Abstract
Inhibition of GSK-3 reduces ischemia-reperfusion injury by mechanisms that involve the mitochondria. The goal of this study was to explore possible molecular targets and mechanistic basis of this cardioprotective effect. In perfused rat hearts, treatment with GSK inhibitors prior to ischemia significantly improved recovery of function. To assess the effect of GSK inhibitors on mitochondrial function under ischemic conditions, mitochondria were isolated from rat hearts perfused with GSK inhibitors and treated with uncoupler or cyanide, or made anoxic. GSK inhibition slowed ATP consumption under these conditions, which could be due to inhibition of ATP entry into the mitochondria through VDAC and/or ANT or to inhibition of the F1F0-ATPase. To determine the site of the inhibitory effect on ATP consumption, we measured the conversion of ADP to AMP by adenylate kinase located in the intermembrane space. This assay requires adenine nucleotide transport across the outer but not the inner mitochondrial membrane, and we found that GSK inhibitors slow AMP production similar to their effect on ATP consumption. This suggests that GSK inhibitors are acting on outer mitochondrial membrane transport. In sonicated mitochondria, GSK inhibition had no effect on ATP consumption or AMP production. In intact mitochondria, cyclosporin A had no effect, indicating that ATP consumption is not due to opening of the mitochondrial permeability transition pore. Since GSK is a kinase, we assessed whether protein phosphorylation might be involved. Therefore, we performed western blot and 1D/2D gel phosphorylation site analysis using phos-tag staining to indicate proteins that had decreased phosphorylation in hearts treated with GSK inhibitors. LC/MS analysis revealed one of these proteins to be VDAC2. Taken together, we found that GSK mediated signaling modulates transport through the outer membrane of the mitochondria. Both proteomics and adenine nucleotide transport data suggest that GSK regulates VDAC and suggest that VDAC may be an important regulatory site in ischemia-reperfusion injury.
Keywords: Mitochondria, GSK-3, VDAC, adenine nucleotide transport
INTRODUCTION
Ischemic heart disease is a major cause of morbidity and mortality worldwide. This has motivated a search for new approaches to reduce ischemic injury, propelled by the discovery of ischemic preconditioning (IPC) (1). Considerable data suggest that IPC leads to the release of adenosine, opioids, or bradykinin (2, 3), which bind to G-protein coupled receptors and initiate a signaling cascade that involves activation of phosphatidylinositol-3 kinase (PI-3 Kinase) and downstream kinases such as Akt, eNOS, PKC, mTOR, p70S6-Kinase, ERK, and GSK-3β (4–6).
Glycogen Synthase Kinase-3 (GSK-3), first identified as a regulator of glycogen metabolism, is also an important regulator of cell function, including gene expression, cell cycle, survival and apoptosis (7). GSK-3 belongs to the serine/threonine kinase family of proteins and the two known isoforms are GSK-3α (51 kDa) and GSK-3β (47 kDa), which share 98% homology and similar substrate specificities.
The activity of GSK-3β is regulated by phosphorylation (7). GSK-3β is active under normal resting conditions when it is not phosphorylated, and phosphorylation at serine 9 leads to its inactivation. The activity of GSK-3β is regulated by several signaling pathways as a number of kinases (Akt, PKC, PKA, and others) can phosphorylate GSK-3β on serine 9 and inactivate it.
In addition to its role in cell survival, proliferation and differentiation, GSK-3β has been proposed to be involved in the cardioprotective effects of IPC (8, 9) and various forms of pharmacologic cardioprotection (10–14). Our laboratory (8) previously demonstrated that the cardioprotection elicited by IPC is mediated by the inhibition of GSK via PI3 Kinase. It has also been reported that opioid-induced cardioprotection occurs via inactivation of GSK-3β by phosphorylation at serine 9 through PI3 Kinase and TOR-dependent pathways (11). Protective effects have been observed when GSK-3β inhibitors were administered immediately prior to ischemia (8), 24 hours prior to ischemia (13), or immediately upon reperfusion (11, 14). GSK inhibition is also protective during heart failure (15).
Recently GSK-3β has been described in mitochondria, and GSK inhibition is thought to block opening of the mitochondrial permeability transition pore in cardiomyocytes (10). However, the molecular targets of GSK-3β in mitochondria and the precise physiologic effects of GSK-3β inhibition on mitochondrial function have not been fully elucidated.
The present study was designed to identify downstream targets of GSK-3 and to link the phosphorylation of downstream targets to physiologic effects on mitochondrial function. We propose that cardioprotection resulting from inhibition of GSK-3 is partly mediated by modulation of mitochondrial bioenergetics.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats were used in this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University.
Langendorff Rat Heart Preparation
Hearts were perfused as described previously (16). Details are provided in the supplement.
Isolated Mitochondria Protocols
Mitochondria were isolated as described previously (16). The supplement provides details of the assays.
Proteomics
Details of the Western Blots, 1D/2D gel electrophoresis, and phospho-protein detection are provided in the supplement.
RESULTS
GSK-3 inhibitors increase recovery of contractile function after ischemia
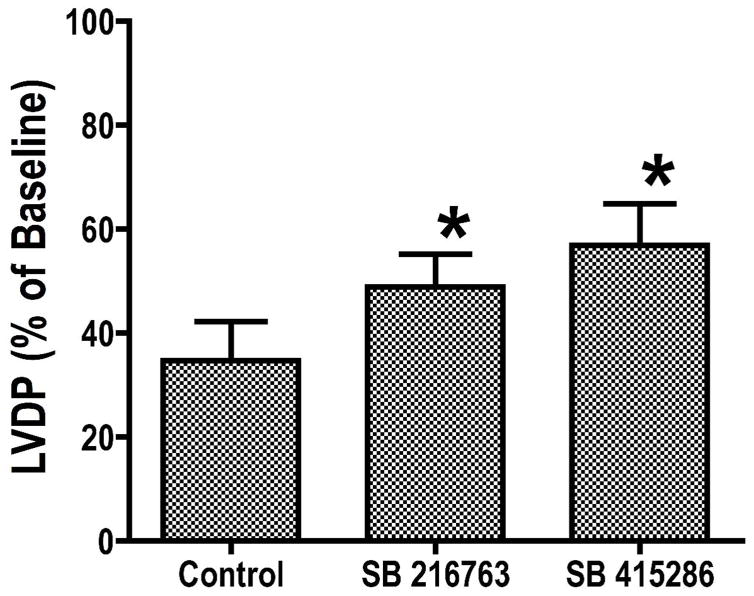
We previously reported that GSK-3 inhibitors improved recovery of function and reduced necrosis following ischemia. In this study, we examined the mechanisms involved, after confirming the protective effects of two GSK-3 inhibitors, SB216763 and SB415286. Dose-response experiments were performed to establish effective concentrations. As shown in Figure 1, either 3 μM SB216763 or 10 μM SB415286 significantly enhanced recovery of left ventricular developed pressure (LVDP) during reperfusion, to 48.9 ± 6.2% and 56.8 ± 8.1% of baseline compared with 34.7 ± 7.5% in control hearts (p< 0.05). Previous work from our laboratory (8) and others (11, 13–14) has shown that GSK-3 inhibitors also reduce necrosis.
