Abstract
The environmental carcinogen 5-methylchrysene (5MC) can be activated to mutagenic metabolites by several isozymes of cytochrome P450 (CYP). The resulting reactive diol-epoxides can be detoxified via conjugation by glutathione transferases (GST). We investigated whether expression of human glutathione transferase P1 (hGSTP1) would differentially protect cells against the cytotoxicity or mutagenicity of 5-methylchrysene (5MC) or its 1,2-dihydrodiol intermediate (5MC-1,2-diol) in V79MZ cells with activation via stably transfected human CYP1B1 (hCYP1B1) as compared to activation by human CYP1A1 (hCYP1A1). The parent compound 5MC was only 2-fold more cytotoxic in the CYP-expressing cell lines than in the V79MZ parental cell line, while 5MC-1,2-dihydrodiol was more than 30-fold more cytotoxic in CYP-transfected cells compared to V79MZ cells. Cells co-expressing either hCYP1B1 or hCYP1A1 together with hGSTP1 were 2-fold less sensitive to 5MC or 5MC-1,2-diol cytotoxicity than their CYP-only parent lines. The 5MC was highly mutagenic with similar potency in both hCYP-transfected cell lines, while 5MC-1,2-diol was 2-fold more mutagenic in hCYP1B1-transfected cells as compared to hCYP1A1 cells. Co-expression of hGSTP1 with either hCYP reduced 5MC or 5MC-1,2-diol mutagenicity by 1.4- to 4.5-fold compared to the corresponding hCYP-only expressing cell lines. The greater protection against mutagenicity of 5MC is in contrast to our previous studies in which we found greater protection by hGSTP1 against cytotoxicity than mutagenicity of benzo[a]pyrene in cells co-expressing hCYP1A1. Protection against mutagenicity by hGSTP1 was greater with activation of either compound by hCYP1B1 than with hCYP1A1 activation. These studies show that the relative efficacy of protection by hGSTP1 against mutagenicity of 5MC or 5MC-1,2-diol is in part determined by the specific CYP pathway that catalyzes activation to the toxic or mutagenic metabolites.
Keywords: cytochrome P-450, glutathione S-transferase, 5-methylchrysene, polycyclic aromatic hydrocarbon, mutgenicity, cytotoxicity
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous carcinogens present in smoke as a result of incomplete combustion. Human populations are exposed to PAHs through inhalation of polluted air or tobacco smoke, as well as via ingestion of contaminated water and smoked or grilled food. PAHs and other environmental chemical carcinogens are often chemically inert until metabolically activated by “phase I” cytochrome P-450 enzymes (CYPs) to more reactive metabolites. The most carcinogenic PAH metabolites are the highly reactive dihydrodiol epoxides (Conney, 1982; Jerina, 1986; Melikian, 1983). Covalent binding of reactive electrophilic metabolites of carcinogens to DNA is a major cause of initiation of carcinogenesis (Dipple, 1995). Previous studies showed that human CYP1A1 and CYP1B1, commonly expressed in extra-hepatic tissues including lungs, mammary glands, ovary, testis and uterus, play an important role in the metabolic activation of PAH and their dihydrodiol metabolites (Hall, 1989; Kim, 1998; Luch, 1998; Schmalix, 1993; Shimada and Fujii-Kuriyama, 2004; Shimada, 1996).
Glutathione-S-transferases (GSTs) are “phase II” enzymes that catalyze the conjugation of reduced glutathione (GSH) with reactive electrophiles, including many chemical carcinogens and environmental pollutants, to reduce their toxic effects (Hayes and Pulford, 1995; Rushmore and Pickett, 1993). Induction of GST expression has been proposed to play a significant role in cellular protection by chemopreventive agents against potentially carcinogenic DNA damage by reactive electrophiles (McMahon, 2001; Wattenberg, 1985). Based on their localization, mammalian GST families are grouped into three types, cytosolic, mitochondrial and microsomal (also referred as membrane-associated proteins in eicosanoid and glutathione metabolism; MAPEG) (Hayes, 2005). Based on sequence similarities, cytosolic GSTs are classified into at least 10 classes in mammalian tissue, with pi, mu and alpha classes the most abundant. The expression of pi-class human GSTP1 (hGSTP1) has been reported to be elevated in many epithelial cells and tumors (Kantor, 1991), and is the principal GST isozyme expressed in the lung, a major target organ for PAH carcinogenesis (Grover, 1975). Overexpression of GST in mammalian tumor cells has been associated with resistance to various anticancer agents and chemical carcinogens (Fields, 1994; Fields, 1999; Hayes and Pulford, 1995).
Although the phase I and phase II activities have been extensively examined separately, the dynamics of metabolic activation by CYP in competition with the protective role of GST detoxification at the cellular level remains incompletely understood. We have developed transgenic cell models via stable transfection to assess the relative protective effects of coexpression of GSTs, together with relevant CYP isozymes implicated in PAH activation, on the cytotoxicity and mutagenicity of PAHs (Townsend, 1998b; Townsend, 2002). Previous studies have demonstrated up to 5-fold reduction in mutagenicity at the hprt locus of benzo[a]pyrene (B[a]P) or dibenzo[a,l]pyrene (DB[a,l]P) in clonal cell lines coexpressing hGSTP1, hGSTM1, or hGSTA1 together with hCYP1A1 or hCYP1B1 (Kushman, 2007a; Kushman, 2007b; Kushman, 2006). However, protection against cytotoxicity was often greater than against mutagenicity, and protection varied with hCYP1A1 vs. hCYP1B1 as the activation pathway. Since the earlier studies examined PAHs with either “bay-region” (B[a]P) or “fjord-region” (DB[a,l]P) structures, the present study was undertaken to examine the effects of activation and detoxification of 5-methylchrysene, a highly carcinogenic representative of a third PAH structural class, the bay-region alkyl-PAHs (Amin, 1985; Hecht, 1987). Based on an earlier report, which showed that hGSTP1 was much more active than mu or alpha class isozymes for conjugation of (+)-anti-5-methylchrysene-1,2-dihydrodiol-3,4-epoxide (Hu, 1998), hGSTP1 was the isozyme selected for the present studies. We compared cytotoxicity or mutagenicity of 5MC or its 1,2-dihydrodiol intermediate (5MC-1,2-diol) in transgenic cell lines expressing either hCYP1B1 or hCYP1A1, alone or together with hGSTP1 expression. In contrast to the our previous results with B[a]P, DB[a,l]P, or their key intermediate diols, the present studies indicated stronger protection by hGSTP1 expression against mutagenicity than cytotoxicity, with less effect of the activating CYP pathway on the magnitude of protection.
