Summary
Biological macromolecules use binding forces to access unfavorable chemical equilibria and stabilize reactive intermediates by temporarily isolating them from the surrounding medium. Certain synthetic receptors, functional cavitands, share these abilities and allow the direct observation of labile intermediates by conventional spectroscopy. The cavitands feature inwardly-directed functional groups that form reversible, covalent bonds with small molecules held inside. Tetrahedral intermediates of carbonyl addition reactions – hemiaminals, hemiacetals and hemiketals – show amplified concentrations and lifetimes of minutes under ambient conditions. Labile intermediates in addition reactions of carboxylic acids to isonitriles are also stabilized by isolation in the space of the cavitands. The restricted environments channel the reactions of intermediates in cavitands along a specific path, and strengthen the parallels between functional synthetic cavitands and enzymes.
Introduction
Ask practically anyone what molecules can fold around their targets, isolate them from the bulk medium, place them in a chiral, hydrophobic environment, and present them with functional groups on a secondary amide scaffold and it’s unlikely the answer will be: Cavitands! Instead, these traits are regarded as exclusive attributes of proteins – receptors, antibodies and enzymes – traits that are widely thought to be the keys to their high binding affinities, exquisite selectivities, catalytic efficiencies and unique signaling abilities. This review intends to show that these traits can now be incorporated into cavitands through synthesis to give receptors that display the behaviors of their naturally-occurring counterparts. Of course, these cavitands are inspired by biological systems, but the present perspective is one of physical organic chemistry, with emphasis on the interplay of intermolecular forces with recognition and reactivity.
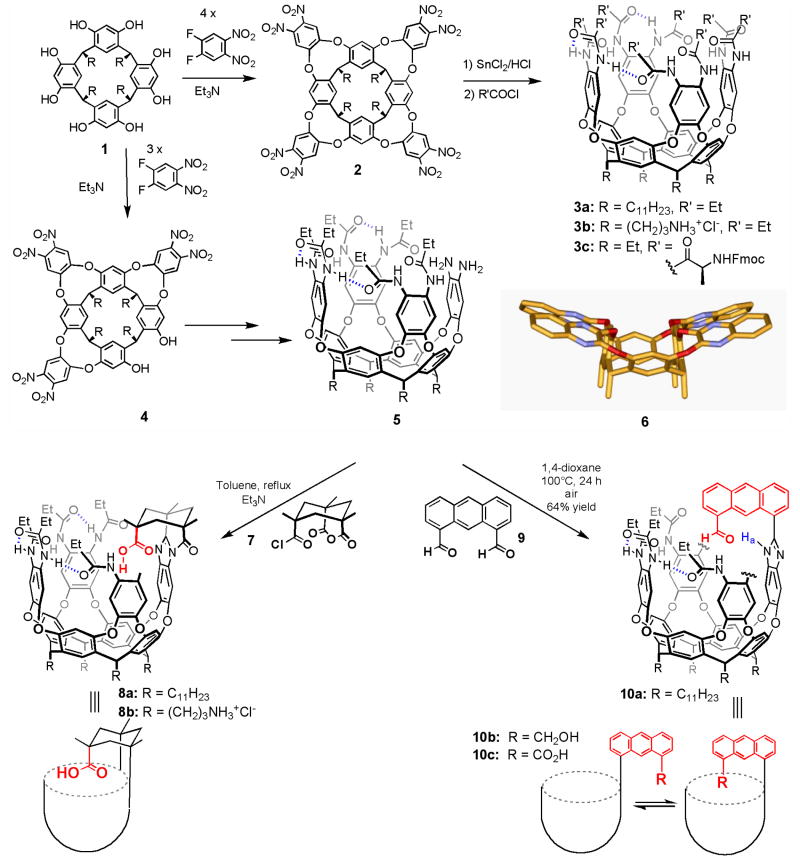
Cavitands are bowl-shaped molecules that feature sizable concave surfaces. Their popularity began with the efficient synthesis of the shallow resorcinarene framework 1, (Figure 1) from which larger structures 2 were elaborated by the addition of aromatic panels (Cram, 1994). The multiple shapes assumed by these limited their use as host structures but some conformations (Moran et al., 1991) offered enough space for small molecules to fit inside (Soncini et al., 1992). The introduction of secondary amides along the periphery folded the structure to the “vase-like” deepened cavitands 3, stabilized by intramolecular hydrogen bonding (Rudkevich et al., 1998). Only one of the head-to-tail arrangements of the amides is shown in 3; it is chiral and it interconverts with its mirror image (cycloenantiomer), about which more later. Apart from the examples presented here, work elsewhere with synthetic receptors has shown how the reactivity of bound species is altered and the lifetimes of otherwise unstable molecules are prolonged (Hou et al., 2008). Remarkable reactivity (Purse et al., 2003), amplified attractions and selective stabilization (Aiegler et al., 2000; Roach et al., 2003) are the results. Examples include isolation (Doun et al., 2006) of iminium ions and catalysis of their rearrangements; (Fiedler et al., 2004) the stabilization of siloxanes (Yoshizawa et al., 2000) in aqueous media, and appearance of unfavored heterocycles (Iwasawa et al., 2006) and unknown reaction courses (Yoshizawa et al., 2006; Warmuth, 2005) imposed by the size and shape of the receptors (Kaanumalle et al., 2005). The early applications of cavitands in encapsulation (Sherman, 1995; Cram et al., 1994b; Cram et al., 1988; Moran et al., 1991) and self-assembly (MacGillivray et al., 1997; Hof, 2002) are reviewed elsewhere; only the progress of the last few years is emphasized here (Purse et al., 2005).
Figure 1.
Cavitands used in this review.
Octamide cavitand 3 presents a chiral, hydrophobic pocket maintained by amide bonds that folds around target molecules and isolates them typically for 0.01-1 second. But where are the functional groups? Most of the atoms that line the interior of 3 are sp2 hybridized with the p orbitals directed inward, resulting in an inner surface that is smooth and concave. Performing chemistry on such a surface is unreasonable, and would leave no room for guests (Goto et al., 1997). But there are structural motifs that present functional groups on concave surfaces: clefts (Galann et al., 1991), armatures (Adrian et al., 1989), tweezers (Zimmerman et al., 1989) and other vehicles (Dixon, 1992) that are without independent binding sites. We fused these shapes onto to the resorcinarene derivatives to arrive at the functional cavitands.
