Abstract
Background
Epidemiologic studies have demonstrated an association between acute exposure to ambient fine particles and both mortality and morbidity. Less is known about the relative impacts of the specific chemical constituents of particulate matter < 2.5 μm in aerodynamic diameter (PM2.5) on hospital admissions.
Objective
This study was designed to estimate the risks of exposure to PM2.5 and several species on hospital admissions for respiratory diseases among children.
Data and Methods
We obtained data on daily counts of hospitalizations for children < 19 and < 5 years of age for total respiratory diseases and several subcategories including pneumonia, acute bronchitis, and asthma for six California counties from 2000 through 2003, as well as ambient concentrations of PM2.5 and its constituents, including elemental carbon (EC), organic carbon (OC), and nitrates (NO3). We used Poisson regression to estimate risks while controlling for important covariates.
Results
We observed associations between several components of PM2.5 and hospitalization for all of the respiratory outcomes examined. For example, for total respiratory admissions for children < 19 years of age, the interquartile range for a 3-day lag of PM2.5, EC, OC, NO3, and sulfates was associated with an excess risk of 4.1% [95% confidence interval (CI), 1.8–6.4], 5.4% (95% CI, 0.8–10.3), 3.4% (95% CI, 1.1–5.7), 3.3% (95% CI, 1.1–5.5), and 3.0% (95% CI, 0.4–5.7), respectively. We also observed associations for several metals. Additional associations with several of the species, including potassium, were observed in the cool season.
Conclusion
Components of PM2.5 were associated with hospitalization for several childhood respiratory diseases including pneumonia, bronchitis, and asthma. Because exposure to components (e.g., EC, OC, NO3, and K) and their related sources, including diesel and gasoline exhaust, wood smoke, and other combustion sources, are ubiquitous in the urban environment, it likely represents an identifiable and preventable risk factor for hospitalization for children.
Keywords: children, EC, hospital admissions, OC, PM2.5, respiratory, species
Fine particles (particles < 2.5 μm in aerodynamic diameter; PM2.5) have been linked to a variety of poor health outcomes, including mortality and cardiorespiratory morbidity (Dominici et al. 2006; Ostro et al. 2006; Schwartz et al. 1996). However, PM2.5 encompasses a chemically heterogeneous mix of solid and liquid particles emitted from a variety of sources, and it is likely that the different chemical species within PM2.5 will have varying effects on specific health end points. Several epidemiologic studies have attempted to address this question, looking at the effects of specific fine particle species on mortality (Burnett et al. 2000; Franklin et al. 2008; Laden et al. 2000; Ostro et al. 2007). However, studies such as those by Burnett et al. (1995) and Peel et al. (2005) examining associations between specific PM2.5 constituents and morbidity outcomes such as hospital admissions, particularly among children, have been less common. With regulatory agencies actively targeting PM constituents for pollution control, a greater understanding of the impacts of current and future control efforts is needed.
Children are a demographic of special concern, as they represent a population at increased risk for air pollution–related respiratory conditions because of their biologic characteristics (e.g., immature lungs and immune system, higher breathing rates) and behavior (e.g., more time spent outdoors) (Miller et al. 2002; Schwartz 2004). Particle deposition, specifically, is higher in both young children and asthmatics (Chalupa et al. 2000; Ginsberg et al. 2005), and several studies have observed associations between PM2.5 and bronchitis and asthma exacerbation in children (Barnett et al. 2005; McConnell et al. 2003). Thus, when designing and instituting pollution controls, the impacts on children from exposure to components of PM2.5 need to be documented.
Our study used speciated PM2.5 monitoring data to examine the association between PM2.5 and its constituents with respiratory hospitalizations among children 0–18 years of age in six California counties. Our goal is to provide further insight into the relative toxicity of fine particle constituents, which would generate multiple benefits. For example, such an analysis would identify specific agents of greater concern within particulate matter (PM), which could be more thoroughly investigated on a mechanistic level in toxicology studies. Second, it would help identify which communities may be at greater risk because of their specific constituent mix of PM2.5. Finally, it could implicate certain fine particle sources as posing a greater health threat based on the chemical profile of the fine particles they emit. In fact, certain constituents can be used as indicators of PM2.5 specific to sources such as gasoline and diesel exhaust, crustal material, and fuel combustion from stationary sources such as power plants, wood smoke, and other biomass burning.
Data and Methods
Hospitalization data
We obtained data for all hospitalizations in California from the Office of Statewide Health Planning and Development, Healthcare Quality and Analysis Division (Sacramento, CA), for the period from 1 January 2000 through 31 December 2003. Based on the International Classification of Diseases, 9th Revision (ICD-9) (World Health Organization 1975), hospital admissions for children < 19 years of age were classified into one or more categories: all respiratory disease (ICD-9 codes 460–519), asthma (ICD-9 code 493), acute bronchitis (ICD-9 code 466), and pneumonia (ICD-9 codes 480–486). Hospital admission counts were then aggregated based on the child’s reported county of residence to yield a daily time-series of relevant hospitalizations for each county. For total respiratory admissions, we further stratified by ages < 5 years and 5–18 years of age. Finally, we examined the same outcomes for the cool season, defined here as October through March.