Materials and Methods
Reagents
All chemicals used in this study were of analytical grade and were obtained from Sigma (St. Louis), Fisher (Atlanta, GA) or Invitrogen (Carlsbad, CA). Advanced Dulbecco's Modified Eagle's Medium (Adv. DMEM) was purchased from Gibco/BRL, Grand Island, NY. Vivid BOMCC substrate was purchased from Invitrogen. 5-Methylchrysene (5MC) was obtained from the National Cancer Institute (NCI) carcinogen repository (Chemsyn), Kansas City, MO and 5-methylchrysene-1,2-dihydrodiolol (5MC-1,2-diol) was synthesized by S. Amin as previously described (Amin, 1988). (Caution: 5MC and its metabolites described herein are potential chemical carcinogens and must be handled with caution as outlined in the NCI guidelines).
Cell Culture and cell lines
The modification of the parental V79MZ (Chinese Hamster lung fibroblast) cell lines to express human CYP1B1 or CYP1A1 has been described previously (Luch, 1998; Schmalix, 1993). Generation of the V79MZ cell lines expressing hCYP1B1 or hCYP1A1 together with stably transfected human GSTP1 (I104, A113 common allele) has also been described (Townsend, 1998a). All cell lines were maintained in Advanced DMEM medium with 2% fetal bovine serum (FBS). The hCYP1B1 and hCYP1A1 expressing cells were selected at alternate passages with 400 μg/mL G-418. Doubling times were similar in all cell lines. Cells were passaged at 1:20 dilution every 2-3 days.
Fluorescent assay for hCYP1B1 or hCYP1A1 activity
Activities of hCYP1B1 or hCYP1A1 in intact cells were measured in intact cells using the fluorescent Vivid BOMCC substrate. All transfected and control cells were plated in 60 mm plates (1.8 × 105 cells per plate in 4 mL Advanced DMEM) and grown without G-418 or hygromycin for two days in a humidified atmosphere of 5% CO2, 95% air at 37°C. At the time of assay all plates were rinsed twice with serum-free regular DMEM and 2 mL of serum free prewarmed regular DMEM added containing 5 μM final concentration of Vivid BOMCC substrate added from 1000X stocks in acetonitrile (0.1% final concentration). All plates were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C for 30 min. At the end of incubation period a 1 mL aliquot of medium was taken from each plate and fluorescence was measured on a Perkin-Elmer LS-3B spectrofluorimeter with excitation at 409 nm and emission detection at 460nm. Activities of hCYP1B1 and hCYP1A1 were measured using a known Vivid blue standard curve (0-100 μM) and final activity was normalized to protein content in each plate, determined by the coomassie dye method (Bio-Rad, Hercules, CA). The hCYP1B1 and hCYP1A1 activity were expressed as pmol/min/mg protein. Although the activity with the BOMCC substrate is 10-fold higher with cells expressing hCYP1A1 than with cells expressing hCYP1B1, this is due to the higher specific activity of hCYP1A1 with this substrate. The relative expression of the two isozymes is similar based on specific activities obtained with the less sensitive ethoxyresorufin O-deethylation (EROD) assay (Luch, 1998).
GST enzyme assay
GST activity in total cell lysate was measured as previously described with slight modifications (Habig, 1974; Townsend, 1989). Cells were cultured in 60 mm plates and harvested after 2-3 days, rinsed twice with ice cold phosphate buffed saline (PBS) and scraped into 1 mL of PBS and collected by centrifugation at 500 × g for 5 min at 4°C. Supernatant was removed and cells were resuspended in 150 μL of 50 mM Tris, 5 mM EDTA buffer and lysed by a 10 second sonication with a microtip probe set at 35% power. The lysate was centrifuged at 14,500 × g for 5 min at 4°C and supernatant (5 – 10 μL) was assayed at room temperature in a solution of 0.1 mM K2PO4, pH 6.5 and 1 mM glutathione (GSH). The reaction was initiated with 1 mM (final concentration) of 1-chloro-2,4-dinitrobenzene (CDNB; 5X in EtOH). The change in absorbance was monitored at 340 nm for 90 seconds (6 intervals) and activity, corrected for nonenzymatic reagent blank, was calculated using the ΔA/min and extinction coefficient [9.6 (mmol/L)-1 cm-1] and expressed as nmol/min/mg protein. Protein concentrations were determined by the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) using bovine serum albumin (BSA) as standard.