Figure 1.
Hearts were perfused for 15 minutes with control buffer, then 15 minutes with GSK inhibitor or vehicle, 20 minutes global ischemia, followed by 40 minutes of reperfusion. Results are Means SEM (n=6). *p<0.05 vs. control.
GSK-3 inhibitors reduce the rate of ATP consumption in isolated mitochondria
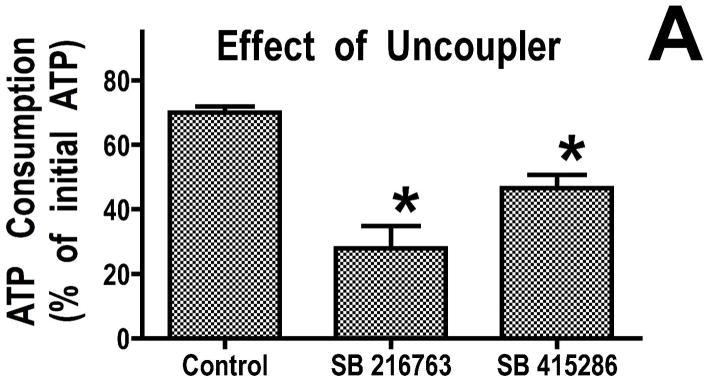
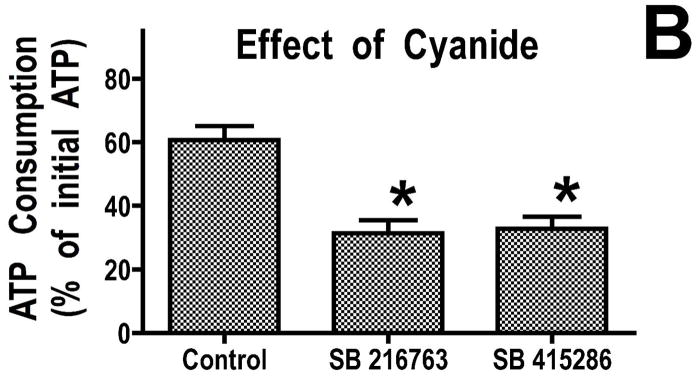
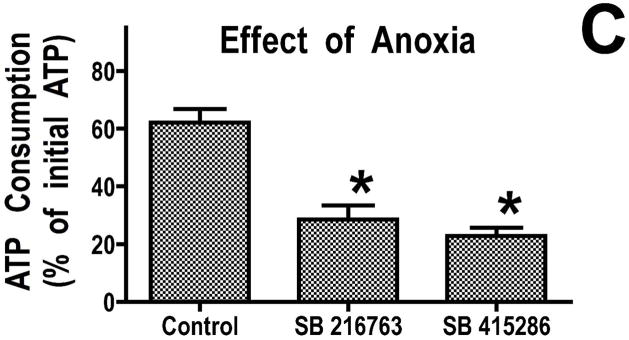
We previously found that addition of recombinant Bcl-2 to mitochondria resulted in reduced breakdown of ATP under de-energized conditions when the mitochondrial F1F0-ATPase runs in reverse (16). This reduced rate of ATP consumption was attributed to altered ATP transport into the mitochondria. To determine whether GSK-3 inhibitors also decreased ATP consumption under de-energized conditions, we studied mitochondria rapidly de-energized using the uncoupler, dinitrophenol. Under these conditions, mitochondria from hearts treated with GSK inhibitors consumed less ATP than control mitochondria (Figure 2A). Similarly, GSK inhibitors significantly reduced ATP consumption when sodium cyanide was used to stop mitochondrial respiration (Figure 2B). In the physiologically relevant model of mitochondrial de-energization, mitochondria were allowed to consume all of the oxygen in an oxygraph chamber, and then the consumption of ATP during the ensuing anoxic period was measured. ATP consumption was linear in both control and GSK inhibitor treated mitochondria, and after 60 minutes of anoxia, the amount of ATP consumed was significantly less in the GSK inhibitor treated mitochondria (Figure 2C). Mitochondria from ischemically preconditioned myocardium showed a similar slowing of ATP consumption (Supplemental Figure 1). This slowing of ATP consumption could be accomplished by attenuating ATP entry into the mitochondria through VDAC and/or the adenine nucleotide transporter (ANT) or by inhibition of the mitochondrial F1F0-ATPase.
Figure 2.
GSK inhibitors slow ATP consumption. Panel A, mitochondria were de-energized with dinitrophenol for 5 minutes, in Panel B, mitochondria were treated with cyanide for 20 minutes, and in Panel C, mitochondria were anoxic for 60 minutes. Both GSK inhibitors significantly decrease the rate of ATP hydrolysis. Results are Means ± SEM (n=6). *p<0.05 vs. control.
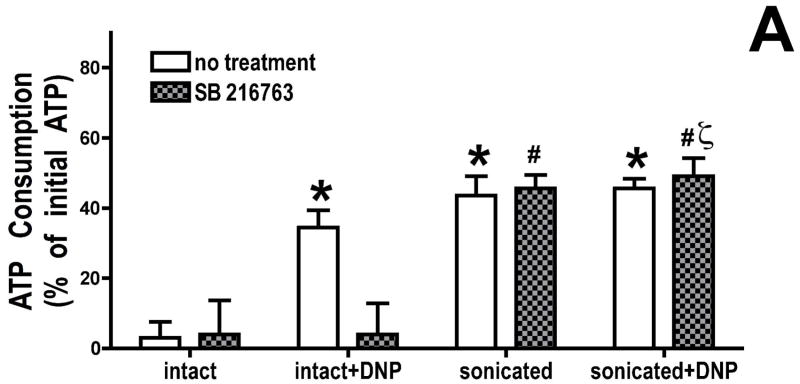
To determine whether this effect is due to inhibition of the mitochondrial F1F0-ATPase, mitochondria were sonicated to disrupt the mitochondria and expose the F1F0-ATPase. We found (figure 3A) that ATPase activity in the sonicated mitochondrial fragments was similar in the presence or absence of uncoupler, as expected if we had fully disrupted the mitochondria, and GSK inhibitors had no effect on ATP consumption by the sonicated mitochondrial fragments. Only in the intact mitochondria was there an inhibitory effect of GSK inhibitors. Thus the effect of GSK inhibitors does not appear to be due to direct inhibition of F1F0-ATPase activity.