Pollutant and meteorologic data
Available PM2.5 speciation data were obtained for 2000–2003 from the California Air Resources Board (Sacramento, CA). The speciation monitors were filter-based Met One Speciation Air Sampling Systems (SASS) (Met One Instruments Inc., Grants Pass, OR) belonging to the Speciation Trends Network (STN). Counties were included if there were ≥ 180 days of observations with PM2.5 species data during the study period, which limited the study to six California counties with a sufficient duration of speciated PM2.5 monitoring. These counties encompass approximately 8.7 million people, or roughly 25% of the state’s population. In Kern, Riverside, and Santa Clara Counties, speciation data from two co-located monitors were available. Fresno, Sacramento, and San Diego Counties each had one monitor with relevant data. Speciation data were generally recorded every 3 days. The number of available days of data over the 4-year period ranged from 227 (San Diego) to 381 (Sacramento), because of varying monitor start dates. The components of PM2.5 were measured as 24-hr averages and included elemental carbon (EC), organic carbon (OC), nitrates (NO3), sulfates (SO4), copper (Cu), iron (Fe), potassium (K), silicon (Si), and zinc (Zn). These species were chosen because they a) include the larger components of PM2.5 in our study sample; b) had a signal-to-noise ratio of ≥ 2, and c) had a majority of their values greater than the detection limit for each county monitor used. Signal-to-noise ratio for a species was defined as
 |
To adjust for the potential confounding between temperature and hospital admissions, average daily temperature and relative humidity data were collected at meteorologic stations at each of the counties using data provided by the California Air Resources Board (unpublished data) or the California Irrigation Management Information System (wwwcimis.water.ca.gov/cimis/data.jsp). The daily hospital admission counts and pollution and meteorologic data were integrated using SAS (version 9.1; SAS Institute Inc., Cary, NC).
Methods
The daily hospital admission counts are non-negative discrete integers that correspond to rare events. Therefore, our analysis used Poisson regression conditional on the explanatory variables: time, day of the week, temperature, relative humidity, and pollutant. Similar model specifications were used for each county and included natural spline smoothers for both a daily time trend and meteorology. The natural spline fits multiple parametric cubic functions evenly placed over the range of the variable, such that controlling the total number of fitting functions, or degrees of freedom (df), controls the smoothness of the resulting spline. The smoothing of the relationship between time and hospital admissions helps to control for the effects of seasonality and other factors that are time dependent. Issues and sensitivity of model and knot selection have been discussed previously (Ostro et al. 2006).
The explanatory variables were day of the week, smoothed splines of daily unlagged average temperature and humidity (each with 3 df), and a smoothing spline of time with 4 df per calendar year. The lags and degrees of freedom for meteorologic variables were chosen based on their effectiveness in controlling for weather variables and promoting best model fits in both this analysis and previous studies (Basu et al. 2008; Ostro et al. 2006). Similarly, 4 df were chosen for the time spline a priori because this number controls well for seasonal and secular patterns (Ostro et al. 2006). For each county, each species was then examined separately in a model using single-day lags of 0–3. Analyses looking at cool season (October–March) effects followed the same structure but included a binary indicator for season (warm vs. cool) and an interaction variable for season and pollutant. County-specific results were later combined in a meta-analysis, using a random-effects model to help account for heterogeneity between county estimates (DerSimonian and Laird 1986).
In contrast to time-series studies with consecutive daily data, different lags in our analyses will relate to different days in the time series of hospital admissions. For example, an unlagged analysis compares pollutant readings with admission counts occurring on the pollutant sampling days. An analysis using 1-day lags would compare those same pollutant readings to admissions occurring 1 day after the sampling day. So although our pollutant data are not available on a daily basis, the daily outcome data afford us the ability to examine different single-day lags.
We calculated the results using R (version 2.6.2; R Development Core Team 2006) for the single-county and meta-analyses. To compare relative impacts of the observed concentrations of the species components of PM2.5, we present the results as the percent excess risk (ER) in daily hospital admissions for the interquartile range (IQR = 75th–25th percentile) of daily exposure for each species, where ER per IQR = [(ebeta × IQR) – 1] × 100 and beta is the combined regression coefficient from the meta-analysis.
Results
Table 1 provides descriptive statistics about the county characteristics, hospitalizations, PM2.5 and its components, and meteorologic data. As displayed in Table 1, the average number of daily respiratory hospital admissions for children < 19 years of age ranged from 3.9 in Kern County to 10.2 in Riverside County, with children < 5 years of age generating about 75% of the admissions. Mean daily PM2.5 concentrations during the study period averaged 19 μg/m3 with a range of 15 μg/m3 in Sacramento to 29 μg/m3 in Riverside County, which exceed the U.S. Environmental Protection Agency annual average PM2.5 standard of 15 μg/m3 in five of the six counties, and the California annual average standard of 12 μg/m3 in all six counties.