Specifically, the hexanitro compound 4, (a side product in the synthesis of 2) was elaborated to the diamine 5 through a short sequence involving reduction and acylation with the appropriate acid chlorides to give a hexamide, then addition of the fourth wall. Figure 1 shows the vase-like conformation of molecules featuring secondary amides that form hydrogen bonded along the upper rims. In contrast molecules without these stabilizing hydrogen bonds exist in a kite-like conformation shown in 6. We then attached the Kemp’s triacid (Kemp et al., 1981) module 7 to the rims (Figure 2) in such a way that when these cavitands were folded around their targets, a carboxylic acid was directed into the cavity (Renslo et al., 2000). This “introverted” functionality is rigidly fixed and has no other choices. Both organic-soluble 8a and water-soluble 8b (Butterfield et al., 2006) versions were synthesized.
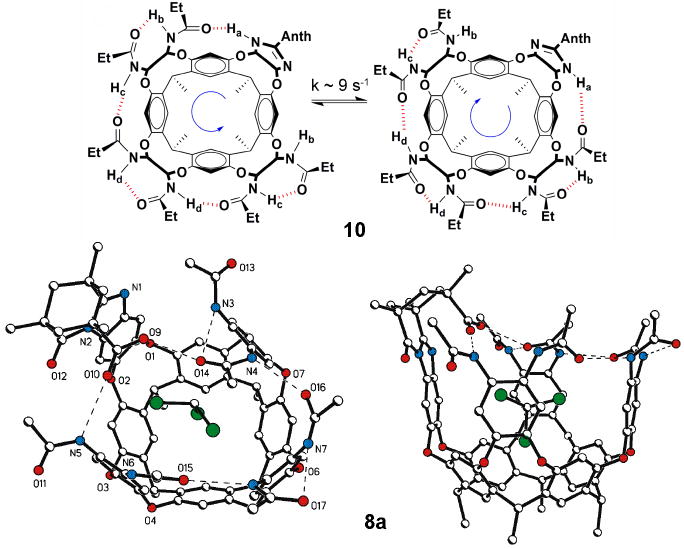
Figure 2.
Top: Schematic of the interconversion of cycloenantiomers of cavitand 10; Bottom) Two views of the crystal structure of acid 8a with chloroform bound inside. Ethyl groups have been shortened to methyl groups for viewing clarity. Hydrogen bonds are shown as dashed lines.
A second system that was useful in this context resulted from the fusion of 5 with the anthracene derivative 9. In this cavitand 10, rotation about the single bond shown provides two conformations, the desired inwardly-directed functionality and the other external functionality. Unlike the Kemp’s triacid derivatives, reagents can access the carbonyl carbon of 10a to perform reduction and oxidation reactions and yield alcohol 10b and acid 10c, respectively.
Structure
In both introverted cavitands the array of amides is affected by the odd wall’s benzimidazole function in a way that leads to a chiral environment. In 8 the amide is the hydrogen bond donor to the oxygen of the Kemp’s triacid, and this fixes the other amides as shown; 8 and its mirror image cannot interconvert without breaking covalent bonds. Accordingly, the interconversion (racemization) is slow and the enantiomers can be separated on a chiral column. In contrast, the benzimidazole is the hydrogen bond donor in 10c, for example, but tautomerization of the hydrogen bond array interconverts the mirror images. This process is slow on the NMR timescale (k = 9 s-1, Figure 2 top) (Hooley et al., 2007a) but fast on the human timescale.
The X-ray crystal structure (Figure 2 bottom) showed the some of the expected intramolecular hydrogen bonds between the adjacent amide units and two others involving the introverted carboxylic acid group. The C–O distances and the pattern of hydrogen bonds clearly indicate that the acid hydrogen atom is bound to acarbonyl oxygen. In addition, two of the corresponding inter-amide hydrogen bonds are broken and an NH is dedicated entirely to donate a hydrogen bond with the acid carbonyl. This donation of a hydrogen bond to the carboxylic acid is expected to increase the acidity when a basic guest is present.
The cavity volume in the X-ray structure is 145 Å3, and provides a good fit for chloroform with a packing coefficient of 0.51. This number is close to that found for a wide array of hosts (0.55) (Mecozzi et al., 1998). The available space and host flexibility is suitable for the binding of one small molecule up to a maximum volume of around 120 Å3. The chloroform inside has its relatively polar C–H directed towards the aromatic cavitand wall. The CH–π interactions, as well as the stronger cation–π interactions, are major contributors to the guest binding energy of cavitands of types 3, 8 and 10, and this is consistent with the orientation observed here. One chlorine atom is situated in the middle of the resorcinarene socket.
Introverted acid 8 provides a fixed functional group that dominates its host-guest interactions, as discussed below. The anthracene cavitands 10 are slightly different; the anthracene arm has one degree of freedom, and rotates over the top of the cavitand rapidly on the NMR timescale. This has the effect of increasing the scope of guests that fit in the cavity (for large species, the anthracene arm can simply rotate out of the way), but reactions are often slower due to the lack of forced proximity between guest and reactant.
Acid/Base Chemistry
Earlier we reported the high affinity of deep cavitands for protonated tertiary amines and quaternary ammonium salts (Hof et al., 2003) so it was expected that 8a would behave likewise. Even so, its complex with [2.2.2.]-diazabicyclooctane (DABCO) showed unexpected features. 1H,1H TOCSY NMR experiments revealed 3JH,H coupling between the quinuclidine methylenes and the proton shared by acid and base. This coupling indicates a bond between nitrogen and hydrogen, requiring a partial transfer of the proton to the base (Lyons et al., 1993), and shows that the cavitand has the ability to stabilize an ion pair. The hydrogen-bonded salt bridge observed in the cavitand’s interior is reminiscent of the stabilization of salt bridges in the hydrophobic interior of some proteins.
