting a common mechanism of p38 MAPK dysregulation in promoting hematopoietic failure. However, the studies reported here are the first to examine whether aberrant p38 MAPK and/or JNK activation contribute to defective Fancc−/− HSC function.
In contrast to the protection afforded Fancc−/− progenitors, JNK inhibition did not enhance Fancc−/− HSC repopulating ability. On the contrary, inhibition of p38 MAPK resulted in an approximate 30% increase in the chimerism of mice transplanted with Fancc−/− reconstituting cells. On the surface, these observations may appear contradictory. However, significant evidence demonstrates that proteins may have distinct functional roles in HSCs versus more differentiated progenitors.20,27,28 Indeed, understanding the molecular regulation of “stemness” is postulated to provide key insights into the pathogenesis of cancer and aging.29 Microarray and proteomic studies show that phenotypically defined HSCs have quantitatively distinct mRNA and protein expression profiles, respectively, compared with more committed progenitor populations.30–33 These important studies identify molecules that are regulated by transcriptional, translational, and protein turnover mechanisms. However, for proteins, such as JNK and p38 MAPK, which are activated primarily through posttranslational modifications, these methodologies provide no information regarding their distinct roles in hematopoietic stem and/or progenitor cell compartments. In addition, HSCs are located in a more hypoxic microenvironment, are equipped with increased expression of proteins that combat oxidant stress, and have lower intracellular ROS concentrations compared with more committed hematopoietic progeny, suggesting that posttranslational redox regulation of protein function will be unique in HSCs compared with progenitors.31,34,35 Unfortunately, current technologies do not exist to evaluate for differences in posttranslational modifications in highly purified HSCs compared with progenitors. Future proteomic advances will be required to address these important questions to further our understanding of the molecular basis of hematologic diseases. Our data showing that p38 MAPK and JNK have distinct functional roles in Fancc−/− HSCs compared with Fancc−/− progenitors are congruent with these previous studies, demonstrating that the molecular repertoire of HSCs is unique compared with progenitors. This is a critical piece of information as we plan toward the future of identifying a molecular target to enhance HSC function in FA patients. Furthermore, these data emphasize the importance of testing potential molecular therapies for FA in HSC functional assays.
For a molecular targeting approach to be successful for FA, it is equally important to demonstrate that the therapy does not enhance the evolution of clonal hematologic disorders. This is especially relevant for FA because persons are at high risk of developing hematologic and nonhematologic malignancies. Importantly, none of the recipient mice transplanted with Fancc−/− donor cells cultured with the p38 MAPK inhibitor (n = 24) developed evidence of clonal hematopoietic disease. This observation was reassuring because blocking an apoptotic protein could theoretically enhance the survival of mutated Fancc−/− HSC clones and increase the risk for leukemia in transplanted mice. Future studies are planned to examine whether prolonged p38 MAPK inhibition enhances Fancc−/− hematopoietic stem and progenitor cell function without the development of clonal evolution.
Several lines of evidence support the concept that p38 MAPK has an integral role in the maintenance of HSC self-renewal, cell cycle regulation, and response to oxidant stress.20,36 Ataxia telangectasia-mutated (Atm)-deficient mice develop BM failure as they age,20 which coincides with increased intracellular ROS and activated p38 MAPK in phenotypically defined HSCs. Importantly, Atm−/− HSC defects are corrected by p38 MAPK inhibition with administration of the same p38 inhibitor used in our studies (SB203580). Furthermore, in normal HSCs, increased ROS and p38 MAPK activation promotes HSC senescence, similar to the effects of aging. For example, serial transplantation of WT HSCs results in enhanced ROS, p38 MAPK activity, and expression of tumor suppressor proteins, p16Ink4a and p19Arf20. Importantly, treatment of serially transplanted mice with a p38 MAPK inhibitor improves HSC reconstitution, directly linking p38 MAPK activity with HSC dysfunction. In addition, when WT c-kit+Sca1+lin−CD34− cells are separated based on intracellular ROS content, ROShigh cells exhibit higher p38 MAPK activity and reduced self-renewal capacity compared with ROSlow cells.34 Furthermore, inhibition of p38 MAPK, but not JNK, improved ROShigh c-kit+Sca1+lin−CD34− cell hematopoietic activity, supporting a differential requirement of stress-activated kinases in the maintenance of HSC function. Our data demonstrating increased donor chimerism in recipients transplanted with WT cells cultured with a p38 MAPK inhibitor, but not a JNK inhibitor, are consistent with these observations and support a central role for p38 MAPK in regulating HSC function. Collectively, these results strongly support a role for aberrant p38 MAPK activation in promoting Fancc−/− HSC dysfunction as well as regulating stress-induced HSC defects.