Table 1.
Mean county daily hospital admissions per day by morbidity category and air quality and meteorologic data, 2000–2003.
| Fresno | Kern | Riverside | Sacramento | San Diego | Santa Clara | |
|---|---|---|---|---|---|---|
| Hospitalizations admission category (age) | ||||||
| Respiratory (< 19 years) | 6.1 | 3.9 | 10.2 | 4.8 | 9.8 | 6.1 |
| Respiratory (< 5 years) | 4.7 | 3.0 | 7.7 | 3.5 | 7.1 | 4.8 |
| Respiratory (5–18 years) | 1.4 | 0.9 | 2.5 | 1.3 | 2.7 | 1.3 |
| Asthma (< 19 years) | 1.7 | 0.8 | 2.4 | 1.3 | 2.8 | 1.4 |
| Pneumonia (< 19 years) | 1.5 | 1.7 | 3.6 | 1.4 | 2.7 | 1.7 |
| Acute bronchitis (< 19 years) | 1.9 | 0.8 | 2.6 | 1.2 | 2.2 | 1.7 |
| County characteristics | ||||||
| Population (1,000s) | 799 | 662 | 1,545 | 1,223 | 2,814 | 1,683 |
| Mean temperature (°F) | 64.7 | 65.5 | 65.4 | 61.0 | 61.4 | 57.5 |
| Mean relative humidity (%) | 56.6 | 57.7 | 58.7 | 68.7 | 74.2 | 68.0 |
| Mean PM2.5 | 22.4 | 21.4 | 28.8 | 14.8 | 15.6 | 15.0 |
| No. of days in analysis | 373 | 283 | 279 | 381 | 227 | 324 |
Table 2 shows the number of useable observations, mean, and IQR of each component of PM2.5. In this data set, PM2.5 is dominated by EC, OC, NO3, and SO4 which are 5%, 36%, 28%, and 10% of the total, respectively. We generally found moderate correlations (r < 0.5) among the species, whereas we observed high correlations between PM2.5 and EC, OC, and NO3 (0.7, 0.8, and 0.9, respectively).
Table 2.
Descriptive statistics for air pollutant species in California counties, 2000–2003.
| No.a | Full-year mean (μg/m3 ) | Full-year IQR (μg/m3 ) | Cool season mean (μg/m3 ) | Cool season IQR (μg/m3 ) | |
|---|---|---|---|---|---|
| PM2.5 | 1,867 | 19.4 | 14.6 | 24.8 | 21.5 |
| EC | 1,867 | 1.0 | 0.8 | 1.4 | 1.2 |
| OC | 1,867 | 7.1 | 4.5 | 9.3 | 6.3 |
| NO3 | 1,805 | 5.4 | 5.7 | 7.3 | 8.1 |
| SO4 | 1,805 | 2.0 | 1.5 | 1.5 | 1.2 |
| Cu | 1,869 | 0.0077 | 0.0074 | 0.0080 | 0.0081 |
| Fe | 1,869 | 0.13 | 0.10 | 0.14 | 0.12 |
| K | 1,869 | 0.12 | 0.08 | 0.14 | 0.11 |
| Si | 1,869 | 0.18 | 0.15 | 0.15 | 0.13 |
| Zn | 1,869 | 0.012 | 0.010 | 0.018 | 0.014 |
Total number of days across the six counties.
Table 3 summarizes the empirical relationships between the health end points and the various lags of PM2.5 and its species. Many species were associated with total hospitalizations for respiratory disease, particularly for those < 5 years of age. We observed associations with multiple lags of PM2.5, OC, NO3, and Fe, and significant associations for EC, SO4, Cu, and Si. Single-day lags of 1–3 days were usually more important than unlagged values. The disease-specific results suggest that PM2.5 and its components were most associated with acute bronchitits and pneumonia, but we observed associations for asthma admissions as well. Analyses of the cool season indicated similar associations between the species and the various health end points. In addition, additional associations were observed between K and admissions for all respiratory disease as well as with pneumonia and bronchitis.
Table 3.