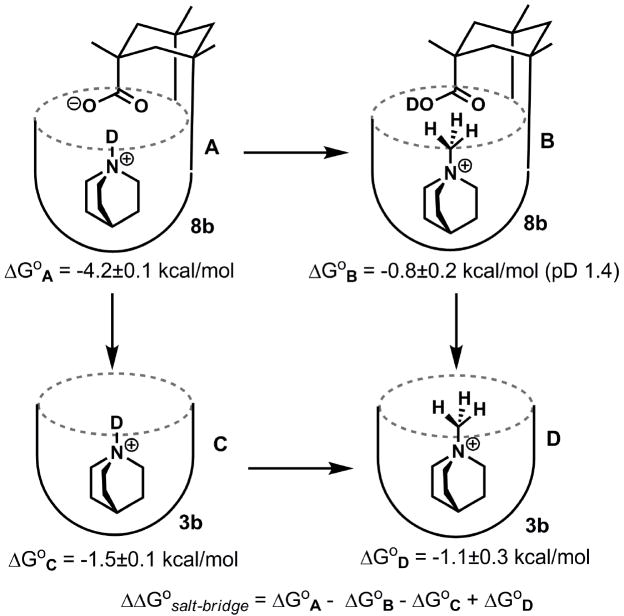
We compared the behavior of acid 8a in organic solution with that of 8b in aqueous solution (Kaanumalle et al., 2005). The conformation of 8b in D2O in the absence of an appropriate guest is different from that of the organic-soluble versions; the NMR spectrum features ill-defined, broad signals indicating aggregation and interconversion of conformations at intermediate rates on the NMR timescale. Evidently, the upper rim hydrogen bonds are not strong enough to hold the vase structure together in competition with the aqueous solvent. The addition of quinuclidinium hydrochloride shifted the host equilibrium to the folded structure through induced-fit recognition, with an affinity of 1300 M−1 in buffered D2O. In contrast, a water soluble version of the octamide receptor 3b bound the same guest with an association constant of only 12 M−1. The difference between the affinities can give an estimate of the buried salt bridge interaction of the introverted acid receptor 8b of −2.7 ± 0.1 kcal mol−1. While this number includes the desolvation of quinuclidinium and its interaction with the host walls, it does not account for the desolvation of the introverted carboxylic acid which occurs during the folding process and accordingly, a “double mutant” cycle analysis (Adams et al., 1996; Carter et al., 1984) must be used (Figure 3). Complex A features the interaction under investigation: that between the introverted acid and the protonated amine guest. Complexes B and C represent the single mutants. In these, the interacting protonated amine has been methylated and the introverted acid has been removed. Complex D is the double mutant where both interacting sites have been deleted. The electrostatic interaction in the complex formed between 8b and the quinuclidinium cation is then −3.0 ± 0.4 kcal mol −1 using equation (4) (Figure 4) (Purse et al., 2008). This figure agrees well with the value determined for a buried electrostatic interaction between Asp and Arg in Barnase (-3.3 kcal/mol) (Vaughan et al., 2002).
Figure 3.
Free energy cycle in deuterated water. Measurements were performed at 300 K in 10 mM sodium phosphate buffer, pD 5.25, except where indicated.
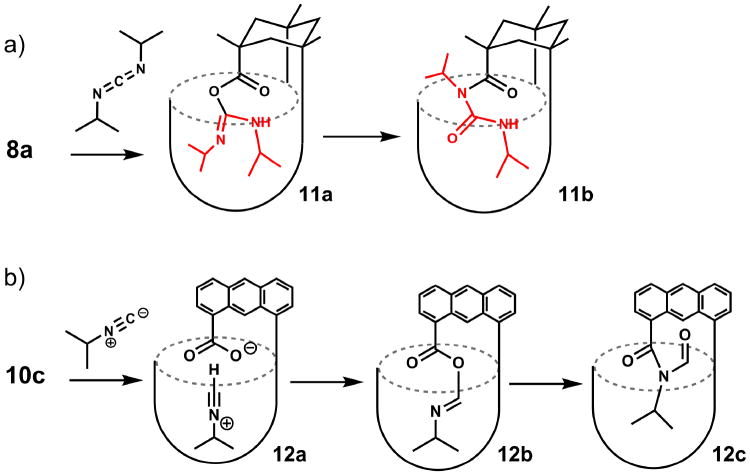
Figure 4.
a) Products obtained on the heating of cavitand 8a with diisopropylcarbodiimide; b) reaction of 10c with aliphatic isonitriles.
Reactions
Are these isolated catalytic sites capable of shifting equilibria toward unstable intermediates (Fersht, 1984)? Enzyme catalyzed reactions show enormous rate enhancements through binding to transition states and, inevitably, to the reaction intermediates that structurally resemble those states. These cavitands are also able to alter reaction pathways by a variety of bonding and non-bonding interactions; these examples are discussed below.
a) Carbodiimides and Isonitriles
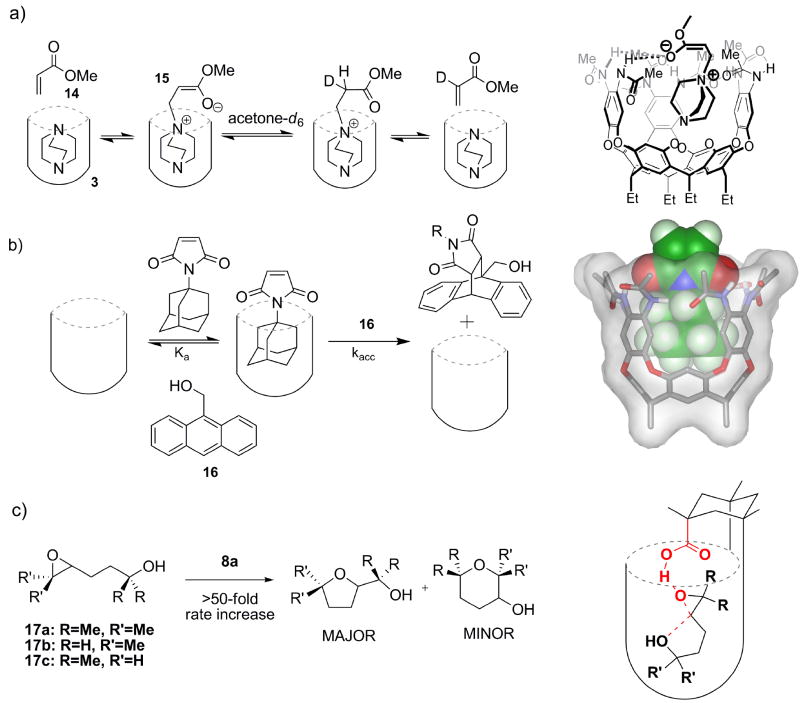
Typical carboxylic acids react with carbodiimides and amines to give amides, a process widely used in peptide synthesis. The acylating agent varies with the conditions (Rebek, 1979), but the initial acylating agent is doubtless the O-acylisourea as deduced by DeTar and Silverstein (De Tar et al., 1966a). Support for this structure comes from kinetic methods (De Tar et al., 1966b) and stereochemical probes (Rebek et al., 1975), rather than direct observation, although a recent publication describes the crystallization of a series of such O-acylisoureas (Bonsignore et al., 1995). The unique structure of the introverted acid suggested that the O-acylisourea intermediate may be trapped in the cavitand. The reaction of acid 8a with diisopropylcarbodiimide at 80°C gave an addition product 11 (Figure 4a). Clear evidence for a covalent adduct was provided by mass spectrometry and 1H NMR (Iwasawa et al., 2007a), but the IR spectrum showed too many carbonyl stretching absorptions in the 1800-1500 cm-1 range to assign to the structure. Accordingly, the product may be the O-acylisourea 11a or the N-acylurea 11b, although an intramolecular O-to-N acyl rearrangement is hard to imagine inside the cramped quarters of the cavitand.
