Abstract
The measurement of antibody to Haemophilus influenzae type b capsular polysaccharide is important in the study of natural immunity and in the immunogenicity evaluation of H. influenzae type b vaccines. Several radioantigen binding assays (RABA) have been developed to measure H. influenzae type b anticapsular antibody, but recent immunogenicity data obtained with structurally similar vaccines suggest major differences in antibody quantitation in different laboratories. To evaluate interlaboratory variability in the measurement of anticapsular antibody levels, we blindly evaluated a sample of 40 pre- and postimmunization sera by eight RABAs in different laboratories. Evaluation of RABA methods revealed differences in polysaccharide antigens, radiolabeling methods, concentration and volume of antigen and antibody, and other assay methods. The reported results of assays varied significantly between laboratories (up to sixfold differences in geometric means), in part because of differences in assay sensitivity and different proportions of samples having undetectable levels of antibody (0 to 65% of specimens with undetectable levels). After standardizing the limit of sensitivity for all assays (0.125 microgram/ml), the results of all combinations of paired analyses of RABA assays correlated well (r = 0.88 to 0.99) but the geometric mean levels still varied as much as twofold. For individual sera, the differences between paired assays often were substantial (P less than or equal to 0.0001, paired t test), with some results varying as much as 64-fold. Differences were greatest for lower levels of antibody. There was good comparability and interlaboratory reproducibility of some assays but not of others. Intrinsic or extrinsic labeling of the antigen was not a major determinant of comparability. In most instances, the current variation in the quantitation of antibody levels by these assays precludes interassay comparisons. A standardized measurement of antibody needs to be developed to adequately compare results between different H. influenzae type b immunogenicity studies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosino D. M., Glode M. P., Williams C. L., Siber G. R. Antibody response of infants to Haemophilus influenzae type b capsular polysaccharide combined with Bordetella pertussis. J Infect Dis. 1985 Jun;151(6):1174–1174. doi: 10.1093/infdis/151.6.1174. [DOI] [PubMed] [Google Scholar]
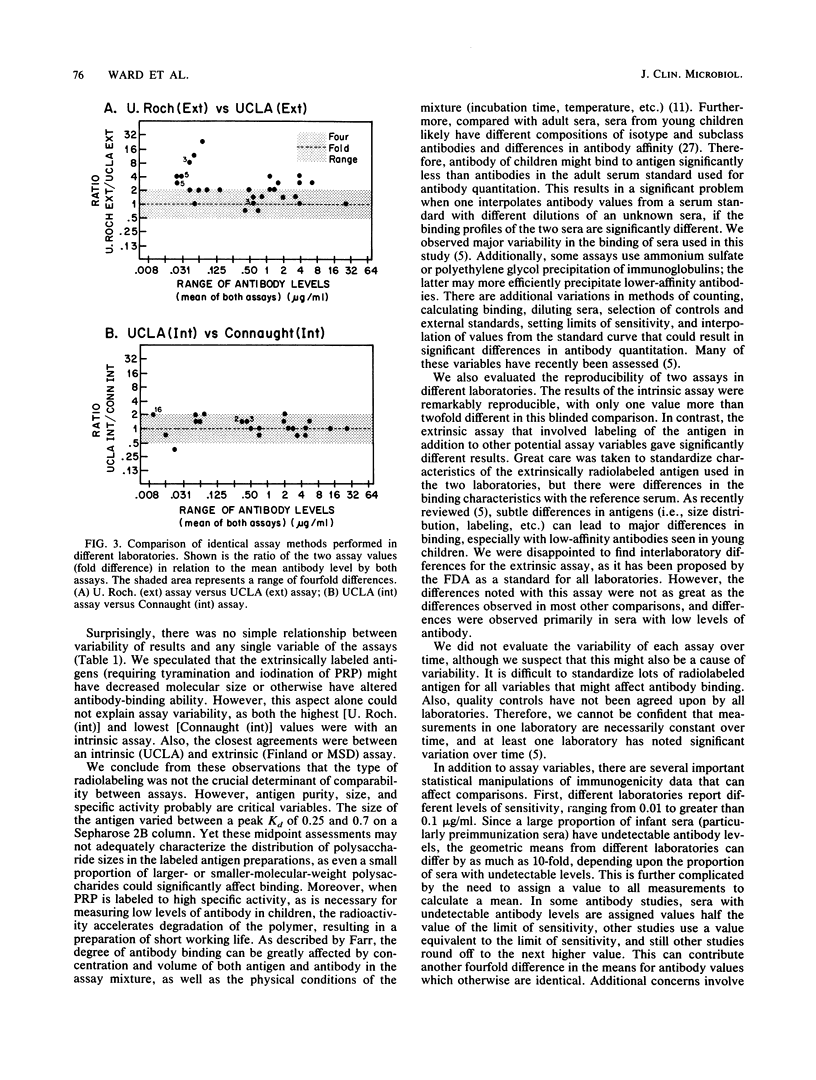
- Anderson P., Insel R. A., Porcelli S., Ward J. I. Immunochemical variables affecting radioantigen-binding assays of antibody to Haemophilus influenzae type b capsular polysaccharide in childrens' sera. J Infect Dis. 1987 Oct;156(4):582–590. doi: 10.1093/infdis/156.4.583. [DOI] [PubMed] [Google Scholar]