Single-day lags asssociated with respiratory admissions by cause and age.
| Respiratory (< 19 years) | Respiratory (< 5 years) | Respiratory (5–18 years) | Asthma (< 19 years) | Pneumonia (< 19 years) | Acute bronchitis (< 19 years) | |
|---|---|---|---|---|---|---|
| Full year | ||||||
| PM2.5 | 1**, 3** | 1*, 2*, 3** | 1**, 3* | 1* | 1**, 3** | |
| EC | 3** | 3** | 3* | |||
| OC | 1**, 3** | 1*, 3** | 1**, 3* | 1**, 3** | ||
| NO3 | 1**, 3** | 3** | 1*, 3* | 1* | 3* | |
| SO4 | 3** | 1*, 3** | 3** | |||
| Cu | 2** | 2** | 2* | 2** | ||
| Fe | 1**, 3** | 1*, 3** | 0*, 1**, 3* | 3** | ||
| K | 2* | |||||
| Si | 3** | 3** | 1* | 3* | 2** | |
| Zn | 3* | 0** | ||||
| Winter | ||||||
| PM2.5 | 1**, 3** | 1**, 2*, 3** | 1* | 1**, 3* | ||
| EC | 3* | 3* | 3* | |||
| OC | 1*, 3** | 3** | 1** | 1**, 3** | ||
| NO3 | 1**, 3** | 3** | 1* | 1**, 3** | ||
| SO4 | 1* | 0* | ||||
| Cu | 1**, 2** | 1**, 2** | 1** | 2** | 1* | |
| Fe | 1*, 3** | 1*, 3** | 1* | |||
| K | 1**, 2*, 3** | 1**, 2*, 3** | 1**, 3* | 1** | ||
| Si | 2* | 1*, 2** | 1* | 2** | ||
| Zn | 0** | |||||
Single-day lag significant at p < 0.10.
Single-day lag significant at p < 0.05.
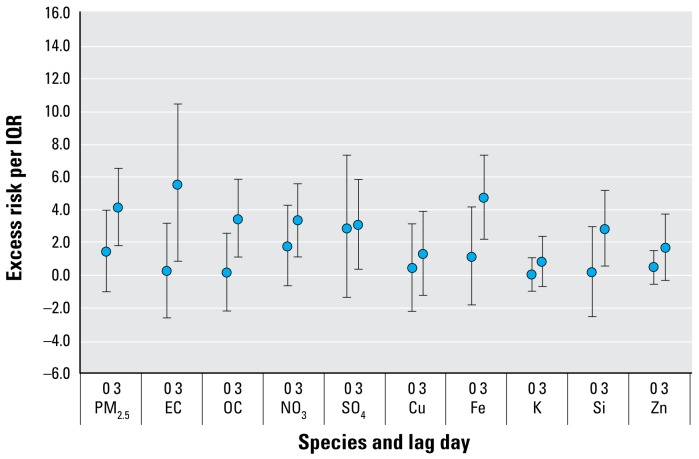
Figure 1 summarizes the excess risks for all-year respiratory hospital admissions among children < 19 years of age. For ease in interpretation, only single-day lags of 0 and 3 are presented in the figures. With collection of species data every third day, these lags correspond to the same count day of hospital admissions. However, additional details on other lags are provided in the Supplemental Material (online at http://www.ehponline.org/members/2008/11848/suppl.pdf). For a 3-day lag, excess risks of 3–6% for the IQR were observed for many of the PM2.5 species, with the highest risks observed for PM2.5, EC, and Fe with respective excess risks of 4.1% [95% confidence interval (CI), 1.8–6.4], 5.4% (95% CI, 0.8–10.3), and 4.7% (95% CI, 2.2–7.2), respectively. Excess risks of approximately 3% were observed for OC, NO3, SO4, and Si.
Figure 1.
Association of PM2.5 species with total respiratory admissions for those < 19 years of age (percent excess risk per IQR and 95% CIs).
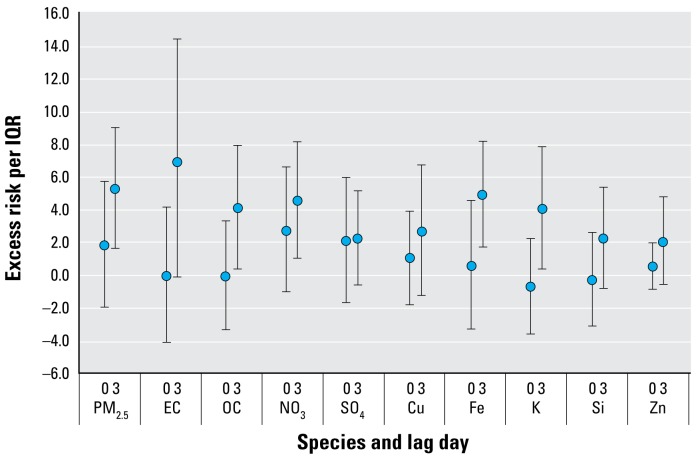
Figure 2 summarizes the excess risks for respiratory hospital admissions during the cool season for those < 19 years of age. Again, higher effect estimates per species IQR were observed for PM2.5, EC, and Fe compared with other species, with the excess risks using the same 3-day lag in the cool season being 5.1% (95% CI, 1.6 to 8.9), 6.8% (95% CI, –0.2 to 14.2), and 4.8% (95% CI, 1.7 to 8.0), respectively. Except for SO4, other species associated with juvenile respiratory admissions in full-year analyses remained significant in cool season analyses, with excess risks per IQR generally falling around 4%. Similarly, K, a species not associated with admissions in full-year analyses, had a significant excess risk of 4.0% (95% CI, 0.3–7.7) during the cool season.