The reaction of carboxylates with nitrilium ions is a key step in the Ugi and Passerini multicomponent condensations (Domling et al., 2000). and as this reaction is much-admired in combinatorial chemistry, its mechanism is well studied (Hegarty et al., 1980). In contrast, the reaction of carboxylic acids and aliphatic isonitriles is obscure (Gautier, 1896). No reaction occurs in dilute solution under ambient conditions but under microwave heating at 150 °C for 30 minutes (Li et al., 2008) showed that this reaction provides an efficient synthesis of imides. The reaction proceeds via the elusive O-acyl isoimide intermediate (Figure 4b) followed by a 1,3-O-to-N acyl transfer to form the N-acylformamide, in a process that has much in common with the reaction of carbodiimides discussed above. We used the acid 10c with small (isopropyl or t-butyl) isonitriles to detect the intermediate (Restorp et al., 2008). On mixing in dilute solution, an intermediate appears in the 1H NMR spectrum and disappears after an hour. We were able to further characterize it by IR spectroscopy, where it showed an IR absorption at 1770 cm-1, consistent with N-acylformamide intermediate 12b. The cavitand facilitates the reaction by amplifying the concentrations of the reacting species: the concentration of an isonitrile in the space is ~8 M, and the reaction becomes effectively unimolecular inside the cavity. The confined space of the cavitand can provide steric barriers that slow the rearrangement of the adduct 12b. Again, these features resemble those of enzyme interiors.
b) Trapping Reactive Intermediates
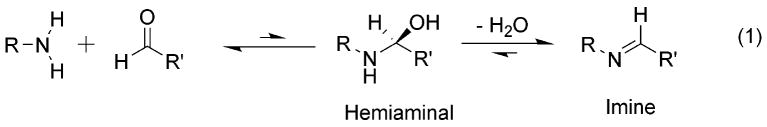
The reaction of carbonyl compounds with nucleophiles invariably involves an addition step that gives an unstable tetrahedral carbon intermediate followed by an elimination step, and the reaction of primary amines with aldehydes to give imines (Figure 5) is no exception. The initial intermediate hemiaminal is – except in very special cases – not observed. It is energetically unfavored as the breaking of the carbonyl bond and the entropic price of bringing the two reactants together is not compensated by the new covalent bonds formed. Accordingly, the unstable hemiaminal dissociates to starting materials or proceeds to imine with loss of water. We arranged for this reaction to take place within a deep cavitand, and found the hemiaminal was stabilized for minutes to hours, long enough to characterize by NMR spectroscopic methods (Iwasawa et al., 2007b).
Figure 5.
The sequence of events for imine formation.
When isobutyl amine is added to a solution of 10a in mesitylene-d12 a complex forms on mixing. Initially two complexes are present, the noncovalent complex 13a and the hemiaminal 13b. The tetrahedral intermediate creates a new asymmetric center; the signal for the C-H bond of the hemiaminal is observed in the 1H NMR spectrum and has a half-life of 27 min before its dehydration to the imine 13c. Hemiaminals were also observed within 10a using a variety of small amines, from cyclopropylamine to n-butylamine (Adrian et al., 1989). The rate of the stabilized hemiaminals depended on the ease of dehydration; the more steric hindrance between the cavitand and the bound amine, the slower the dehydration (and special reorganization) of the hemiaminal. For example, the half-life of the hemiaminal of n-butylamine was 20 min, whereas that of more rigid cyclobutylamine was over 6 hours! Larger amines could also form imines, but this occurred outside the cavity. If n-hexylamine was added, the extroverted n-hexylimine cavitand was formed under standard steady-state kinetics, with no stabilization of the hemiaminal; the stabilization only occurs on the interior of the cavity.
The cavitand’s environment must preferentially stabilize the tetrahedral intermediate through hydrogen bonding and other attractive interactions with the amide seam. This situation is regarded as commonplace in enzymes, where the catalytic site is capable of shifting equilibria toward unstable intermediates. The cavitand provides a nearly ideal chamber for this reaction: the reactants are confined in a limited space, properly oriented and the environment favors the reactive intermediate.
Further stabilization was also possible. By adding amines with extra hydrogen-bonding groups (such as ethanolamine or ethylenediamine), the resulting hemiaminals were stabilized not only by the cavity, but also by a hydrogen bond with the remaining OH/NH2 group. These hemiaminals were exceedingly stable, with half-lives up to 100 hours at ambient temperature. They were persistent enough to study by 2D NOESY NMR, which showed the close proximity of the hemiaminal OH and the terminal NH2 (Figure 6b). This fixed intramolecular hydrogen bond adds a barrier of almost 3 kcal mol-1 to the dehydration event.