Figure 3.
Effect of GSK inhibition on mitochondrial adenine nucleotide metabolism. Panel A, mitochondria were de-energized using dinitrophenol, and ATP remaining after 2.5 minutes of incubation was measured. GSK inhibitors have no effect on ATPase activity in sonicated mitochondria. Panel B, cyclosporin A (200 μM) was added to de-energized mitochondria; this had no effect on ATP hydrolysis, in control and GSK inhibitor groups. Results are Means ± SEM (n=6). *p<0.05 vs. intact control. #p<0.05 vs. intact SB216763 treated. ζp<0.05 vs. intact+DNP, SB 216763 treated.
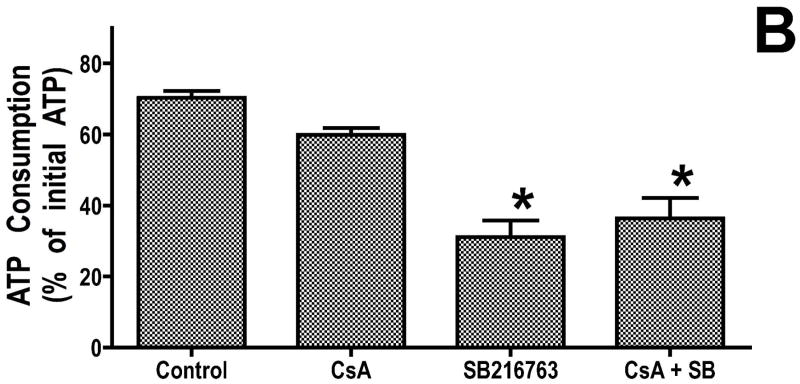
The effect of GSK-3 inhibitors on mitochondrial adenine nucleotide metabolism is not due to a direct effect on the mitochondrial permeability transition pore
To examine whether the effect of GSK inhibitors on adenine nucleotide metabolism is due to a direct effect on the mitochondrial permeability transition pore (mPTP), we examined the effect of cyclosporin A (CsA) on ATP consumption under de-energized conditions. To establish that an effective dose of CsA was being used, we measured mitochondrial swelling induced by calcium, and we found that 200 μM CsA markedly reduced mitochondrial swelling in response to calcium compared to control. However, this concentration of CsA had no effect on adenine nucleotide transport (figure 3B). Thus GSK inhibitors are inhibiting transport through physiologic transport mechanisms, and there is no evidence that adenine nucleotide entry into the mitochondria during early anoxia is related to mPTP opening. However, inhibiting physiologic transport could indirectly protect the mitochondria and reduce the probability of mPTP opening as injury progresses.
GSK-3 inhibitors reduce adenine nucleotide transport through the outer mitochondrial membrane
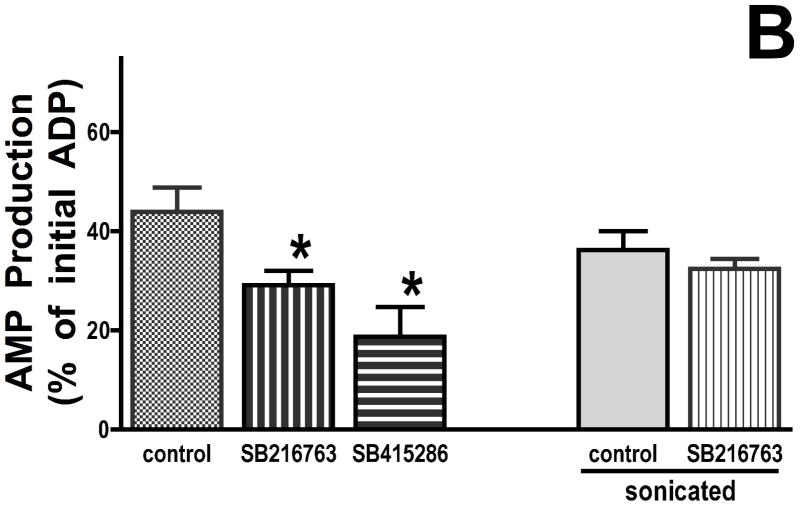
To further explore the mechanism whereby GSK inhibitors slow ATP consumption under de-energized conditions, an assay was developed that isolated effects of outer mitochondrial membrane transport from effects on ANT or the F1F0-ATPase. This assay took advantage of adenylate kinase, which resides in the intermembrane space. Under de-energized conditions, comparable to the conditions used to measure mitochondrial ATP consumption but in the presence of oligomycin to block matrix adenine nucleotide metabolism, ADP was added to isolated mitochondria, and AMP generation was measured. This assay is independent of ANT or F1F0-ATPase activity; it relies solely on VDAC to transport ADP into the mitochondria, adenylate kinase to convert ADP into AMP and ATP, and VDAC to transport AMP out of the mitochondria. If the effect of GSK inhibitors is on adenine nucleotide transport across the outer mitochondrial membrane, then there should be less ADP consumed and less AMP produced in the mitochondria treated with GSK inhibitors compared to control. There is significantly less ADP consumed (Figure 4A) and less AMP produced (Figure 4B) in the GSK inhibitor treated mitochondria, as compared to the untreated mitochondria. In a separate series of experiments, we assessed the effect of GSK inhibitors on adenylate kinase activity in sonicated mitochondria where the outer membrane would be disrupted, and GSK inhibitors had no effect on ADP consumption or AMP production (right side, Figure 4A 4B). The data suggest that GSK inhibitors do not inhibit adenylate kinase and that the ATP-sparing effect of GSK inhibitors is related to decreased adenine nucleotide transport across the outer mitochondrial membrane.
Figure 4.
GSK inhibitors slow the rate of ADP consumption (Panel A) and the rate of AMP production (Panel B) by cyanide-treated mitochondria. Results are Means ± SEM (n=6). *p<0.05 vs. control.