Figure 2.
Association of PM2.5 species with total respiratory admissions during the cool season for those < 19 years of age (percent excess risk per IQR and 95% CIs).
Discussion
Our analysis of hospital admissions for children indicates that PM2.5 and several of its components have important effects on hospital admissions for respiratory diseases, especially acute bronchitis and pneumonia. Acute exposure to total PM2.5 was associated with total respiratory hospital admissions for those < 19 years and < 5 years of age. Children < 5 years of age were particularly sensitive to PM2.5 and several of its components including EC, OC, NO3, SO4, Fe, and Si. We observed excess risks of 3–7% for many of the respiratory outcomes, based on species IQR. We observed similar risks during the cool season, where associations with K were also demonstrated.
The associations demonstrated for total PM2.5 levels corroborate previous studies that observed effects of fine PM on respiratory hospitalizations in children (Barnett et al. 2005; Lin et al. 2002; van der Zee et al. 1999). Earlier studies also found associations between PM < 10 μm in aerodynamic diameter (PM10) and total respiratory, asthma, and pneumonia hospitalizations (Pope 1989; Tolbert et al. 2000). Despite the direct causes of pneumonia and other respiratory infections being biologic in nature, the effect of air pollution on the development of severe cases requiring hospitalization is especially plausible in children, because particulates likely hamper the ability of an already immature immune system to clear bacteria and other pathogens from the lung (Dietert et al. 2000). Other studies have also indicated that short-term incidences of respiratory disease may not be the only impact on children’s health. Lung development itself may be compromised, as PM2.5 and, more specifically, EC were among pollutants strongly associated with decreased lung function attainment in school children (Gauderman et al. 2004).
These studies of children also complement previous studies on elderly populations reporting associations between PM2.5 and PM10 levels and hospitalizations for respiratory diseases, including respiratory tract infections, chronic obstructive pulmonary disease, and pneumonia (Dominici et al. 2006; Zanobetti et al. 2000).
Because data collection on the chemical components of PM2.5 has only recently begun, however, fewer studies are available on the effects related to specific species. Most of these studies link specific components of PM2.5 to mortality. For example, in analyses of six California counties and of Phoenix, Arizona, acute exposure to several of these components, including EC, OC, Fe, Zn, and K, was associated with increases in daily counts of mortality (Mar et al. 2004; Ostro et al. 2007). In contrast, previous epidemiologic studies looking at fine particle constituents and morbidity have been sparse. Associations between OC and emergency department visits for pneumonia have been reported (Peel et al. 2005), as well as asthma attacks in children and exhaled nitric oxide, a biomarker for airway inflammation (Delfino et al. 2003, 2006; Peel et al. 2005; Sinclair and Tolsma 2004). A recent study of pre-school-age children in the Czech Republic reported associations between bronchitis and both PM2.5 and polycyclic aromatic hydrocarbons (PAHs) (Hertz-Picciotto et al. 2007).
To date, studies focusing on NO3 and SO4 have been mixed in their findings (Schlesinger et al. 2006). We observed associations between daily exposure to NO3 and respiratory disease for both those < 19 years and those < 5 years of age and more modest associations between SO4 and acute bronchitis. NO3 constitute a significantly larger, and SO4 a smaller, share of PM2.5 in California than observed in most of the rest of the United States (Bell et al. 2007). However, because of lack of available data, NO3 have not been extensively examined in epidemiologic studies of air pollution. In one of the few previous studies examining the association between acute exposure to SO4 and respiratory morbidity in children, an association was observed for children with chronic respiratory symptoms between SO4 and both lower respiratory tract symptoms and decrements in lung function (van der Zee et al. 1999). In addition, Burnett et al. (1995) reported associations between SO4 and respiratory hospital admissions for children < 15 years of age among hospitals in the province of Ontario, Canada, and Sarnat et al. (2008) found associations between emergency department visits for respiratory disease with SO4, but not with any other species, in an Atlanta-based study. Although motor vehicle fuel combustion sources of NO3 are, of course, ubiquitous throughout the state, there are only a few major sources of SO4 in California, because most state utilities do not use either coal or oil. These sources are found primarily in the Los Angeles Air Basin and are associated with the local port facilities. Major SO4 sources include petroleum refining (both fuel combustion and industrial processes), heavy duty diesel trucks, and fuel combustion of ships and commercial boats (California Air Resources Board 2007).