Figure 6.
a) The stabilization of the hemiaminal formed from addition of isobutylamine to cavitand 10a; b) Minimized structure of the hemiaminal formed by addition of ethylenediamine to 10a; the hydrogen bond between the terminal amine and hemiaminal OH is shown; c) Minimized structure of the hemiacetal formed by addition of N,N’-dimethylaminoethanol to 10a; the newly formed OH group is located near the amides and is stabilized by a factor of 5800; d) Minimized structure of the hemiacetal formed by addition of isopropionaldehyde to 10b; the newly formed OH group is located near the aromatic walls and is only stabilized by a factor of 140.
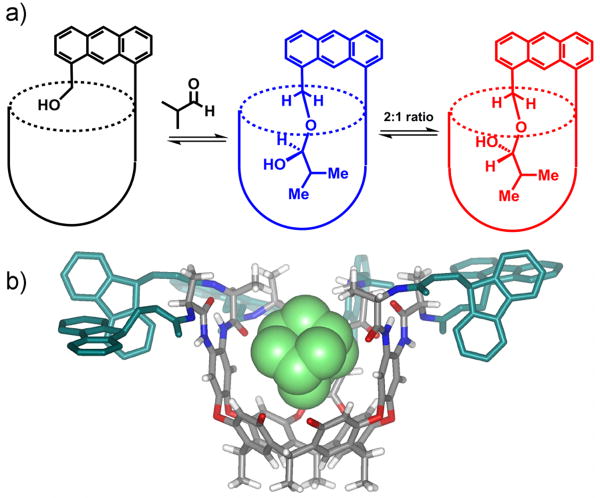
Amines are not the only nucleophiles available; by addition of alcohols, the equilibrium constant for hemiacetal formation can be enhanced almost 5000-fold (Figure 6c) (Hooley et al., 2007b). The aldehyde and alcohol functions can be incorporated into both cavitand and guest; either adding alcohols to 10a, or small aldehydes to 10b. The equilibrium enhancement is due to hydrogen bonding between the hemiacetal OH and the amide seam; consequently the equilibrium is strongly dependent on the position of the hemiacetal. If the bound guest is small (eg. isopropionaldehyde), the hemiacetal is positioned deeper in the cavity, near the aromatic walls (Figure 6d) and the equilibrium enhancement is only 13-fold.
c) Catalysis
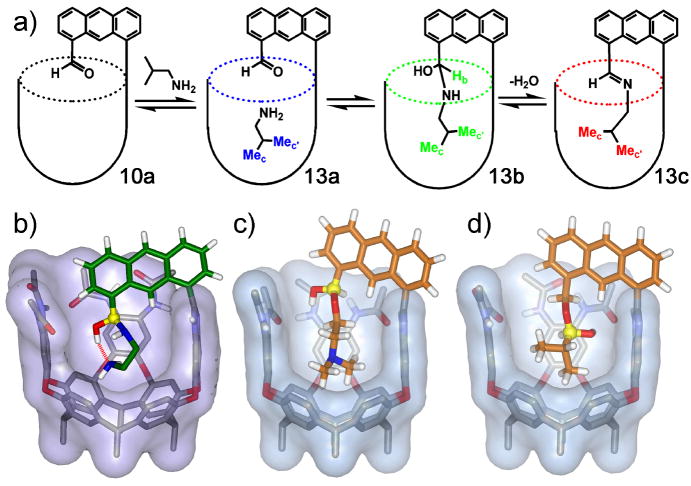
By positioning functional groups towards the interior of the cavity, great changes in guest behavior can be observed. The amide rim, however, can also have an effect on guest behavior. The amide seam acts as both hydrogen bond donor and acceptor, and can stabilize charges during reactions that occur inside the cavity (Hooley et al., 2005). Figure 7 shows two examples of catalysis controlled by the hydrogen-bonding ability of the amide seam of cavitand 3. By complexing a molecule of DABCO, the reactive amine center is positioned near the cavitand rim. When electron-deficient olefins such as methyl acrylate 14 are added to a catalytic amount of this complex in acetone-d6, the rate of α-deuteration increased by as much as 1400-fold due to the stabilization of the intermediate enolate complex 15 by the amide seam (Figure 7b). Similarly, by positioning a dienophilic maleimide group at the rim of the cavitand, the hydrogen-bonding groups accelerate the cycloaddition between the maleimide and 9-anthracenemethanol 16 (Hooley et al., 2007c). Apparently, the seam is less suited for interaction with neutral species (as opposed to charged enolates) and the rate acceleration is modest at 60-fold. Even so, turnover occurs: the products are larger and poorer guests for the cavitand than the starting materials and catalysis is observed. There are a number of examples of the use of hydrogen bonds to catalyze reactions (Taylor et al., 2006; Pihko, 2004; Huang et al., 2003) - the cavitands add a recognition element to this and can discriminate between differently sized molecules that display the same functionality; something for which small-molecule catalysts are ill-suited.
Figure 7.
Catalytic processes in the cavitands a) The use of DABCO in cavitand 3a to catalyze the α-deuteration of activated olefins; b) Catalytic Diels-Alder reaction of maleimides bound in 3a and suitable dienes; c) Catalyzed rearrangement of suitable 1,5-epoxyalcohols in acid 8a.
If the amide seam itself is capable of accelerating catalytic reactions, combining that with an introverted group should be even more effective. We used the introverted acid 8a to trigger the cyclization reaction of 1,5-epoxyalcohols. Three types of 1,5-epoxyalcohol (17a-c) were suitably sized, and immediate host-guest complexation was observed upon their addition to cavitand 8a. Reaction of alcohols 17a and 17c produced the tetrahydrofuran (THF) derivatives 18a/c as the exclusive products of the reaction, whereas the solution-phase reactions with camphorsulfonic acid (as a non-binding acid analog) afforded a ratio of 87:13 for (THF) and tetrahydropyran (THP) products (Shenoy et al., 2008). The regiocontrol for alcohol 17b was not as effective, giving similar products to the control. As well as the benefits of regioselectivity, significant rate accelerations of 50-300 fold were observed over the control reactions with pivalic acid.
The rate enhancement observed in this system can be understood by considering the molecular recognition events involved. Upon host-guest complexation, the epoxy-alcohol substrates are exposed to a high local concentration of Brönsted acid. At the same time, CH-π contacts between the aromatic walls of the host and the alkyl backbone of the guest induce the coiling of the substrate inside the cavitand. Such coiling brings the reactive centers of the epoxy-alcohols in close proximity, folding the substrates into conformations resembling the transition-state structures of the cyclization reactions. Together, these factors contribute to the overall increase in reaction rate.
