Abstract
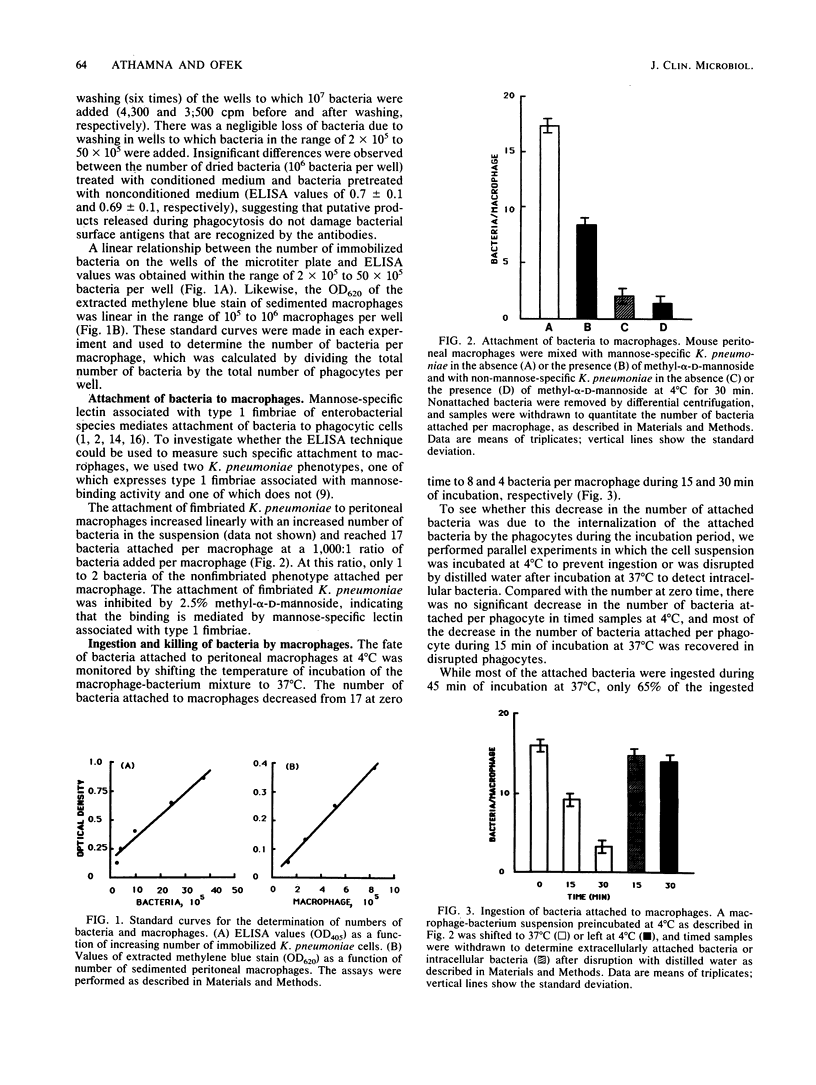
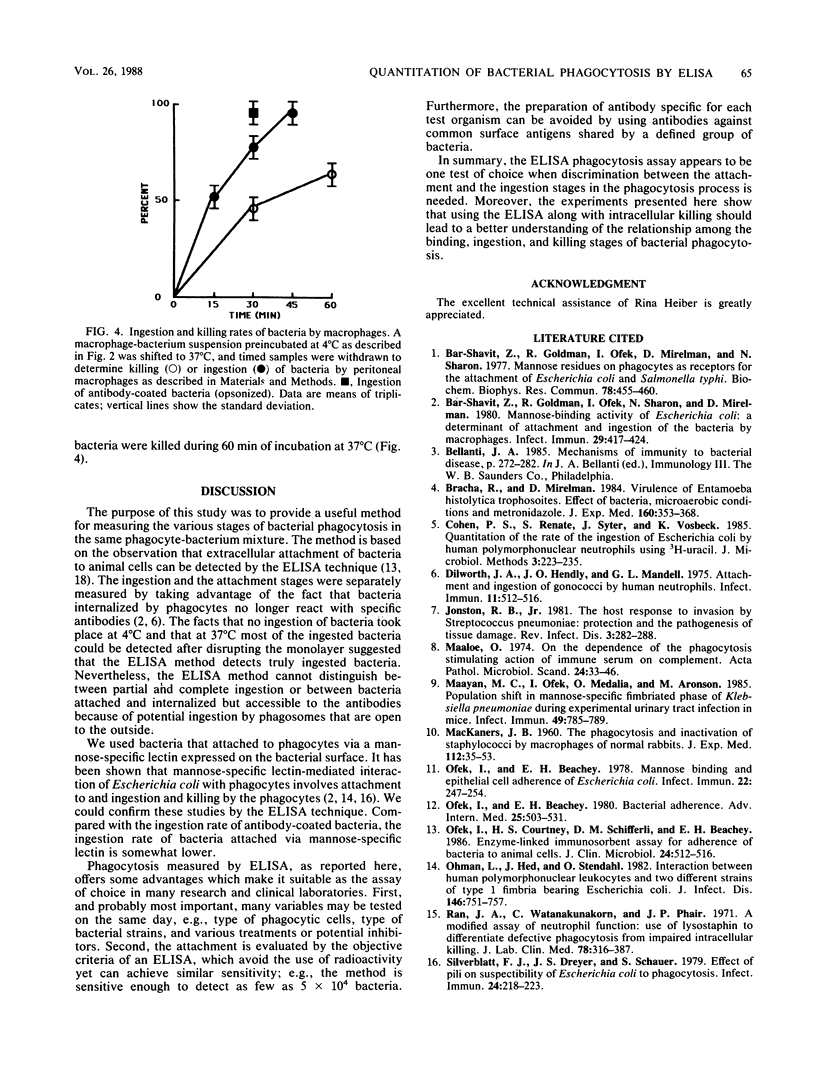
Research on phagocytosis of bacteria is often hampered by the inability to distinguish quantitatively between bacteria that have been ingested by phagocytic cells and those which are attached to the surface of the cells. A method using the enzyme-linked immunosorbent assay technique to simply and accurately measure the rate of bacterial ingestion by phagocytic cells is described. The method is based on the ability of antibacterial antibodies to bind to bacteria attached to but not internalized by phagocytic cells. The attached bacteria were quantitated by enzyme-linked immunosorbent assay. Compared with the number of bacteria at zero time (17 bacteria attached per phagocyte) only 10 to 20% of the bacteria remained attached to phagocytic cells after incubation for 30 min at 37 degrees C. The decrease in detected attached bacteria at 37 degrees C was due to internalization of the bacteria by phagocytic cells, since upon disruption of the monolayer, most of the ingested bacteria were recovered, and at 4 degrees C, most of the bacteria remained extracellularly attached. The proposed attachment and ingestion assay is easy to perform, allows the detection of specific attachment of test bacteria, and provides objective quantitation of attached and ingested bacteria. Most importantly, the assay allows testing of ingestion rates of bacteria under many variables on the same day.