We also found associations between hospital admissions and several metals including Cu, Fe, and, to a lesser extent, Zn. Existing studies indicate that Fe is a marker for brake wear and is found in road dust and soil, and Cu and Zn may be generated from vehicular-related sources including brake wear, emissions from lubrication oil, and tire wear (Schauer et al. 2006). Several human and animal studies have reported associations of ferric iron and other metallic compounds with the generation of oxidative stress and pulmonary inflammation (Ghio 2004; Pritchard et al. 1996), and another study in laboratory rats demonstrated that pulmonary Fe exposure impaired both immune function and the ability to clear bacteria (Zelikoff et al. 2002). Burnett et al. (2000) reported associations between mortality and daily exposures to several metals, including Zn, nickel, and Fe in their study of eight Canadian cities. In addition, Hirshon et al. (2008) found associations between ambient Zn and asthma urgent care visits and hospitalization among a cohort of individuals < 18 years of age. Intratracheal instillation studies in rodents demonstrated that pulmonary neutrophilic inflammation and protein leakage could be partially reproduced by zinc sulfate and copper sulfate, both major transition metals in the PM samples (Gottipolu et al. 2008; Kodavanti et al. 2002). Cu itself induced pulmonary inflammation, and in combination with Zn produced higher numbers of total lavageable cells and neutrophils than either treatment alone (Gottipolu et al. 2008). Our findings for Si are supported by evidence that crystalline silica is a well-known occupational fibrosis hazard and can produce oxidative stress, lung inflammation, and cell death at relatively low exposures (Kaewamatawong et al. 2006).
Although the above evidence presents the case that specific constituents cause direct effects, our study examines levels of PM2.5 constituents under real-world conditions, and our observed effects for one constituent may be attributable to its correlation with other constituents from a similar generating source. Source apportionment was not performed in this current analysis; however, a study of source apportionment methods found that correctly selected single-species tracers can be almost as informative in identifying source contributions as more rigorous methods (Sarnat et al. 2008).
EC, the tracer for diesel particulates used by Sarnat et al. (2008), is likely a reasonable marker for diesel exhaust in this study as well. Because there are few other sources of primary emissions of carbonaceous material such as coal and fuel oil combustion in California, approximately 80% or more of the EC is derived from diesel exhaust (Schauer and Cass 2000). Existing toxicologic and human clinical studies (Salvi et al. 1999) indicate that diesel exhaust particles induce both pulmonary inflammation and cell damage, including death of alveolar macrophages, through the generation of reactive oxygen species (Hiura et al. 2000; Mar et al. 2000; Nel et al. 2001). Alveolar macrophage death may lessen defense against pulmonary pathogens. Atopic individuals challenged intranasally with diesel exhaust particles and an allergen exhibited increased antigen-specific immunoglobulin E, cytokine, and chemokine levels and increased mast cell degranulation, indicating enhanced allergic inflammation, the principal hallmark of asthma (Diaz-Sanchez et al. 1997, 2000; Nel et al. 1998). So in relation to the EC associations observed in our study, EC could be serving as a surrogate for the larger category of diesel exhaust particles, which, in addition to EC, can include trace metals or adherent organic hydrocarbons such as PAHs, any or all of which could be acting to cause physiologic effects (Li et al. 2003).
Other studies apportioning ambient PM2.5 levels to sources suggest that acute exposure to particles from motor vehicle exhaust, fossil fuel combustion, and vegetative burning—all sources marked by emissions of EC and OC (Sarnat et al. 2008)—is associated with both respiratory morbidity (Andersen et al. 2007) and daily mortality (Laden et al. 2000; Mar et al. 2000).
Our findings suggesting effects for metals, NO3, SO4, EC, and OC implicate man-made combustion sources (i.e., emissions from motor vehicle and industrial fuel combustion, refineries, and industrial processes) and related processes (i.e., brake and tire wear) as significant producers of bioactive fine particulate. K, generally regarded as a marker for wood and biomass burning (Khalil and Rasmussen 2003; Laden et al. 2000; Sarnat et al. 2008), was not consistently related to respiratory admissions in our full-year analyses. However, cold season–specific K levels were significantly associated with respiratory admissions, implying that fine particles from wood smoke may also contribute to respiratory morbidity, as has been suggested in previous research (Naeher et al. 2007). Si is generally regarded as a good tracer for crustal or soil particles (Laden et al. 2000; Marcazzan et al. 2001), and the significant associations we found between Si and respiratory admissions suggest that crustal particulates can also affect respiratory health.
We found that for many of the respiratory outcomes, stronger associations tended to occur with a 1- to 3-day lag of the species. Although there is little to justify an appropriate lag structure a priori for the vast majority of PM2.5 constituents, it is of note that Peel et al. (2005) also found that lags of several days were significant for respiratory ER visits for children. Additional support for longer lags for respiratory disease outcomes is provided by two multicity studies, where hospital admissions for pneumonia were more strongly associated with lags of 2 days or more (Braga et al. 2001; Zeka et al. 2005). In our analysis, as in the others cited, it is unclear whether the longer lag associations are attributable to biologic mechanisms or simply to stochastic variability. However, it is plausible that a latency of several days may occur between exposure and exacerbation of infection because of inflammation and immune suppression. In addition, there may be a delay between exposure and actual day of hospital admission, as opposed to an emergency department visit.