Cytotoxicity assays
Cytotoxicity was assessed by the sulforhodamine B method (Skehan, 1990). Cells were plated in Advanced DMEM with 2% FBS at a density of 250 cells/well on 96-well plates and allowed to attach and grow without G-418 or hygromycin selection for 16-24 hours. The 5MC (0-5 μM) or 5MC-1,2-diol (0-1 μM) was added from 1000X stocks in ethanol (0.1% final concentration of ethanol) and incubated continuously for 72 hours, medium removed and cells fixed in 200 μL cold 5% TCA. After removal of the TCA, plates were stained with 0.4% sulforhodamine B dye in 1% glacial acetic acid for 10 minutes, then washed 4-5 times with 1% glacial acetic acid. The stained plates were dried in a 55°C oven for several hours. The dye was solubilized by adding 100 μL of 10 mM Tris base and absorbance measured with a microplate reader (Molecular Devices. Sunnyvale, CA) at 560 nm to quantitate protein as an indirect measure of cell number.
hprt Mutagenicity Assay
Cells were plated at a density of 5 × 105 cells per 100 mm plate in the absence of G-418 or hygromycin selection, incubated overnight and exposed to 5MC (0.1, 0.3 or 1 μM) or 5MC-1,2-diol (0.01, 0.03 or 0.1 μM) in culture medium for the 48-hour dose-response study. Cells were then rinsed twice with cell culture medium and 10 mL fresh medium were added to each plate and allowed to recover overnight. Cells were then sub-cultured for phenotypic development at their original density (5 × 105 cells/plate) for 6-7 days with sub-culturing at 5 × 105 cells/plate every 2-3 days. After this period, cells were plated at a density of 5 × 105 cells per 100 mm plate and the next day 6-TG was added (10 μg 6-TG/mL medium) in order to select for hprt mutant colonies. 6-TG selection was carried out for 10 days with one medium change and fresh 6-TG after 3 days. After 10 days, mutant hprt colonies were stained with 5 mM methylene blue in methanol and mutant colonies were counted. Mutants are expressed per million cells plated for 6-TG selection.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD) of three or more independent experiments. Statistical differences were evaluated by ANOVA followed by Tukey's post-hoc test. The criterion for statistical significance was set at P < 0.05.
Results
hCYP and GST enzyme activity in transgenic cell lines
The modified cell lines exhibited closely matched hCYP1B1 or hCYP1A1 activities. The CYP activity was greatly increased in cell lines expressing transfected hCYP1B1 (8.5 – 9.5 pmol/min/mg) or hCYP1A1 (79-90 pmol/min/mg), as compared to essentially background levels in V79MZ control cells (0.32 pmol/min/mg). There were no significant differences in CYP enzyme activity between the hCYP1B1, hCYP1B1+hGSTP-25 and hCYP1B1+hGSTP-15, or between hCYP1A1 and hCYP1A1+hGSTP-23 transfected cell lines. Cells expressing hCYP1B1 together with hGSTP1 had 2.6-fold (hCYP1B1+hGSTpi-25) or 7-fold (hCYP1B1+hGSTpi-15) higher GST specific activity as compared to the hCYP1B1 only expressing cells (Table 1). The cells expressing hCYP1A1 together with hGSTP1 (hCYP1A1+hGSTpi-23) had 5-fold higher GST specific activity as compared to the hCYP1A1 only expressing cells. The hGSTP1 activities in the V79MZ, hCYP1B1 and hCYP1A1 cell lines were not significantly different.
Table 1. Cytochrome P450 and GST activities in parental and transfected cell lines.
Cytochrome P-450 activity was measured in intact cells using the Vivid™ BOMCC fluorescent substrate as described in Materials and Methods. Results are the mean ± SD of three or more independent assays.
| Cell lines | Cyt P450 activity (pmol/min/mg protein) |
GST activity (nmol/min/mg protein) |
|---|---|---|
| V79MZ | 0.32 ± 0.12 | 108 ± 5 |
| hCYP1B1 | 8.5 ± 1.0 a | 127 ± 16 c |
| hCYP1B1+GSTpi-25 | 9.5 ± 0.4 b | 329 ± 23 d |
| hCYP1B1+GSTpi-15 | 8.9 ± 0.5 b | 867 ± 114 d |
| hCYP1A1 | 79 ± 10 a | 147 ± 48 c |
| hCYP1A1+hGSTpi-23 | 90 ± 14b | 824 ± 104 e |
Significantly different (P < 0.05) from the mean for control V79MZ;
Not significantly different (p ≥ 0.1) from the mean for the respective hCYP1A1 or hCYP1B1 control;
Not significantly different (p > 0.1) from the mean for control V79MZ;
Significantly different (P < 0.05) from the mean for hCYP1B1 control;
Significantly different (P < 0.05) from the mean for hCYP1A1 control.
Cytotoxicity of 5MC or 5MC-1,2-diol
The dose-response of the cytotoxicity of 5MC or 5MC-1,2-diol was compared in V79MZ control and all CYP- and/or GSTP-transfected cell lines. 5MC was more cytotoxic in both hCYP1B1-expressing and hCYP1A1-expressing cell lines (p < .05) as compared to V79MZ control cells (Table 2). The IC50 values of 5MC in V79MZ, hCYP1B1, hCYP1B1+hGSTP-25, and hCYP1B1+hGSTP-15 were 3.1 ± 0.2, 1.6 ± 0.2, 3.1 ± 0.3, and 2.9 ± 0.4 μM, respectively (Table 2). The IC50 values of 5MC in hCYP1A1 or hCYP1A1+hGSTP-23 were 1.6 ± 0.2 or 3.2 ± 0.3 μM, respectively. Thus, a statistically significant 2-fold protection by hGSTP1 against 5MC cytotoxicity was observed in all cell lines expressing hGSTP1 together with either hCYP1B1 or hCYP1A1 (Table 2). In contrast to 5MC, the metabolite 5MC-1,2-diol was at least 10-fold more cytotoxic than 5MC to all hCYP1B1-, hCYP1A1- and hCYP+hGSTP1-transfected clones. The IC50 values of 5MC-1,2-diol for hCYP1B1, hCYP1B1+hGSTP-25, hCYP1B1+hGSTP-15, and V79MZ control cell lines were 0.11 ± 0.03, 0.16 ± 0.03, 0.23 ± 0.03, and > 3.0 μM, respectively (Table 3). The IC50 values of 5MC-1,2-diol for hCYP1A1 and hCYP1A1+hGSTP-23 cell lines were 0.076 ± 0.02 and 0.13 ± 0.04, respectively (Table 3). Thus the protection against 5MC-1,2-diol by coexpression of hGSTP1 ranged from 1.4-fold to 2.1-fold in the cell lines expressing either hCYP1B1 or hCYP1A1, but was statistically significant only in the hCYP1B1-15 line.