In the context of FA, previous studies support the idea that p38 MAPK and JNK have distinct roles in promoting hematopoietic pathology.5 Our studies are the first to suggest that p38 MAPK and JNK inhibition protects Fancc−/− HSCs to different degrees. Hematopoietic progenitor survival is improved by inhibition of either p38 MAPK or JNK, whereas HSC repopulating ability is enhanced only by p38 MAPK inhibition. Understanding how dysregulated activation of p38 MAPK and JNK in terminally differentiated Fancc−/− hematopoietic cells affects their function will be equally important because it is well established that signaling through both of these stress-activated protein kinases increases inflammatory cytokine production, which are elevated in FA patients and Fancc−/− mice treated with LPS.2–5 Indeed, recent studies in human FA type C lymphoblasts demonstrate that aberrant activation of both p38 MAPK and JNK contributes to TNF-α overproduction.37
In conclusion, an in-depth interrogation of molecular mechanisms that promote altered hematopoiesis in FA is required to understand hematopoietic disease pathogenesis. We propose that, by defining the molecular pathways responsible for reduced survival of FA hematopoietic stem and progenitor cells, the potential may exist to develop novel therapeutic strategies for the treatment and/or prevention of BM failure in FA. The studies reported are one step toward this overall goal. Although enhanced activity of both p38 MAPK and JNK contributes to Fancc−/− progenitor survival defects, only inhibition of p38 MAPK enhances Fancc−/− HSC repopulating ability. Future preclinical studies assessing the effectiveness of prolonged p38 MAPK inhibition on improving Fancc−/− hematopoietic stem and progenitor cell defects without enhancing clonal evolution will be an important next step toward achieving the long-term goal of identifying new therapies for persons with FA.
Acknowledgments
The authors thank Dr Helmut Hanenberg (University of Dusseldorf) for thoughtful critique of the manuscript and Janice Walls and Marilyn Wales for excellent administrative support.
This work was supported by US Public Health Services (grant R01 HL077175, L.S.H.; grant R01 HL077177, R.K.; grant P01 HL53586, L.S.H.; and grant P30 CA82709, L.S.H.), the Fanconi Anemia Research Fund (Eugene, OR; L.S.H.), and the Riley Children's Foundation (Indianapolis, IN; R.K., L.S.H.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.R.S. and K.B.-V. designed research, performed research, and analyzed data; R.K. designed research and interpreted data; and L.S.H. designed research, analyzed data, and wrote manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laura S. Haneline, Indiana University School of Medicine, Herman B Wells Center for Pediatric Research, 1044 West Walnut Street, R4-476, Indianapolis, IN 46202; e-mail: lhanelin@iupui.edu.