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Bracha R., Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984 Aug 1;160(2):353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr The host response to invasion by Streptococcus pneumoniae: protection and the pathogenesis to tissue damage. Rev Infect Dis. 1981 Mar-Apr;3(2):282–288. doi: 10.1093/clinids/3.2.282. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan M. C., Ofek I., Medalia O., Aronson M. Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun. 1985 Sep;49(3):785–789. doi: 10.1128/iai.49.3.785-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Courtney H. S., Schifferli D. M., Beachey E. H. Enzyme-linked immunosorbent assay for adherence of bacteria to animal cells. J Clin Microbiol. 1986 Oct;24(4):512–516. doi: 10.1128/jcm.24.4.512-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman L., Hed J., Stendahl O. Interaction between human polymorphonuclear leukocytes and two different strains of type 1 fimbriae-bearing Escherichia coli. J Infect Dis. 1982 Dec;146(6):751–757. doi: 10.1093/infdis/146.6.751. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O. Protection of phagocytized bacteria against antibiotics. A new method for the evaluation of neutrophil granulocyte functions. Acta Med Scand. 1972 May;191(5):383–387. [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- van den Broek P. J., Dehue F. A., Leijh P. C., van den Barselaar M. T., van Furth R. The use of lysostaphin in in vitro assays of phagocyte function: adherence to and penetration into granulocytes. Scand J Immunol. 1981 May;15(5):467–473. doi: 10.1111/j.1365-3083.1982.tb00672.x. [DOI] [PubMed] [Google Scholar]