Table 2. Cytotoxicity of 5-methylchrysene (5MC) in cell lines expressing hCYP1B1 or hCYP1A1, alone or together with hGSTP1.
Cytotoxicity was assayed by the sulforhodamine dye method as described in Materials and Methods. Each value represents the mean ± standard deviation of three or more independent assays.
| Cell lines | IC50 values (μM 5MC) |
Fold-Resistance |
|---|---|---|
| V79MZ | 3.1 ± 0.2 | (N/A) |
| hCYP1B1 | 1.6 ± 0.2 a | (1.0) |
| hCYP1B1 + hGSTP1-25 | 3.1 ± 0.3 b | 2.0 |
| hCYP1B1 + hGSTP1-15 | 2.9 ± 0.4 b | 1.9 |
| hCYP1A1 | 1.6 ± 0.2 a | (1.0) |
| hCYP1A1 + hGSTP1-23 | 3.2 ± 0.3 c | 2.0 |
Significantly different (P ≤ 0.05) from the mean for V79MZ;
Significantly different (P < 0.05) from the mean for hCYP1B1;
Significantly different (P < 0.05) from the mean for hCYP1A1.
Table 3. Cytotoxicity of 5-methylchrysene-1,2-diol in cell lines expressing hCYP1B1 or hCYP1A1, alone or together with hGSTP1.
Cytotoxicity was assayed by the sulforhodamine dye method as described in Materials and Methods. Each value represents the mean ± standard deviation of three or more independent assays.
| Cell lines | IC50 values (μM 5MC-1,2-diol) |
Fold-Resistance |
|---|---|---|
| V79MZ | > 3.0 | (N/A) |
| V79hCYP1B1 | 0.11 ± 0.03 a | (1.0) |
| V79hCYP1B1/hGSTP1-25 | 0.16 ± 0.03 b | 1.4 |
| V79hCYP1B1/hGSTP1-15 | 0.23 ± 0.03 c | 2.1 |
| V79hCYP1A1 | 0.076 ± 0.02 a | (1.0) |
| V79hCYP1A1/hGSTP1-23 | 0.13 ± 0.04 d | 1.7 |
Significantly different (P ≤ 0.05) from the mean for V79MZ;
Not significantly different (P > 0.1) from the mean for hCYP1B1;
Significantly different (P < .05) from the mean for hCYP1B1;
Not significantly different (P > 0.1) from the mean for hCYP1A1.
Mutagenicity of 5MC and 5MC-1,2-diol
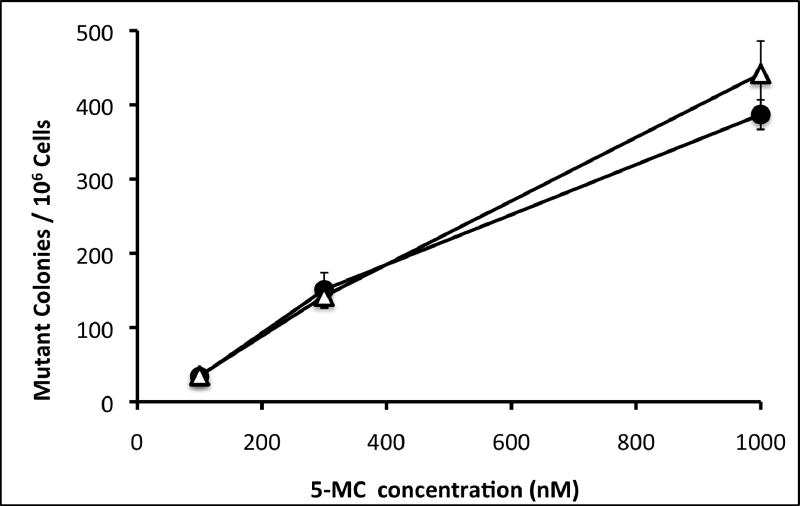
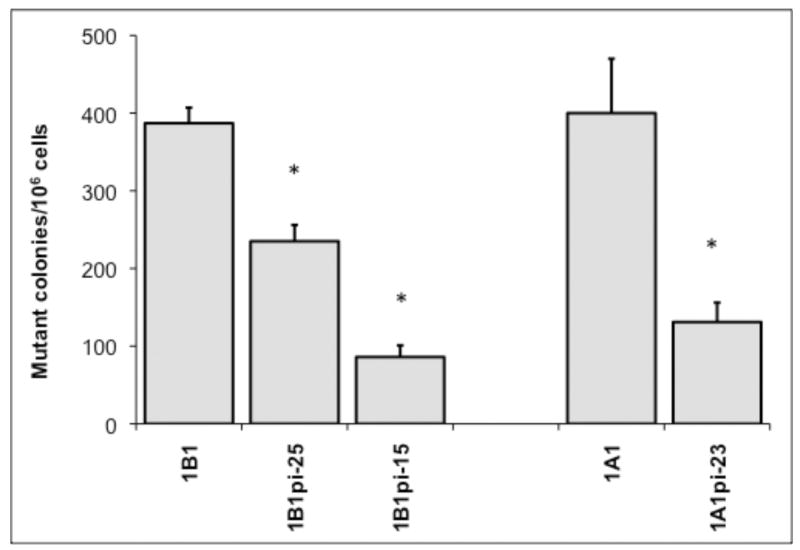
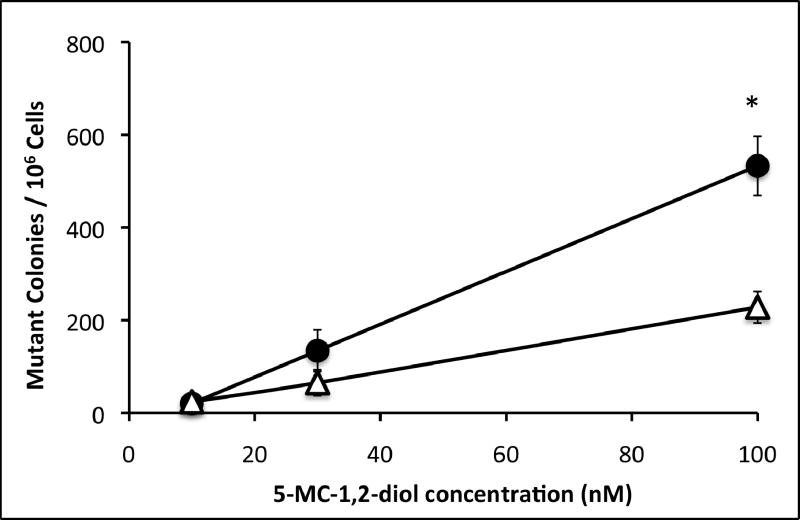
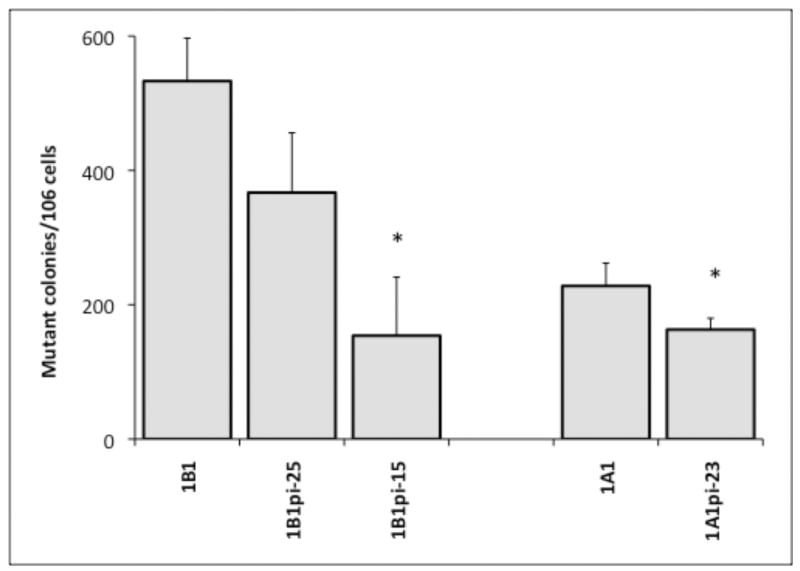
Mutagenicity was measured by frequency of mutation at the hprt locus following exposure to 5MC or 5MC-1,2-diol in the transfected cell lines and compared with the V79MZ group (no CYP). The control hCYP1B1- and hCYP1A1-expressing cell lines exhibited a dose-dependent frequency of mutation after exposure to 0.1, 0.3, or 1.0 μM 5MC (Figure 1). The