GSK-3 inhibitors reduce phosphorylation of VDAC
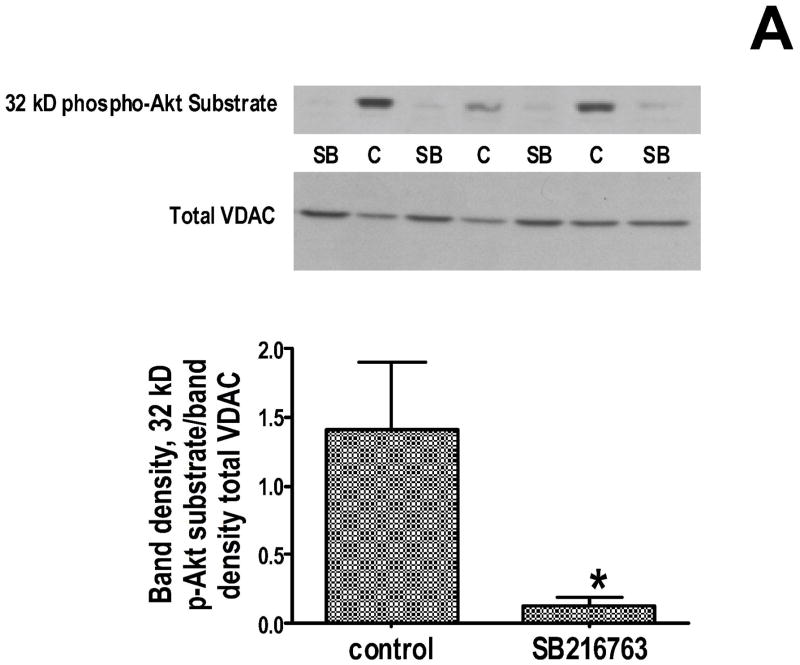
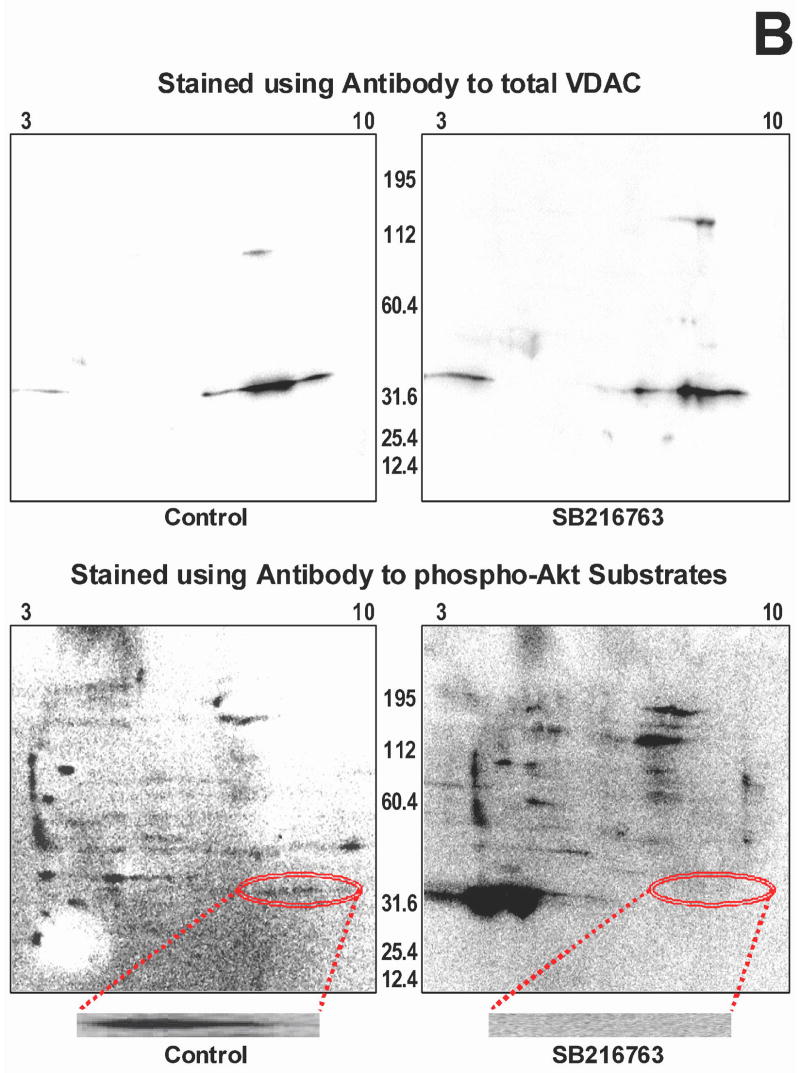
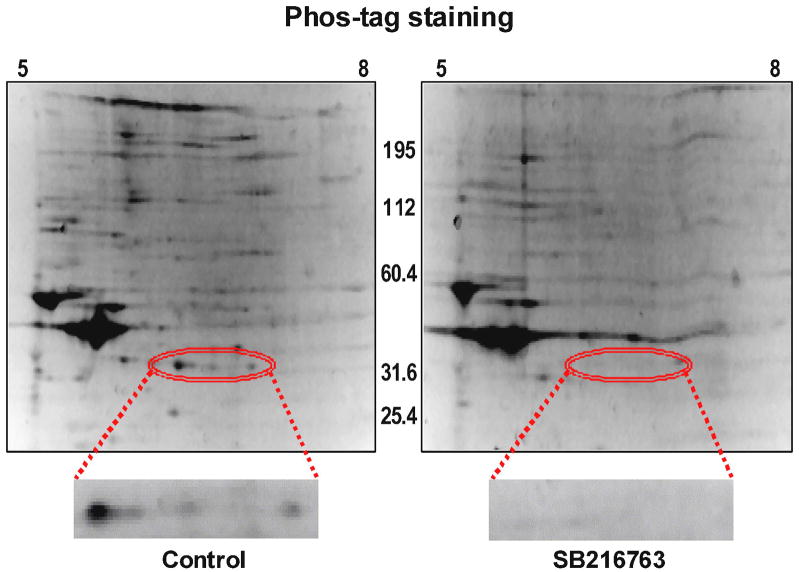
Since the functional data suggest that GSK inhibitors are slowing adenine nucleotide transport through the outer mitochondrial membrane, and VDAC is the channel that is responsible for this transport, we explored the effect of GSK inhibitors on VDAC phosphorylation. We used several approaches. First, we used an antibody that recognizes proteins that are phosphorylated at serine/threonine residues of Akt substrates, and we did western blotting to determine if GSK inhibition affects protein phosphorylation. We observed a dramatic change in a protein band at ~32 kD. As shown in figure 5A (top), there is much less phosphorylation of this band when the hearts were treated with a GSK inhibitor than in untreated hearts. The molecular weight of VDAC is 32kD. The gel was stripped and re-probed with antibody to VDAC, and the phosphorylated protein overlaps with VDAC, and as shown in figure 5A (bottom), there is the same amount of VDAC in each lane. Next we performed 2D gel electrophoresis to verify this finding when proteins are better separated. As shown in figure 5B, the location of VDAC in the 2D gel was established with VDAC antibody (upper panels), and phosphorylation was assessed using phospho-Akt substrate antibodies (lower panels). The lower panels show VDAC phosphorylation in the control extracts on the left but not in the GSK inhibitor treated extracts on the right. To examine this further, we used Phos-tag to identify phosphorylated proteins, and we performed 1D as well as 2D gel electrophoresis. In the 1D gels, the GSK inhibitors significantly reduced protein phosphorylation in the 32 kD region. To validate this finding, 2D gel electrophoresis was performed with narrow pH range strips that focused the VDAC region more clearly, and then stained with Phos-tag. Figure 6 shows several spots that had high levels of phosphorylation in untreated heart extracts but very little phosphorylation in GSK inhibitor treated heart extracts. Mass spectrometry demonstrated that VDAC2 is present in all 3 spots in the 2D gel.