The coiling of substrates inside the cavitand also influences the regioselectivity of the reaction. The stronger CH-π interactions garnered by the geminal methyl substituents of alcohols 17a and 17c folds the substrate into a more compressed five-membered-ring transition-state structure, leading to THF products. Alternatively, fewer CH-π contacts between the primary alcohol 17b and the cavitand allows reactions through more relaxed transition states which also affords THP products.
Chiral Recognition
Virtually all enzymes can discriminate between different enantiomers of substrate: multiple asymmetric centers confront the target when the linear sequence of the peptide or nucleotide folds around it. The use of flat aromatic panels to create the binding pocket makes the synthesis of chiral spaces in cavitands difficult. Chiral cavitands can be easily synthesized, but the difficulty is in presenting that chirality effectively to the guest.
As we described before, the seam of amides around the rim of 8 and 10 confers chirality on the cavitands; the amide seam is chiral as a whole, and the swirl of their electrons is detected, but the amides themselves are not chiral and present flat surfaces to the cavity. As the cavitands are overall racemic, only diastereoselectivity is possible, and is seen in certain covalent addition products. Addition of sec-diazobutane to acid 8a leads to a 2:1 ratio of diastereomers of the resulting sec-butyl esters (Purse et al., 2005). The hemiacetals formed by addition of isobutyraldehyde to alcohol 10b are also formed in a 2:1 ratio (Domling et al., 2000). The hemiacetal formation is an equilibrium process, but the interconversion between the two diastereomers does not occur inside the cavity – the aldehyde is eliminated and disassociates before re-addition. By orienting the stereogenic center near the chiral cavitand rim, maximal diastereoselectivity is obtained. Other, smaller aldehydes did not show comparable diastereoselectivities upon addition.
In an attempt to present steric aspects of chirality to non-covalently bound guests, chiral amides were attached to the cavitand rim (Mann et al., 2008). Fmoc-derived amino acid chlorides are easily accessed, and can be attached to the symmetrical cavitand using the same techniques as before to give cavitand 3c (Figure 8). The increased steric bulk of the amides does not interfere with vase formation in non-protic organic solvents. Two cyclodiastereomers are now possible, as the amides themselves are homochiral and the amide seam can adopt the two standard cycloenantiomers. Nonetheless, only one of the two possible cyclodiastereoisomers is present in solution at ambient temperatures. In other words, the intramolecular hydrogen bond seam is unidirectional, fixed by the asymmetric centers of the amides. Discrimination between different enantiomers of nopinone was achieved with a moderate diastereoselectivity of 3.5:1.
Figure 8.
Chiral recognition in cavitands; a) Diastereoselectivity in cavitand 10b; b) Structure of chiral cavitand 3c, illustrating the difficulty in conferring chirality from the cavitand to the guest.
Conclusion
A recent perspective by Ringe and Petsko (Ringe et al., 2008) identifies the key features of catalysis that have emerged from the study of enzymes over the last decades. These comprise positioning of reactive species, isolation in a special microenvironment, distortion of the substrate to a reactive shape and stabilizing transition states and intermediates that channel the reaction along a specific path. Another proposal implicates the formation of covalent bonds between enzyme and substrate for large rate effects (Zhang et al., 2005). These features are products of billions of years of evolution that can involve trillions of iterative refinement cycles. In comparison, the chemist concerned with synthetic catalysts works within narrow constraints: the timescale is decades, resources allow only a few systems to be constructed. Second generation molecules are synthesized only when structural features are identified that result in a desired behavior. Moreover, this bottoms-up approach does not allow much fine-tuning: adding or removing a single carbon atom causes changes in distance of more than 1Å and alters the orientation of an attached group. There are no methods for separating functional groups continuously at distances of tenths of Ångstroms. If we are permitted to call these notional enzymes, it is seen that several of the characteristics are present. Unfavored conformations of guests (Scarso et al., 2003) can be imposed by the size and shape of the host. When covalent bonds are formed between host and guest, the cavitands are stoichiometric reagents, yet these they show behavior believed to be exclusive to enzyme active sites. These systems operate at equilibrium, in the solution phase and at ambient temperatures, and are the products of imagination…
Acknowledgments
We are grateful to the Skaggs Institute and the National Institutes of Health (GM 50174) for financial support. R. J. H. is a Skaggs Postdoctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams H, Carver FJ, Hunter CA, Morales JC, Seward EM. Chemical Double-Mutant Cycles for the Measurement of Weak Intermolecular Interactions: Edge-to-Face Aromatic Interactions. Angew Chem Intl Ed. 1996;35:1542–1544. [Google Scholar]
- Adrian JC, Jr, Wilcox CS. Chemistry Of Synthetic Receptors And Functional Group Arrays. 10. Orderly Functional Group Dyads. Recognition Of Biotin And Adenine Derivatives By A New Synthetic Host. J Am Chem Soc. 1989;111:8055–8057. [Google Scholar]