frequency of mutation at the hprt locus was similar in hCYP1A1 transfected cell as compared to hCYP1B1 transfected cell at dosing conc. of 5MC (0.3 or 1 μM). No mutant colony formation was observed in V79MZ control cells exposed to 5MC (not shown). Comparisons of cells with only hCYP expression to the derivative lines with coexpression of hGSTP1 indicated that hGSTP1 provided strong protection against mutagenicity of 1.0 μM 5MC, with 1.7-fold reduction in mutant colonies in hCYP1B1+GSTpi-25, and 4.5-fold reduction in hCYP1B1+hGSTpi-15 cells as compared to control cells expressing only hCYP1B1 (Figure 2). Similarly, 3.1-fold protection against 5MC mutagenicity was observed in the hCYP1A1+GSTpi-23 line as compared to control cells expressing only hCYP1B1. The mutation frequency in these cell lines following exposure to 5MC-1,2-diol was also dose-dependent, but in contrast to 5MC, the metabolite was more than twice as mutagenic in the hCYP1B1-expressing cell line as in the hCYP1A1-expressing line (Figure 3). Protection against mutagenicity by hGSTP1 was 1.5-fold in hCYP1B1+hGSTpi-25 cells, and 3.5-fold in hCYP1B1+hGSTpi-15 cells as compared cells expressing only hCYP1B1 (Figure 4). However, the protection by hGSTP1 expression at the same level as in the hCYP1B1+hGSTpi-15 line was less (1.4-fold), though significant in cells expressing hCYP1A1.
Figure 1.
Dose dependent mutagenicity of 5MC was determined in hCYP1B1 and hCYP1A1 cell lines. Cells were exposed to the indicated concentrations of 5MC for 48 hours and mutagenicity assays were performed as described in Materials and Methods. Symbols: closed circles, V79MZh1B1 cell line; open triangles, V79MZh1A1 cell line. Each value represents the mean ± SD of three independent assays.
Figure 2.
Effect of hGSTP1 expression on mutagenicity of 5MC in cell lines expressing hCYP1B1 or hCYP1A1. Cells were exposed to 1.0 μM 5MC for 48 hours and processed for mutagenicity assays as described in Materials and Methods. Results are expressed as mutant colonies per million cells. Each value represents the mean ± SD of three independent assays. * Significantly different (P < 0.05) from the mean of the corresponding hCYP1B1 or hCYP1A1 cell lines, respectively.