Figure 5.
Effect of GSK inhibition on phosphorylation of a 32 kD protein. Panel A-top is a representative 1D gel showing the amount of 32 kD phosphorylated Akt substrate protein in control and GSK inhibitor treated heart extracts. Panel A-middle shows the gel re-probed with VDAC antibody, showing no significant difference in total VDAC expression. Gel densitometry was performed for quantitation, and the ratio of 32 kD phospho-protein to total VDAC is plotted. *p<0.05 vs control. In Panel B, whole heart homogenates were further separated using 2D gel electrophoresis. In the upper panels, control homogenate (left) and GSK inhibitor treated homogenate (right) is probed with VDAC antibody. Then the membranes were stripped and reprobed for phosphorylated Akt substate protein (lower images). There is phosphorylated protein in the VDAC region in control but not in GSK inhibitor treated homogenate. The region of interest is circled and shown at higher magnification at the bottom.
Figure 6.
Whole heart homogenate protein was separated by 2D gel electrophoresis and stained with phos-tag to detect phosphorylated proteins. Three spots showing marked differences in phosphorylation level, in the 32 kD region (shown in higher magnification), were analyzed by LC-MS, and VDAC-2 was identified by at least 4 peptide fragments, in all three spots.
VDAC can be phosphorylated by either Akt or GSK-3β
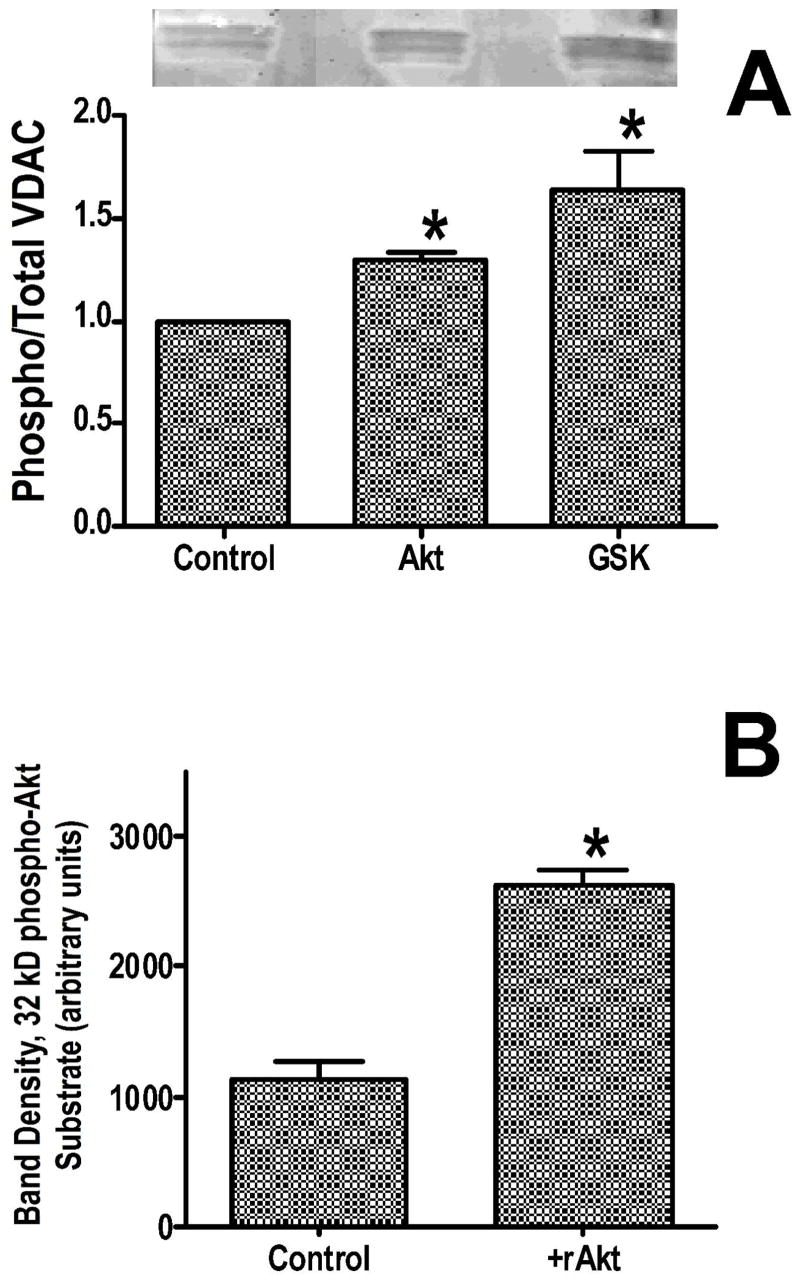
To determine whether Akt or GSK-3β can phosphorylate VDAC in vitro, we partially purified VDAC and performed an in vitro kinase assay using recombinant active Akt and recombinant GSK-3β. VDAC was partially purified using a hydroxyapatite/celite column as used by others (17). We then performed an in vitro kinase assay, and measured the extent of phosphorylation using Pro-Q Diamond staining. Although there was some endogenous phosphorylation, this was further increased by either Akt or GSK-3β (figure 7A). We also examined the ability of recombinant active Akt to phosphorylate VDAC using isolated mitochondria. Recombinant Akt added to the medium, in the presence of ATP, increased phosphorylation of the ~32 kD protein band (figure 7B). Thus external Akt can phosphorylate the protein, indicating that the phosphorylation site is on the outside of the mitochondria, consistent with the location of VDAC.