It is important to note the limitations of our study. First, spatial coverage of the monitors is limited for several of the counties. This is likely to introduce random measurement error into the analysis and the potential for downwardly biasing the effect estimates. In addition, differences in measurement techniques or spatial heterogeneity among constituents could introduce different levels of measurement error. Second, this study shares a shortcoming with other particulate studies relying on nondaily data. Because readings are taken every 3 days, comparisons of effect between lags are less direct than they would be with daily data. For this analysis, a same-day lag analysis compares measurements with hospitalizations that occurred on days when readings were taken. A 1-day lag analysis looks at the association between those same readings and all hospitalizations taking place 1 day after a measurement day, or what is in essence a different risk set. This introduces a greater possibility of chance differences in comparisons between different lags. Third, given the numbers of pollutants and end points examined and the relatively low number of observations, it is possible that some of the results may have occurred by chance or that the power to detect effects is limited. This likelihood is diminished somewhat by using a multicounty study design, which minimizes the likelihood that an unusual chance finding observed in one geographic area would heavily influence our conclusions. Also, in view of previous studies, our results seem quite plausible in terms of both the end points and the components for which associations were observed. Finally, associations for a given component may reflect its own toxicity or the toxicity of a correlated, measured or unmeasured, co-pollutant. Therefore, some caution is justified in attributing effects to any single component.
Our findings indicate that hospital admissions for childhood respiratory disease, especially pneumonia, are associated with exposure to PM2.5 and several of its components. Because these components and their related sources, including diesel exhaust, motor vehicle emissions, biomass burning, and other fuel combustion processes, are ubiquitous in the urban environment, small reductions in their concentrations could have a noticeable effect on childhood respiratory morbidity.
Footnotes
Supplemental Material is available online at http://www.ehponline.org/members/2008/11848/suppl.pdf
The opinions expressed in this article are solely those of the authors and do not represent the policy or position of the State of California or the California Environmental Protection Agency.
References
- Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Scheike T, Loft S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol. 2007;17(7):625–636. doi: 10.1038/sj.jes.7500546. [DOI] [PubMed] [Google Scholar]
- Barnett AG, Williams GM, Schwartz J, Neller AH, Best TL, Petroeschevsky AL, et al. Air pollution and child respiratory health: a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171(11):1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- Basu R, Feng WY, Ostro BD. Characterizing temperature and mortality in nine California counties. Epidemiology. 2008;19(1):138–145. doi: 10.1097/EDE.0b013e31815c1da7. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Ebisu K, Zeger SL, Samet J. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115:989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga A, Zanobetti A, Schwartz J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med. 2001;43(11):927–933. doi: 10.1097/00043764-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Brook J, Dann T, Delocla C, Philips O, Cakmak S, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Dales R, Krewski D, Vincent R, Dann T, Brook J. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol. 1995;142(1):15–22. doi: 10.1093/oxfordjournals.aje.a117540. [DOI] [PubMed] [Google Scholar]
- California Air Resources Board. 2007 Almanac Data, Oxides of Sulfur Projected Emission Inventory. 2007. [[accessed 3 Sept 2008]]. Available: http://www.arb.ca.gov/app/emsinv/fcemssumcat2007.php.
- Chalupa D, Morrow P, Oberdorster G, Utell M, Frampton M. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2000;112:879–882. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Gone H, Linn WS, Pellizzari ED, Hu Y. Asthma symptoms in Hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ Health Perspect. 2003;111:647–656. doi: 10.1289/ehp.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D, Penichet-Garcia M, Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J Allergy Clin Immunol. 2000;106(6):1140–1146. doi: 10.1067/mai.2000.111144. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158(5):2406–2413. [PubMed] [Google Scholar]
- Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, et al. Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect. 2000;108(suppl 3):483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–689. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Ghio AJ. Biological effects of Utah Valley ambient air particles in humans: a review. J Aerosol Med. 2004;17(2):157–164. doi: 10.1089/0894268041457200. [DOI] [PubMed] [Google Scholar]
- Ginsberg G, Foos B, Firestone M. Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J Toxicol Environ Health A. 2005;68(8):573–615. doi: 10.1080/15287390590921793. [DOI] [PubMed] [Google Scholar]