- Bonsignore L, Cottiglia F, Maccioni AM, Secci D, Lavagna SM. The synthesis of coumarin 3-O acylisoures by dicyclohexylcarbodiimide. J Heterocyclic Chem. 1995;32:573. [Google Scholar]
- Butterfield SM, Rebek J., Jr A Synthetic Mimic of Protein Inner Space: Buried Polar Interactions in a Deep Water-Soluble Host. J Am Chem Soc. 2006;128:15366–15367. doi: 10.1021/ja0663374. [DOI] [PubMed] [Google Scholar]
- Carter PJ, Winter G, Wilkinson AJ, Fersht AR. The use of double mutants to detect structural changes in the active site of the tyrosyl-tRNA synthetase (Bacillus stearothermophilus) Cell. 1984;38:835–40. doi: 10.1016/0092-8674(84)90278-2. [DOI] [PubMed] [Google Scholar]
- Cram DJ, Cram JM. In: Container Molecules and Their Guests. Stoddart F, editor. The Royal Society of Chemistry; London: 1994. [Google Scholar]
- Cram DJ, Karbach S, Kim H-E, Knobler CB, Maverick EF, Ericson JL, Helgeson RC. Host-guest complexation. 46. Cavitands as open molecular vessels form solvates. J Am Chem Soc. 1988;110:2229–2237. [Google Scholar]
- De Tar DF, Silverstein R. Reactions of Carbodiimides 1. The Mechanism of the reaction of acetic acid with dicyclohexylcarbodiimide. J Am Chem Soc. 1966a;88:1013–1019. [Google Scholar]
- De Tar DF, Silverstein R. Reactions of Carbodiimides 2. The reaction of dicyclohexylcarbodiimide with carboxylic acids in the presence of amines and phenols. J Am Chem Soc. 1966b;88:1020–1023. [Google Scholar]
- Dixon RP, Geib SJ, Hamilton AD. Molecular Recognition: bisacylguanidiniums provide a simple family of receptors for phosphodiesters. J Am Chem Soc. 1992;114:365–366. [Google Scholar]
- Dömling A, Ugi I. Multicomponent Reactions with Isocyanides. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dong VM, Fiedler D, Carl B, Bergman RG, Raymond KN. Molecular Recognition and Stabilization of Iminium Ions in Water. J Am Chem Soc. 2006;128:14464–14465. doi: 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. Chemical Catalysis in Enzyme Structure and Mechanism. W.H. Freeman and Company; New York, NY: 1984. pp. 47–97. [Google Scholar]
- Fiedler D, Bergman RG, Raymond KN. Supramolecular Catalysis of a Unimolecular Transformation: Aza-Cope Rearrangement within a Self-Assembled Host. Angew Chem Int Ed. 2004;43:6748–675. doi: 10.1002/anie.200461776. [DOI] [PubMed] [Google Scholar]
- Galán A, de Mendoza J, Toiron C, Bruix M, Deslongchamps G, Rebek J., Jr A Synthetic Receptor for Dinucleotides. J Am Chem Soc. 1991;113:9424–9425. [Google Scholar]
- Gautier A. Ueber die einwirkung der Sauern auf die carbylamine. Liebig’s Annalen. 1896;151:240–243. [Google Scholar]
- Goto K, Holler M, Okazaki R. Synthesis, Structure, and Reactions of a Sulfenic Acid Bearing a Novel Bowl-Type Substituent: The First Synthesis of a Stable Sulfenic Acid by Direct Oxidation of a Thiol. J Am Chem Soc. 1997;119:1460–1461. [Google Scholar]
- Hegarty AF. Stereospecific Reactions of Nitrilium Ions and Analogous 1,3-Dipoles Acc. Chem Res. 1980;13:448–454. [Google Scholar]
- Hof F, Craig SL, Nuckolls C, Rebek J., Jr Molecular Encapsulation. Angew Chem Int Ed. 2002;41:1488–1508. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Hof F, Trembleau L, Ullrich EC, Rebek J., Jr Acetylcholine Recognition by a Deep, Biomimetic Pocket. Angew Chem Int Ed. 2003;429:3150–3153. doi: 10.1002/anie.200351174. [DOI] [PubMed] [Google Scholar]
- Hooley RJ, Rebek Jr. A Deep Cavitand Catalyzes the Diels-Alder Reaction of Bound Maleimides. Org Biomol Chem. 2007a;5:3631–3636. doi: 10.1039/b713104f. [DOI] [PubMed] [Google Scholar]
- Hooley RJ, Rebek J., Jr Deep Cavitands Provide Organized Solvation of Reactions. J Am Chem Soc. 2005;127:11904–11905. doi: 10.1021/ja052910s. [DOI] [PubMed] [Google Scholar]
- Hooley RJ, Iwasawa T, Rebek J., Jr Detection of Reactive Tetrahedral Intermediates in a Deep Cavitand with an Introverted Functionality. J Am Chem Soc. 2007b;129:15330–15339. doi: 10.1021/ja0759343. [DOI] [PubMed] [Google Scholar]
- Hooley RJ, Restorp P, Iwasawa T, Rebek J., Jr Cavitands with Introverted Functionality Stabilize Tetrahedral Intermediates. J Am Chem Soc. 2007c;129:15639–15643. doi: 10.1021/ja0756366. [DOI] [PubMed] [Google Scholar]
- Hou J-L, Ajami D, Rebek J., Jr Reaction of Carboxylic Acids and Isonitriles in Small Spaces. J Am Chem Soc. 2008;130:7810–7811. doi: 10.1021/ja802288k. [DOI] [PubMed] [Google Scholar]
- Huang Y, Unni AK, Thadani AN, Rawal VH. Hydrogen bonding: Single enantiomers from a chiral-alcohol catalyst. Nature. 2003;424:146. doi: 10.1038/424146a. [DOI] [PubMed] [Google Scholar]
- Iwasawa T, Hooley RJ, Rebek J., Jr Isolation and Observation of Unstable Intermediates in Carbonyl Addition Reactions. Science. 2007a;317:493–496. doi: 10.1126/science.1143272. [DOI] [PubMed] [Google Scholar]
- Iwasawa T, Mann E, Rebek J., Jr A Reversible Reaction Inside a Self-Assembled Capsule. J Am Chem Soc. 2006;128:9308–9309. doi: 10.1021/ja062768a. [DOI] [PubMed] [Google Scholar]
- Iwasawa T, Wash P, Gibson C, Rebek J., Jr Reaction of an Introverted Carboxylic Acid with Carbodiimide. Tetrahedron. 2007b;63:6506–6511. doi: 10.1016/j.tet.2007.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. A Hydrophobic Nanocapsule Controls the Photophysics of Aromatic Molecules by Suppressing Their Favored Solution Pathways. J Am Chem Soc. 2005;127:3674–3675. doi: 10.1021/ja0425381. [DOI] [PubMed] [Google Scholar]
- Kemp DS, Petrakis KS. Synthesis and Conformational Analysis of cis, cis - 1,3,5 Trimethylcyclohexane-1,3,5-tricarboxylic Acid. J Org Chem. 1981;46:5140–5143. [Google Scholar]