Figure 7.
Panel A illustrates in vitro phosphorylation of semi-purified VDAC, by Akt and GSK-3β. Panel B shows increased 32 kD Akt substrate phosphorylation in isolated mitochondria following addition of recombinant Akt (rAkt). *p<0.05 vs control.
GSK-3 inhibitors increase Bcl-2 binding to mitochondria
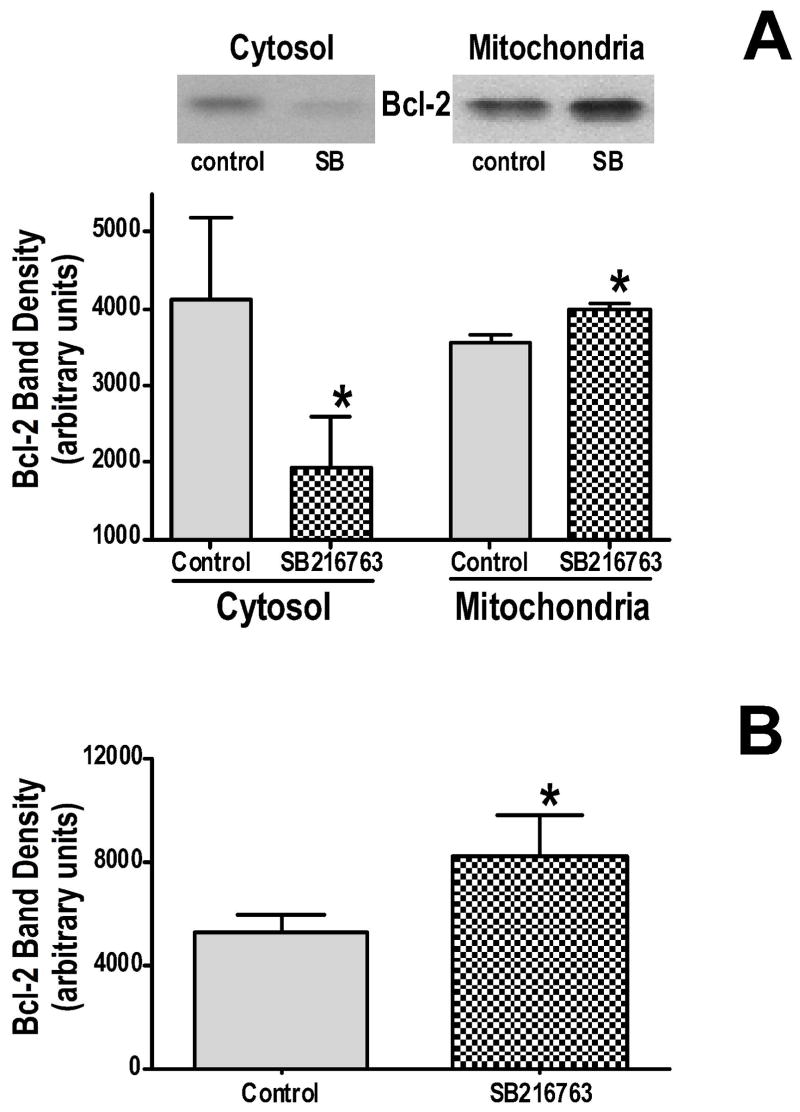
Previous work (16) had demonstrated that cardiac overexpression of Bcl-2 protects the heart from ischemia-reperfusion injury, and this protection is associated with inhibited mitochondrial ATP consumption under de-energized conditions and with binding of Bcl-2 to VDAC. To determine if GSK inhibitors are protective, at least in part, by enhancing Bcl-2 binding to VDAC, cell fractionation experiments were performed, and the amount of Bcl-2 in the mitochondrial and cytosolic fractions were determined by western blotting. GSK inhibition causes a significant loss of Bcl-2 from the cytosol and a significant increase in the mitochondrial fraction (figure 8A). This indicates that binding of Bcl-2 to mitochondrial targets increases in the presence of GSK inhibitors. To test whether this increased binding of Bcl-2 to mitochondria is specific binding to VDAC, we added equal amounts of recombinant Bcl-2 to GSK inhibitor treated mitochondria and untreated mitochondria, immunoprecipitated VDAC, and measured the amount of Bcl-2 that was bound to VDAC. As shown in figure 8B, there was significantly more Bcl-2 bound to VDAC in the GSK inhibitor treated mitochondria. Thus phosphorylation of VDAC may affect the binding affinity for Bcl-2, which may regulate outer mitochondrial membrane transport.
Figure 8.
Panel A shows the effect of GSK inhibition on Bcl-2 levels in cytosolic and mitochondrial fractions. Panel B shows the effect of GSK inhibition on the amount of Bcl-2 that is immunoprecipitated by VDAC antibodies. *p<0.05 vs control.
DISCUSSION
Previously, our group has demonstrated that ischemic preconditioning results in phosphorylation and inactivation of GSK-3β and that this is mediated by the PI3-kinase pathway (8). Furthermore, pretreatment with GSK-3 inhibitors is approximately as protective as ischemic preconditioning. Others (11–14) have shown that GSK-3β is involved in a variety of forms of pharmacologic preconditioning, and the GSK-3β inhibitors are protective when added at the start of reperfusion (11, 14). This suggests that GSK-3β may play a central role in a final pathway of cardioprotection, as suggested by Sollott (10). Furthermore, previous work has suggested that the effects of GSK-3β inhibition are primarily focused on the mitochondria, although the molecular target has not been determined.
The present study suggests a possible molecular target for GSK-3β in the mitochondria that may be involved in cardioprotection, and a possible mechanism whereby protection is achieved. The molecular target appears to be VDAC in the outer mitochondrial membrane and phosphorylation may be a mechanism for regulating VDAC activity, either directly or by altering the binding of Bcl-2. There are 3 VDAC isoforms in mammalian cells (18–20), which are primarily responsible for transport of molecules up to 5000 kD across the outer mitochondrial membrane. Transport of anions is highly voltage sensitive with high conductance at low voltages and low conductance when voltage is increased, either positively or negatively. In contrast, cation conductance is relatively voltage insensitive. Nucleotides such as ATP are readily permeable when VDAC is in the open state, and are virtually impermeant when VDAC is closed. Ablation of the mouse VDAC1 gene results in altered mitochondrial sensitivity for ADP in muscle, but since VDAC2 and VDAC3 are also present at high levels in the heart, the mice are viable (21). Mice deficient for VDAC3 are viable, but deficiency of VDAC2 is embryologically lethal. Our proteomic studies suggest that it is VDAC2 that is a primary GSK target in the outer mitochondrial membrane.