Figure 3.
Dose dependent mutagenicity of 5MC-1,2-diol was determined in hCYP1B1 and hCYP1A1 cell lines. Cells were exposed to the indicated concentrations of 5MC-1,2-diol for 48 hours and mutagenicity assays were performed as described in Materials and Methods. Symbols: closed circles, V79MZh1B1 cell line; open triangles, V79MZh1A1 cell line. Each value represents the mean ± SD of three independent assays.
* significantly different from V79MZh1A1 value (p < .05).
Figure 4.
Effect of hGSTP1 expression on mutagenicity of 5MC-1,2-diol in cell lines expressing hCYP1B1 or hCYP1A1. Cells were exposed to 0.1 μM 5MC-1,2-diol for 48 hours and processed for mutagenicity assays as described in Materials and Methods. Results are expressed as mutant colonies per million cells. Each value represents the mean ± SD of three independent assays except for the V79MZh1B1-. * Significantly different (P < 0.05) from the mean of the corresponding hCYP1B1 or hCYP1A1 cell lines, respectively.
Discussion
We have utilized genetically modified cell lines that express single human cytochrome P450 isozymes alone or in combination with specific human GST isozymes, as model systems to examine the dynamic interaction between the phase I activation of carcinogens by hCYPs and the competing phase II detoxification by GSTs. The capacity of GST expression to serve as a chemopreventive mechanism can then be assessed by assays of relevant endpoints such as cytotoxicity, mutagenicity, formation of stable adducts, or other measures of cellular functional integrity. The V79MZ hamster lung fibroblast cell line is a useful platform for reconstitution of these metabolic pathways, as it lacks any endogenous or inducible CYP expression (Doehmer, 1993; Glatt, 1987). The background hamster GST activity is relatively low in this cell line and apparently also ineffective for the conjugation of PAH diol epoxides (Swedmark, 1992). The transgenic cell lines exhibited closely matched hCYP1B1 or hCYP1A1 activities, and heterologous expression of hGSTP1 increased the total cellular GST activity by up to 7-fold or 5-fold, respectively (Table 1).
The environmental PAH 5MC is one of the most potent rodent carcinogens among the PAHs with an alkyl side group (Amin, 1985; Hecht, 1985), and hence was chosen for this study as a representative of this group along with its intermediate metabolite, 5MC-1,2-diol (Amin, 1988; Hecht, 1978). While V79MZ cells are insensitive to mutagenicity of the parent compound 5MC, expression of either hCYP1B1 or hCYP1A1 resulted in potent activation to highly mutagenic 5MC metabolites (Fig. 1). The cytotoxicity of 5MC was increased only moderately, as indicated by the 2-fold lower IC50 in either cell line (Table 2). This is in contrast to 17- to 40-fold enhancement of cytotoxicity of B[a,l]P in cells expressing hCYP1B1 or hCYP1A1, respectively, in a previous study (Kushman, 2007a). Exposure of cells to the penultimate carcinogenic metabolite 5MC-1,2-diol also resulted in strong mutagenicity, at 10-fold lower concentration than 5MC, and the 10-fold lower IC50 also indicated an order of magnitude increase in cytotoxicity. Coexpression of hGSTP1 conferred strong (∼ 4-fold) protection against 5MC or 5MC-1,2-diol mutagenicity. However, protection by hGSTP1 against cytotoxicity was only 2-fold or less for either PAH (Tables 2 and 3). While this 2-fold protection fully reversed the moderate 2-fold enhancement of 5MC cytotoxicity by expression of hCYP1A1 or hCYP1B1, the 10-fold enhancement of 5MC-1,2-diol cytotoxicity by expression of hCYP1A1 or hCYP1B1 was also reversed by only 2-fold or less (Table 3). This pattern of stronger protection against mutagenicity would seem to augur well for a function of GST expression in chemoprevention of cancer due to PAH mutagenicity.
Previous studies in this lab with the cell lines expressing hCYP1A1 together with either hGSTP1 or hGSTM1 indicated that protection against cytotoxicity of B[a]P was more effective (8- to 16-fold) than protection against B[a]P mutagenicity (2- to 4-fold) (Kushman, 2007b; Kushman, 2006)(S. Kabler, et al., submitted). However, protection against the intermediate metabolite (+)-B[a]P-7,8-diol was similar (up to 5-fold) for both endpoints with either hGSTP1 or hGSTM1, and less effective (2- to 3-fold) against the more carcinogenic (−)-B[a]P-7,8-diol enantiomer (Kushman, 2007b). Protection by hGSTA1 against cytotoxicity of DB[a,l]P or the DB[a,l]P-11,12-diol metabolite was effective (5-fold and 9-fold, respectively) with activation by hCYP1B1 but not hCYP1A1. Protection against mutagenicity of DB[a,l]P or DB[a,l]P-11,12-diol by hGSTA1 was 2-fold or less with either activation pathway (Kushman, 2007a). Thus, the present study, in contrast to our earlier results with B[a]P or DB[a,l]P, shows greater protection against mutagenicity (∼ 4-fold) than against cytotoxicity (2-fold or less) of 5MC activated by either hCYP1B1 or hCYP1A1 (Figure 2), or against 5MC-1,2-diol activated by hCYP1B1 (Figure 4).