References
- 1.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8:735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 2.Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi's anemia. Am J Hematol. 1993;42:196–201. doi: 10.1002/ajh.2830420211. [DOI] [PubMed] [Google Scholar]
- 3.Rosselli F, Sanceau J, Gluckman E, Wietzerbin J, Moustacchi E. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia: II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood. 1994;83:1216–1225. [PubMed] [Google Scholar]
- 4.Dufour C, Corcione A, Svahn J, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood. 2003;102:2053–2059. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 5.Sejas DP, Rani R, Qiu Y, et al. Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice. J Immunol. 2007;178:5277–5287. doi: 10.4049/jimmunol.178.8.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence. J Cell Sci. 2007;120:1572–1583. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haneline LS, Broxmeyer HE, Cooper S, et al. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac−/− mice. Blood. 1998;91:4092–4098. [PubMed] [Google Scholar]
- 8.Otsuki T, Nagakura S, Wang J, Bloom M, Grompe M, Liu JM. Tumor necrosis factor-alpha and CD95 ligation suppress erythropoiesis in Fanconi anemia C gene knockout mice. J Cell Physiol. 1999;179:79–86. doi: 10.1002/(SICI)1097-4652(199904)179:1<79::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS. Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J Biol Chem. 2004;279:16805–16812. doi: 10.1074/jbc.M313721200. [DOI] [PubMed] [Google Scholar]
- 10.Du W, Adam Z, Zhang X, Rani R, Pang Q. Oxidative stress in Fanconi anemia hematopoiesis and disease progression. Antioxid Redox Signal. 2008;10:1909–1921. doi: 10.1089/ars.2008.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjur S, Ung K, Wadsworth L, et al. Defective hematopoiesis and hepatic steatosis in mice with combined deficiencies of the genes encoding Fancc and Cu/Zn superoxide dismutase. Blood. 2001;98:1003–1011. doi: 10.1182/blood.v98.4.1003. [DOI] [PubMed] [Google Scholar]
- 12.Bijangi-Vishehsaraei K, Saadatzadeh MR, Werne A, et al. Enhanced TNF-alpha-induced apoptosis in Fanconi anemia type C-deficient cells is dependent on apoptosis signal-regulating kinase 1. Blood. 2005;106:4124–4130. doi: 10.1182/blood-2005-05-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Sejas DP, Zhang X, et al. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest. 2007;117:3283–3295. doi: 10.1172/JCI31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Tomkins DJ, Auerbach W, et al. Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nat Genet. 1996;12:448–451. doi: 10.1038/ng0496-448. [DOI] [PubMed] [Google Scholar]
- 16.Haneline LS, Li X, Ciccone SL, et al. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc−/− hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- 17.Haneline LS, Gobbett TA, Ramani R, et al. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94:1–8. [PubMed] [Google Scholar]
- 18.Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Le Beau MM, Ciccone S, et al. Ex vivo culture of Fancc−/− stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005;105:3465–3471. doi: 10.1182/blood-2004-06-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 21.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 22.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant. 2008;41:127–132. doi: 10.1038/sj.bmt.1705960. [DOI] [PubMed] [Google Scholar]
- 24.Dufour C, Svahn J. Fanconi anaemia: new strategies. Bone Marrow Transplant. 2008;41(suppl 2):S90–S95. doi: 10.1038/bmt.2008.63. [DOI] [PubMed] [Google Scholar]
- 25.Navas TA, Mohindru M, Estes M, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–4177. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A, Deb DK, Sassano A, et al. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. J Biol Chem. 2002;277:7726–7735. doi: 10.1074/jbc.M106640200. [DOI] [PubMed] [Google Scholar]
- 27.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 28.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 29.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 31.Unwin RD, Smith DL, Blinco D, et al. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood. 2006;107:4687–4694. doi: 10.1182/blood-2005-12-4995. [DOI] [PubMed] [Google Scholar]
- 32.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 33.Zhong JF, Zhao Y, Sutton S, et al. Gene expression profile of murine long-term reconstituting vs. short-term reconstituting hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:2448–2453. doi: 10.1073/pnas.0409459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 37.Briot D, Mace-Aime G, Subra F, Rosselli F. Aberrant activation of stress-response pathways leads to TNF-alpha oversecretion in Fanconi anemia. Blood. 2008;111:1913–1923. doi: 10.1182/blood-2007-07-099218. [DOI] [PubMed] [Google Scholar]