- Li X, Danishefsky S. New Chemistry with Old Functional Groups: On the Reaction of Isonitriles with Carboxylic Acids—A Route to Various Amide Types. J Am Chem Soc. 2008;130:5446–5447. doi: 10.1021/ja800612r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons BA, Montelione GT. An HCCNH Triple-Resonance Experiment Using Carbon-13 Isotropic Mixing for Correlating Backbone Amide and Side-Chain Aliphatic Resonances in Isotopically Enriched Proteins. J Magn Reson. 1993;101:206–208. [Google Scholar]
- MacGillivray LR, Atwood JL. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature. 1997;389:469–472. [Google Scholar]
- Mann E, Rebek J., Jr Deepened Chiral Cavitands. Tetrahedron. 2008;64:8484–8487. [Google Scholar]
- Mecozzi S, Rebek J., Jr The 55% Solution: A Formula for Molecular Recognition in the Liquid State. Chemistry-A European Journal. 1998;4:1016–1022. [Google Scholar]
- Moran JR, Ericson JL, Dalcanale E, Bryant JA, Knobler CB, Cram DJ. Vases and kites as cavitands. J Am Chem Soc. 1991;113:5707–5714. [Google Scholar]
- Moran J, Ericson J, Dalcanale E, Bryant J, Knobler C, Cram D. Vases and Kites as Cavitands. J Am Chem Soc. 1991;113:5707–5714. [Google Scholar]
- Pihko PM. Activation of carbonyl compounds by double hydrogen bonding: An emerging tool in asymmetric catalysis. Angew Chem, Int Ed. 2004;43:2062. doi: 10.1002/anie.200301732. [DOI] [PubMed] [Google Scholar]
- Purse B, Rebek J., Jr Functional Cavitands: Chemical Reactivity in Structured Environments. Proc Natl Acad Sci U S A. 2005;102:10777–10782. doi: 10.1073/pnas.0501731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purse BW, Ballester P, Rebek J., Jr Reactivity and Molecular Recognition – Amine Methylation by an Introverted Ester. J Am Chem Soc. 2003;125:14682–14683. doi: 10.1021/ja036595q. [DOI] [PubMed] [Google Scholar]
- Purse BW, Butterfield SM, Ballester P, Shivanyuk A, Rebek J., Jr Interaction Energies and Dynamics of Acid-Base Pairs Isolated in Cavitands. J Org Chem. 2008 doi: 10.1021/jo8008534. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebek J., Jr Mechanistic Studies Using Solid Supports: The Three-Phase Test. Tetrahedron. 1979;35:723. [Google Scholar]
- Rebek J, Jr, Zimmerman S, Brown D. New Probes for the Study of Acylation Reactions. J Am Chem Soc. 1975;97:4407. [Google Scholar]
- Renslo AR, Rebek J., Jr Molecular Recognition and Introverted Functionality. Angew Chemie Int Ed Engl. 2000;39:3281–3283. doi: 10.1002/1521-3773(20000915)39:18<3281::aid-anie3281>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Restorp P, Rebek J., Jr Reaction of Isonitriles with Carboxylic Acids in a Cavitand: Observation of Elusive Isoimide Intermediates. J Am Chem Soc. 2008 doi: 10.1021/ja803854r. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe D, Petsko GA. How Enzymes Work. Science. 2008;320:1428–1429. doi: 10.1126/science.1159747. [DOI] [PubMed] [Google Scholar]
- Roach P, Warmuth R. The room-temperature stabilization of bicyclo[2.2.2]oct-1-ene and bicyclo[3.2.1]oct-1-ene. Angew Chem Int Ed. 2003;42:3039–3042. doi: 10.1002/anie.200351120. [DOI] [PubMed] [Google Scholar]
- Rudkevich DM, Hilmersson G, Rebek J., Jr Self-Folding Cavitands. J Am Chem Soc. 1998;120:12216–12225. [Google Scholar]
- Scarso A, Trembleau L, Rebek J., Jr Encapsulation Induces Helical Folding of Alkanes. Angew Chemie Intl Ed Engl. 2003;42:5499–5502. doi: 10.1002/anie.200352235. [DOI] [PubMed] [Google Scholar]
- Shenoy SR, Pinacho Crisostomo FR, Iwasawa T, Rebek J., Jr Organocatalysis in a synthetic receptor with an Inwardly-Directed Carboxylic Acid. J Am Chem Soc. 2008;130:5658–5659. doi: 10.1021/ja801107r. [DOI] [PubMed] [Google Scholar]
- Sherman JC. Carceplexes and hemicarceplexes: Molecular encapsulation—From hours to forever. Tetrahedron. 1995;51:3395–3422. [Google Scholar]
- Soncini P, Bonsignore S, Dalcanale E, Ugozzoli F. Cavitands as Versatile Molecular Receptors. J Org Chem. 1992;57:4608–4612. [Google Scholar]
- Taylor MS, Jacobsen EN. Asymmetric catalysis by chiral hydrogen-bond donors. Angew Chem Int Ed. 2006;45:1520. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]
- Vaughan CK, Harryson P, Buckle AM, Fersht AR. A structural double-mutant cycle: estimating the strength of a buried salt bridge in barnase. Acta Cryst. 2002;D58:591–600. doi: 10.1107/s0907444902001567. [DOI] [PubMed] [Google Scholar]
- Warmuth R, Makowiac S. The phenyinitrene rearrangement in the inner phase of a hemicarcerand. J Am Chem Soc. 2005;127:1084–1085. doi: 10.1021/ja044557g. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Kusukawa T, Fujita M, Yamaguchi K. Ship-in-a-bottle Synthesis of Otherwise Labile Cyclic Trimers of Siloxanes in a Self-assembled Coordination Cage. J Am Chem Soc. 2000;122:6311. [Google Scholar]
- Yoshizawa M, Tamura M, Fujita M. Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis. Science. 2006;312:251–254. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- Zhang X, Houk KN. Why Enzymes Are Proficient Catalysts: Beyond the Pauling Paradigm. Acc Chem Res. 2005;38:379–385. doi: 10.1021/ar040257s. [DOI] [PubMed] [Google Scholar]
- Ziegler M, Brumaghim JL, Raymond KN. Stabilization of a Reactive Cationic Species by Supramolecular Encapsulation. Angew Chem Int Ed. 2000;39:4119–4121. doi: 10.1002/1521-3773(20001117)39:22<4119::aid-anie4119>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman SC, Wu W. A Rigid Molecular Tweezers With An Active Site Carboxylic Acid: Exceptionally Efficient Receptor For Adenine In An Organic Solvent. J Am Chem Soc. 1989;111:8054–8055. [Google Scholar]