The variable degree of protection against cytotoxicity as compared to mutagenicity may have several potential implications. One possibility is that these endpoints are mediated by different metabolite(s) or metabolite stereoisomers of the PAHs examined, each of which may be detoxified to different extents by GSTs (Sundberg, 2002; Sundberg, 1998). Another implication is that cytotoxicity induced by PAH metabolite(s) that are substrates for detoxification by GSTs is not solely the result of the DNA damage that results in mutagenesis by the same metabolite(s). Otherwise the patterns of protection would be parallel for the two endpoints, even with activation by different CYP isozymes. Another possibility could be that the same reactive metabolites are intercepted by cytosolic GSTs with differential efficacy depending on the compartmental location of the most sensitive target(s) for induction of cytotoxicity. However, our initial investigations along these lines have indicated similar reductions of about 2-fold in [3H]-B[a]P-labeled protein adducts by hGSTP1 with activation by hCYP1A1 (S. Ahmad, unpublished results). Yet another consideration is the potential for amplification or attenuation effects for either endpoint. Thus DNA damage signaling may facilitate recruitment of DNA repair mechanisms while also initiating a process that will lead to cell death if repair is not effective in reversing the damage signal. On the other hand, the critical protein targets involved in cytotoxicity of PAHs may differ among various PAHs or activation pathways, and these damaged protein targets may be replaced at different rates.
In summary, we have shown that coexpression of hGSTP1 together with hCYP1B1 or hCYP1A1 results in strong protection against mutagenicity, or moderate protection against cytotoxicity of the potent environmental carcinogen 5MC or its intermediate metabolite 5MC-1,2-diol. Further studies will be required to distinguish among these possibilities and clarify the mechanisms that underlie the differential protection by GST expression against cytotoxicity, mutagenicity, and other endpoints. These differences in relative protection against cytotoxicity or mutagenicity in this may have important biological implications for the protective efficacy of GST expression against carcinogenicity of different PAHs.
Acknowledgments
This work was supported by a grant from the National Institute for Environmental Health Sciences, NIH, # RO1-ES-10175, and by training grant # T32-ES-07331 (S.A).
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin S, Huie K, Balanikas G, Hecht S. Synthesis and mutagenicity of 5-alkyl-substituted chrysene-1,2-diol-3,4- epoxides. Carcinog. 1988;9:2305–2308. doi: 10.1093/carcin/9.12.2305. [DOI] [PubMed] [Google Scholar]
- Amin S, Huie K, Melikian A, Leszczynska J, Hecht S. Comparative metabolic activation in mouse skin of the weak carcinogen 6-methylchrysene and the strong carcinogen 5-methylchrysene. Cancer Res. 1985;45:6406–6442. [PubMed] [Google Scholar]
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes memorial lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- Dipple A. DNA adducts of chemical carcinogens. Carcinog. 1995;16:437–441. doi: 10.1093/carcin/16.3.437. [DOI] [PubMed] [Google Scholar]
- Doehmer J. V79 chinese hamster cells genetically engineered for cytochrome p450 and their use in mutagenicity and metabolism studies. Toxicol. 1993;82:105–118. doi: 10.1016/0300-483x(93)90063-x. [DOI] [PubMed] [Google Scholar]
- Fields WR, Li Y, Townsend AJ. Protection by transfected glutathione S-transferase isozymes against carcinogen-induced alkylation of cellular macromolecules in human MCF-7 cells. Carcinog. 1994;15:1155–1160. doi: 10.1093/carcin/15.6.1155. [DOI] [PubMed] [Google Scholar]
- Fields WR, Morrow CS, Doehmer J, Townsend AJ. Expression of stably transfected murine glutathione S-transferase A3-3 protects against nucleic acid alkylation and cytotoxicity by aflatoxin B1 in hamster V79 cells expressing rat cytochrome p450-2b1. Carcinog. 1999;20:1121–1125. doi: 10.1093/carcin/20.6.1121. [DOI] [PubMed] [Google Scholar]
- Glatt H, Gemperlein I, Turchi G, Heinritz H, Doehmer J, Oesch F. Search for cell culture systems with diverse xenobiotic-metabolizing activities and their use in toxicological studies. Molec Toxicol. 1987;1:313–334. [PubMed] [Google Scholar]
- Grover PL, Sims P, Mitchley BC, Roe FJ. The carcinogenicity of polycyclic hydrocarbon epoxides in newborn mice. Brit J Cancer. 1975;31:182–188. doi: 10.1038/bjc.1975.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W, Pabst M, Jakoby W. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hall M, Forrester LM, Parker DK, Grover PL, Wolf CR. Relative contribution of various forms of cytochrome p450 to the metabolism of benzo[a]pyrene by human liver microsomes. Carcinog. 1989;10:1815–1821. doi: 10.1093/carcin/10.10.1815. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Ann Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Molec Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hecht S, LaVoie E, Mazzarese R, Amin S, Bedenko V, Hoffmann D. 1,2-dihydro-1,2-dihydroxy-5-methylchrysene, a major activated metabolite of the environmental carcinogen 5-methylchrysene. Cancer Res. 1978;38:2191–2194. [PubMed] [Google Scholar]
- Hecht S, Radok L, Amin S, Huie K, Melikian A, Hoffmann D, Pataki J, Harvey R. Tumorigenicity of 5-methylchrysene dihydrodiols and dihydrodiol epoxides in newborn mice and on mouse skin. Cancer Res. 1985;45:1449–1452. [PubMed] [Google Scholar]