Several investigators have suggested that VDAC can be an important regulator of mitochondrial function. Under conditions of anoxia, there is evidence of inhibition of mitochondrial anion exchange (22). Others (23–24) have found evidence for VDAC regulation of mitochondrial function under conditions that stimulate apoptosis. In the apoptosis studies, it has been suggested that early in the apoptotic program, the outer mitochondrial membrane becomes impermeable to small molecules such as ATP and creatine phosphate, and that this leads to the eventual loss of outer mitochondrial membrane integrity and cytochrome c release, which can be prevented by anti-apoptotic Bcl-2 family members (23–24). Although the conditions and mechanisms are different in our study, the findings support the concept that VDAC transport can be regulated, and plays a role in cell injury. Under conditions leading to apoptosis, there is some controversy concerning Bcl-2 regulation of VDAC. Some reports suggest that during apoptosis, VDAC closes and that Bcl-2 is protective by maintaining VDAC in an open configuration (23–24), whereas others suggest that Bcl-2 promotes VDAC closure (25). This could be related to the specific experimental conditions or the timing of the measurements. Regardless of the controversy, our data as well as the apoptosis literature support the concept that altered VDAC conductance can be involved in the evolution of cell injury.
Although our data suggest that VDAC phosphorylation under the control of GSK-3 is an important mechanism that alters mitochondrial function under energy deficient conditions, others (26) suggest that mitochondrial protein phosphorylation is not involved in the protective effect of ischemic preconditioning. One might expect that ischemic preconditioning and GSK inhibition would involve many of the same mechanisms since ischemic preconditioning inhibits GSK through phosphorylation. However, this thorough study by Clarke et al (26) was performed on extracts following mitochondrial purification, and we found that we had to use whole-tissue extracts, immediately placed in detergent, to see the phosphorylation clearly. If we purified the mitochondria and then prepared extracts, the phosphorylation was barely detectable. This suggests that the phosphorylation is highly labile, presumably because the phosphatases must be separated from the substrate rapidly to preserve the phosphorylated state. To minimize this problem, we used a rapid isolation procedure for preparing mitochondria and analyzing mitochondrial function. It is possible that even under these conditions, our mitochondrial functional assays are underestimating the magnitude of the effect that is present in situ.
Functional Consequences of GSK Signaling in the Mitochondria
One of the downstream effects of GSK inhibition is delayed opening of the mitochondrial permeability transition pore (mPTP) in response to oxygen radicals (10), and opening of the mPTP has been suggested to be a causative event in cell death during myocardial ischemia-reperfusion injury (27–28). This effect on mPTP opening could be a direct or indirect effect of GSK inhibition. Since VDAC does not appear to be necessary for mPTP formation (29), it seems unlikely that altered phosphorylation of VDAC would necessarily prevent mPTP formation. Also the studies of Sollott’s group show that mPTP opening occurs even in the presence of GSK inhibitors, just more slowly (10). In accordance with these observations, our present study has also revealed that cyclosporin A treatment does not change the rate of ATP hydrolysis following de-energization, indicating an effect independent of mPTP opening. Alternatively, GSK inhibitors could alter mitochondrial function in a way that makes the mPTP less likely to form or open, possibly by limiting the factors that are known to activate the mPTP, oxidant stress and high calcium concentrations (30). If GSK inhibitors either reduced mitochondrial calcium uptake, reduced endogenous oxygen radical production, or both, this could account for the ability of GSK inhibitors to slow mPTP opening. The importance of calcium loading in mPTP opening in situ has been questioned (31) and ROS may be a more important factor in intact tissue.
One mechanism whereby GSK inhibitors could reduce mitochondrial calcium loading would be by inhibiting ATP influx through VDAC during ischemia. During ischemia, ATP is produced by anaerobic glycolysis, and this ATP can enter the mitochondria and be hydrolyzed by the matrix F1F0-ATPase. This is a significant ATP consuming process; when ischemic myocardium is treated with oligomycin, ATP consumption slows (16, 32), by as much as 50%. Although inhibition of this process may be protective by preserving ATP, this is not likely to be its major effect. Rather, mitochondrial ATP hydrolysis is used to preserve the mitochondrial membrane potential (ΔΨ), and direct measurement of ΔΨ in isolated myocytes subjected to simulated ischemia (33) shows that ΔΨ falls much more rapidly in the presence of oligomycin than in its absence, indicating that the matrix F1F0-ATPase is using cytosolic ATP to slow the decay of ΔΨ. Since ΔΨ is the primary driving force for calcium uptake into the mitochondria, it is not surprising that there is virtually complete elimination of mitochondrial calcium uptake in isolated myocytes subjected to simulated ischemia in the presence of oligomycin, whereas mitochondrial calcium uptake is observed under similar conditions in the absence of oligomycin (33). Although this calcium uptake may be transient and may not directly open the mPTP, it could facilitate mPTP opening in response to other stimuli.
Another possible mechanism whereby inhibition of VDAC transport by GSK inhibitors may be protective under anoxic conditions, is by reducing oxygen radical production during early reperfusion (34). Agents that reduce ΔΨ by a small amount are cardioprotective, such as mitochondrial K-ATP channel openers, presumably because production of oxygen radicals is enhanced when the electrochemical proton gradient is high (35). However, there is no consensus that a decrease in ΔΨ is critical for the protective effect of K-ATP channel openers. A decrease in ΔΨ during ischemia could result in less oxygen radical production during early reperfusion, which would reduce the probability of mPTP opening. Therefore, GSK inhibitors could be protective by allowing ΔΨ to decrease during ischemia, reducing mitochondrial calcium loading during ischemia and reducing oxygen radical production during reperfusion, both of which would tend to reduce the probability of mPTP opening and thereby be protective. Others have suggested that the protective effect of ischemic preconditioning involves suppression of oxidant stress (26). These effects on calcium and oxidant stress would not be direct effects on the mPTP but rather effects on mPTP activators.