- Anderson P. Intrinsic tritium labeling of the capsular polysaccharide antigen of Haemophilus influenzae type B. J Immunol. 1978 Mar;120(3):866–870. [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1984 Jun;149(6):1034–1035. doi: 10.1093/infdis/149.6.1034. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Edwards K. M., Decker M. D., Palmer P., Porch C. R., Sell S. H., Wright P. F. Lack of comparability between commonly used serological assays of immune response to Haemophilus influenzae vaccine. J Infect Dis. 1987 Feb;155(2):283–291. doi: 10.1093/infdis/155.2.283. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Boies E. G., Munson R. S., Jr Immunogenicity of Haemophilus influenzae type b polysaccharide--diphtheria toxoid conjugate vaccine in adults. J Pediatr. 1984 Jul;105(1):22–27. doi: 10.1016/s0022-3476(84)80350-9. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Cates K. L. Haemophilus influenzae type b polysaccharide vaccines. J Pediatr. 1985 Sep;107(3):330–336. doi: 10.1016/s0022-3476(85)80502-3. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Pandey J. P., Boies E. G. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986 Aug;154(2):257–264. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- Greenberg D. P., Ward J. I., Burkart K., Christenson P. D., Guravitz L., Marcy S. M. Factors influencing immunogenicity and safety of two Haemophilus influenzae type b polysaccharide vaccines in children 18 and 24 months of age. Pediatr Infect Dis J. 1987 Jul;6(7):660–665. doi: 10.1097/00006454-198707000-00008. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Anderson P., Rosen F. S., Smith D. H. Characterization of human antibody to polyribophosphate, the capsular antigen of Hemophilus influenzae, type B. Clin Immunol Immunopathol. 1973 Jan;1(2):234–240. doi: 10.1016/0090-1229(73)90024-x. [DOI] [PubMed] [Google Scholar]
- King S. D., Ramlal A., Wynter H., Moodie K., Castle D., Kuo J. S., Barnes L., Williams C. L. Safety and immunogenicity of a new Haemophilus influenzae type b vaccine in infants under one year of age. Lancet. 1981 Oct 3;2(8249):705–709. doi: 10.1016/s0140-6736(81)91045-x. [DOI] [PubMed] [Google Scholar]
- Kuo J. S., Monji N., Schwalbe R. S., McCoy D. W. A radioactive antigen-binding assay for the measurement of antibody to Haemophilus influenzae type b capsular polysaccharide. J Immunol Methods. 1981;43(1):35–47. doi: 10.1016/0022-1759(81)90034-x. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Mäkelä P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984 Nov;74(5):857–865. [PubMed] [Google Scholar]
- Käyhty H., Peltola H., Karanko V., Mäkelä P. H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983 Jun;147(6):1100–1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Peter G., Glode M. P., Daum R. S., Calnen G., Knight K. M., Mayer D., Kuo J. S., Lui N. S. Response of infants to Haemophilus influenzae type b polysaccharide and diphtheria-tetanus-pertussis vaccines in combination. J Infect Dis. 1984 Jun;149(6):950–955. doi: 10.1093/infdis/149.6.950. [DOI] [PubMed] [Google Scholar]
- Lepow M. L., Samuelson J. S., Gordon L. K. Safety and immunogenicity of Haemophilus influenzae type b-polysaccharide diphtheria toxoid conjugate vaccine in infants 9 to 15 months of age. J Pediatr. 1985 Feb;106(2):185–189. doi: 10.1016/s0022-3476(85)80284-5. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Schreiber J. R., Barrus V., Cates K. L., Siber G. R. Functional characterization of human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986 Jan;153(1):8–16. doi: 10.1093/infdis/153.1.8. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]