- Gottipolu R, Landa E, Schladweiler M, Mcgee J, Ledbetter A, Richards J, et al. Cardiopulmonary responses of intratracheally instilled tire particles and constituent metal components. Inhal Toxicol. 2008;20(5):473–484. doi: 10.1080/08958370701858427. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Baker RJ, Yap PS, Dostal M, Joad JP, Lipsett M, et al. Early childhood lower respiratory illness and air pollution. Environ Health Perspect. 2007;115:1510–1518. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshon J, Shardell M, Alles S, Powell J, Squibb K, Ondov J, et al. Elevated ambient air zinc increases pediatric ashma morbidity. Environ Health Perspect. 2008;116:826–831. doi: 10.1289/ehp.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura T, Li N, Kaplan R, Horwitz M, Seagrave J, Nel A. The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. J Immunol. 2000;165(5):2703–2711. doi: 10.4049/jimmunol.165.5.2703. [DOI] [PubMed] [Google Scholar]
- Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K, et al. Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol. 2006;34(7):958–965. doi: 10.1080/01926230601094552. [DOI] [PubMed] [Google Scholar]
- Khalil MAK, Rasmussen RA. Tracers of wood smoke. Atmos Environ. 2003;37(9–10):1211–1222. [Google Scholar]
- Kodavanti U, Schladweiler M, Ledbetter A, Hauser R, Samet J, McGee J, et al. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: multiple exposure scenarios. Toxicol Sci. 2002;70(1):73–85. doi: 10.1093/toxsci/70.1.73. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The influence of ambient coarse particulate matter on asthma hospitalization in children: case-crossover and time-series analyses. Environ Health Perspect. 2002;110:575–581. doi: 10.1289/ehp.02110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. 2004;16(13):809–815. doi: 10.1080/08958370490506646. [DOI] [PubMed] [Google Scholar]
- Mar T, Norris G, Koenig J, Larson T. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcazzan GM, Vaccaro S, Valli G, Vecchi R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy) Atmos Environ. 2001;35(27):4639–4650. [Google Scholar]
- McConnell R, Berhane K, Gilliland F, Molitor J, Thomas D, Lurmann F, et al. Prospective study of air pollution and bronchitic symptoms in children with asthma. Am J Respir Crit Care Med. 2003;168(7):790–797. doi: 10.1164/rccm.200304-466OC. [DOI] [PubMed] [Google Scholar]
- Miller M, Marty M, Arcus A, Brown J, Morry D, Sandy M. Differences between children and adults: implications for risk assessment at California EPA. Int J Toxicol. 2002;21(5):403–418. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, et al. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19(1):67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Nel A, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Nel A, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114:29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- Pope CA., III Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79(5):623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard RJ, Ghio AJ, Lehman JR, Winsett DW, Tepper JS, Park P, et al. Oxidant generation and lung injury after particulate air pollutant exposure increases with the concentrations of associated metals. Inhal Toxicol. 1996;8(5):457–477. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing . Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Rubin JI, Brown SG, Wade KS, Hafner HR. Final report prepared for the U.S. Environmental Protection Agency, Research Triangle Park, NC. Petaluma, CA: Sonoma Technology, Inc; 2006. Dec, Apportionment of PM25 and air toxics in Detroit, Michigan. STI-906201.06-3103-FR. [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelley F, Sandstrom T, Holgate S, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159(3):702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, et al. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer J, Cass G. Source apportionment of wintertime gas-phase and particle-phase air pollutants using organic compounds as tracers. Environ Sci Technol. 2000;34:1821–1832. [Google Scholar]
- Schauer J, Lough G, Shafer M, Christensen W, Arndt M, DeMinter J, et al. Research Report: Characterization of Metals Emitted from Motor Vehicles . Boston, MA: Health Effects Institute; 2006. [PubMed] [Google Scholar]
- Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M. The health relevance of ambient particulate matter characteristics: coherence of toxicological and epidemiological inferences. Inhal Toxicol. 2006;18(2):95–125. doi: 10.1080/08958370500306016. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113:1037–1043. [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manag Assoc. 1996;46(10):927–939. [PubMed] [Google Scholar]
- Sinclair AH, Tolsma D. Associations and lags between air pollution and acute respiratory visits in an ambulatory care setting: 25-month results from the aerosol research and inhalation epidemiological study. J Air Waste Manag Assoc. 2004;54(9):1212–1218. doi: 10.1080/10473289.2004.10470979. [DOI] [PubMed] [Google Scholar]
- Tolbert P, Mulholland J, MacIntosh D, Xu F, Daniels D, Devine O, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151(8):798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- van der Zee S, Hoek G, Boezen H, Schouten J, van Wijnen J, Brunekreef B. Acute effects of urban air pollution on respiratory health of children with and without chronic respiratory symptoms. Occup Environ Med. 1999;56(12):802–812. doi: 10.1136/oem.56.12.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Diseases. 9th Revision. Geneva: World Health Organization; 1975. [Google Scholar]
- Zanobetti A, Schwartz J, Gold DR. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect. 2000;108:841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. Short term effects of particulate matter on cause specific mortality: effects of lags and modification by city characteristics. Occup Environ Med. 2005;62(10):718–725. doi: 10.1136/oem.2004.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikoff JT, Schermerhorn KR, Fang K, Cohen MD, Schlesinger RB. A role for associated transition metals in the immunotoxicity of inhaled ambient particulate matter. Environ Health Perspect. 2002;110(suppl 5):871–875. doi: 10.1289/ehp.02110s5871. [DOI] [PMC free article] [PubMed] [Google Scholar]