One interesting aspect of this study is that we found that GSK inhibitors reduced VDAC phosphorylation using an antibody that detects proteins phosphorylated at Akt sites. This could be because the antibody is not entirely specific for Akt phosphorylation sites and also recognizes other serine/threonine phosphorylations. However, it is also possible that VDAC is phosphorylated at multiple sites. GSK-3β is known to prefer substrates that have already been phosphorylated by other kinases (9, 36–37). Thus it is possible that physiologic regulation of VDAC is achieved through a dual phosphorylation mechanism involving Akt as the priming kinase and GSK-3β as the secondary kinase that is required for regulation of VDAC function. An alternative possibility is that VDAC is phosphorylated by Akt and that GSK-3β regulates the phosphatase that dephosphorylates VDAC. If GSK-3β inactivates the phosphatase that dephosphorylates VDAC, then GSK inhibitors could decrease VDAC phosphorylation by increasing the activity of the phosphatase. Further characterization of the phosphorylation sites of VDAC will be necessary to resolve this issue.
Although dephosphorylation of VDAC by GSK inhibition may be protective by altering channel conductance directly, an alternative mechanism would involve differential protein binding to the phosphorylated versus dephosphorylated VDAC. In a previous study (16), we found that Bcl-2 overexpression inhibited mitochondrial ATP consumption under de-energized conditions, similar to what we report here with GSK inhibitors. One possible explanation for the similar effect of Bcl-2 overexpression and GSK inhibition is that an important mechanism of VDAC regulation involves Bcl-2 binding, and this binding can be increased either by overexpression or by altering affinity, and one mechanism for altering affinity may be through phosphorylation. Data presented here suggests that Bcl-2 affinity for mitochondria and for VDAC specifically, is increased by GSK inhibitors, consistent with a regulatory role for VDAC phosphorylation. Others have provided convincing evidence that Bcl-2 binding to a component of the mPTP is critical for cardioprotection, and that GSK-3 is a critical regulator of this interaction (31). An alternate possibility is that binding of hexokinase to VDAC could have important regulatory properties (38). The binding of hexokinase to VDAC appears to require activated Akt (39), although it is not clear if phosphorylation plays a role.
CONCLUSIONS
The data suggest that VDAC is an important site of regulation of mitochondrial metabolism and function under de-energized conditions. GSK inhibition decreases adenine nucleotide entry through the outer mitochondrial membrane, most likely through VDAC, under anoxic or similar conditions. This reduction in adenine nucleotide transport is likely to result in a decrease in ΔΦ, less mitochondrial calcium loading during anoxic conditions, and less endogenous oxygen radical production during reoxygenation/reperfusion. Furthermore, GSK inhibitors alter the phosphorylation status of VDAC and alter the affinity for Bcl-2, which may also contribute to the decrease in transport through VDAC. All of these factors, less calcium loading, less oxidant stress, and more Bcl-2 binding, likely contribute to the cardioprotective effect of GSK inhibitors.
Acknowledgments
Supported in part by NIH grant HL39752. RW, NR, and EM were supported by the NIH intramural program.
Footnotes
CONFLICTS OF INTEREST: None
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–221. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 4.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res. 2004;94:1133–1141. doi: 10.1161/01.RES.0000126048.32383.6B. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signaling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 6.Liem DA, Honda MH, Zhang J, Woo D, Ping P. Past and present course of cardioprotection against ischemia-reperfusion injury. J Appl Physiol. 2007;103:2129–2136. doi: 10.1152/japplphysiol.00383.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen Synthase Kinase-3 – An overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 8.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3β during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ Res. 2002;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 9.Murphy E, Steenbergen C. Inhibition of GSK-3β as a target for cardioprotection: the importance of timing, location, duration, and degree of inhibition. Expert Opin Ther Targets. 2005;9:447–456. doi: 10.1517/14728222.9.3.447. [DOI] [PubMed] [Google Scholar]
- 10.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 12.Nishihara M, Miura T, Miki T, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Ohori K, Takahashi A, Shimamoto K. Erythropoietin affords additional cardioprotection to preconditioned hearts by enhanced phosphorylation of glycogen synthase kinase-3β. Am J Physiol Heart Circ Physiol. 2006;291:H748–H755. doi: 10.1152/ajpheart.00837.2005. [DOI] [PubMed] [Google Scholar]
- 13.Gross ER, Hsu AK, Gross GJ. Delayed cardioprotection afforded by the glycogen synthase kinase 3 inhibitor SB-216763 occurs via a KATP- and MPTP-dependent mechanism at reperfusion. Am J Physiol. 2008;294:H1497–H1500. doi: 10.1152/ajpheart.01381.2007. [DOI] [PubMed] [Google Scholar]
- 14.Gross ER, Hsu AK, Gross GJ. GSK3 inhibition and KATP channel opening mediate acute opioid-induced cardioprotection at reperfusion. Bas Res Cardiol. 2007;102:341–349. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- 15.Hirotani S, Zhai P, Tomita H, Galeotti J, Marquez JP, Go S, Hong C, Yatani A, Avila J, Sadoshima J. Inhibition of glycogen synthase kinase 3β during heart failure is protective. Circ Res. 2007;101:1164–1174. doi: 10.1161/CIRCRESAHA.107.160614. [DOI] [PubMed] [Google Scholar]
- 16.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 17.Lai JC, Tan W, Benimetskaya L, Miller P, Colombini M, Stein CA. A pharmacologic target of G3139 in melanoma cells may be the mitochondrial VDAC. Proc Natl Acad Sci U S A. 2006;103:7494–7499. doi: 10.1073/pnas.0602217103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Columbini M. VDAC: The channel at the interface between mitochondria and the cytosol. Mol Cell Biochem. 2004;256/257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 19.Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ERB, Adleman J, Columbini M, Forte M. Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem. 1993;268:1835–1841. [PubMed] [Google Scholar]
- 20.Sampson MJ, Lovell RS, Davison DB, Craigen WJ. A novel mouse mitochondrial voltage-dependent anion channel gene localizes to chromosome 8. Genomics. 1996;36:192–196. doi: 10.1006/geno.1996.0445. [DOI] [PubMed] [Google Scholar]
- 21.Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 22.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 24.Vander Heiden MG, Chandel NS, Li XX, Schumacker PT, Colombini M, Thompson CB. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc Nat Acad Sci USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 26.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102 doi: 10.1161/CIRCRESAHA.107.167072. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition port causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 28.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion – a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 29.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore. Where the known meets the unknown. Ann N Y Acad Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 32.Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol. 1991;23:1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Meana M, Garcia-Dorado D, Miró-Casas E, Abellán A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 35.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 36.Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–14048. [PubMed] [Google Scholar]
- 37.Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisani OM. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J Biol Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- 38.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome C release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 39.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]