- Hecht SS, Amin S, Huie K, Melikian AA, Harvey RG. Enhancing effect of a bay region methyl group on tumorigenicity in newborn mice and mouse skin of enantiomeric bay region diol epoxides formed stereoselectively from methylchrysenes in mouse epidermis. Cancer Res. 1987;47:5310–5315. [PubMed] [Google Scholar]
- Hu X, Pal A, Krzeminski J, Amin S, Awasthi YC, Zimniak P, Singh SV. Specificities of human glutathione s-transferase isozymes toward anti-diol epoxides of methylchrysenes. Carcinog. 1998;19:1685–1689. doi: 10.1093/carcin/19.9.1685. [DOI] [PubMed] [Google Scholar]
- Jerina DM, Sayer JM, Agarwal SK, Yagi H, Levin W, Wood AW, Conney AH, Pruess SD, Baird WM, Pigott MA, et al. Reactivity and tumorigenicity of bay-region diol epoxides derived from polycyclic aromatic hydrocarbons. Adv Exper Med Biol. 1986;197:11–30. doi: 10.1007/978-1-4684-5134-4_2. [DOI] [PubMed] [Google Scholar]
- Kantor R, Giardina SL, Bartolazzi A, Townsend AJ, Myers CE, Cowan KH, Longo DL, Natali PG. Monoclonal antibodies to glutathione s-transferase pi-immunohistochemical analysis of human tissues and cancers. Int J Cancer. 1991;47:193–201. doi: 10.1002/ijc.2910470206. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR. Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinog. 1998;19:1847–1853. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- Kushman ME, Kabler SL, Ahmad S, Doehmer J, Morrow CS, Townsend AJ. Cytotoxicity and mutagenicity of dibenzo[a,l]pyrene and (+/-)-dibenzo[a,l]pyrene-11,12-dihydrodiol in V79MZ cells co-expressing either hCYP1A1 or hCYP1B1 together with human glutathione-s-transferase a1. Mutat Res/Fund Molec Mech Mutag. 2007a;624:80–87. doi: 10.1016/j.mrfmmm.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushman ME, Kabler SL, Ahmad S, Doehmer J, Morrow CS, Townsend AJ. Protective efficacy of hGSTM1-1 against B[a]P and (+)- or ( )-B[a]P-7,8-dihydrodiol cytotoxicity, mutagenicity, and macromolecular adducts in v79 cells coexpressing hCYP1A1. Toxicol Sci. 2007b;99:51–57. doi: 10.1093/toxsci/kfm133. [DOI] [PubMed] [Google Scholar]
- Kushman ME, Kabler SL, Fleming MH, Ravoori S, Gupta RC, Doehmer J, Morrow CS, Townsend AJ. Expression of human glutathione S-transferase P1 confers resistance to benzo[a]pyrene or benzo[a]pyrene-7,8-dihydrodiol mutagenesis, macromolecular alkylation and formation of stable N2-Gua-BPDE adducts in stably transfected V79MZ cells co-expressing hCYP1A1. Carcinog. 2006;28:207–214. doi: 10.1093/carcin/bgl125. [DOI] [PubMed] [Google Scholar]
- Luch A, Coffing SL, Tang YM, Schneider A, Soballa V, Greim H, Jefcoate CR, Seidel A, Greenlee WF, Baird WM, et al. Stable expression of human cytochrome P450 1B1 in V79 chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem Res Toxicol. 1998;11:686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The cap ‘n’ collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-Related Factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Melikian A, LaVoie E, Hecht S, Hoffmann D. 5-methylchrysene metabolism in mouse epidermis in vivo, diol epoxide--DNA adduct persistence, and diol epoxide reactivity with DNA as potential factors influencing the predominance of 5-methylchrysene-1,2-diol-3,4-epoxide--DNA adducts in mouse epidermis. Carcinog. 1983;4:843–849. doi: 10.1093/carcin/4.7.843. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Glutathione s-transferases, structure, regulation, and therapeutic implications. J Biol Chem. 1993;268:11475–11478. [PubMed] [Google Scholar]
- Schmalix WA, Maser H, Kiefer F, Reen R, Wiebel FJ, Gonzalez F, Seidel A, Glatt H, Greim H, Doehmer J. Stable expression of human cytochrome p450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. Eur J Pharmacol. 1993;248:251–261. doi: 10.1016/0926-6917(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes p450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Sundberg K, Dreij K, Seidel A, Jernstrom B. Glutathione conjugation and DNA adduct formation of dibenzo[a,l]pyrene and benzo[a]pyrene diol epoxides in v79 cells stably expressing different human glutathione transferases. Chem Res Toxicol. 2002;15:170–179. doi: 10.1021/tx015546t. [DOI] [PubMed] [Google Scholar]
- Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinog. 1998;19:433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- Swedmark S, Romert L, Morgenstern R, Jenssen D. Studies on glutathione transferases belonging to class pi in cell lines with different capacities for conjugating (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Carcinog. 1992;13:1719–1723. doi: 10.1093/carcin/13.10.1719. [DOI] [PubMed] [Google Scholar]
- Townsend AJ, Fields WR, Haynes RL, Karper AJ, Li Y, Doehmer J, Morrow CS. Chemoprotective functions of glutathione s-transferases in cell lines induced to express specific isozymes by stable transfection. Chem Biol Interact. 1998a;111-112:389–407. doi: 10.1016/s0009-2797(97)00175-0. [DOI] [PubMed] [Google Scholar]
- Townsend AJ, Fields WR, Doss AJ, Clapper ML, Doehmer J, Morrow CS. Modeling the chemoprotective functions of glutathione s-transferases in cultured cell lines by heterologous expression. Drug Metab Rev. 1998b;31:43–69. doi: 10.1081/dmr-100101907. [DOI] [PubMed] [Google Scholar]
- Townsend AJ, Goldsmith ME, Pickett CB, Cowan KH. Isolation, characterization, and expression in escherichia coli of two murine mu class glutathione s-transferase cDNAs homologous to the rat subunits 3 (yb1) and 4 (yb2) J Biol Chem. 1989;264:21582–21590. [PubMed] [Google Scholar]
- Townsend AJ, Kabler SL, Doehmer J, Morrow CS. Modeling the metabolic competency of glutathione s-transferases using genetically modified cell lines. Toxicol. 2002;181-182:265–269. doi: 10.1016/s0300-483x(02)00294-9. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW. Chemoprevention of cancer. Cancer Res. 1985;45:1–8. review. [PubMed] [Google Scholar]