Abstract
Adalimumab is a biological agent, one of the tumour necrosis factor-alpha inhibitors. Pivotal studies evaluating its efficacy in plaque psoriasis (CHAMPION, REVEAL) and psoriatic arthritis (PsA) (ADEPT) were carried out in recent years. Adalimumab proved highly effective in psoriasis patients and in PsA patients previously unresponsive to nonsteroidal anti-inflammatory drugs. Results of smaller studies suggest therapy with the drug may be successful in psoriasis resistant to other biologics and PsA unresponsive to disease-modifying antirheumatic drugs. Adalimumab has also been shown to improve patients' quality of life significantly. Although they should be further extended as far as dermatological conditions are concerned, available data indicate adalimumab is safe and well tolerated. Numerous case reports featuring its off-label use suggest the drug could be helpful in treating hidradenitis suppurativa, pyoderma gangrenosum, Sweet's syndrome, cutaneous sarcoidosis, pemphigus, systemic vasculitides, multicentric reticulohistiocytosis and stomatitis.
Keywords: adalimumab, biological agent, off-label use, psoriasis, psoriatic arthritis
Introduction
Adalimumab is the first fully human monoclonal antitumour necrosis factor (TNF)-α antibody and has been in use since 2003. Until recently it had four authorized indications: rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosoid spondylitis and Crohn's disease. In December 2007 the European Commission approved it for therapy of moderate to severe plaque psoriasis. Adalimumab is one of the biologics that contributed to huge progress in the treatment of autoimmune diseases (such as RA and Crohn's disease) in recent years. The TNF-α inhibitors infliximab, etanercept, adalimumab and T-cell-specific agents such as alefacept and efalizumab are increasingly often used in dermatology.
Clinical pharmacology
Pharmacodynamic profile
Adalimumab is a highly specific TNF-α inhibitor. It binds to both soluble and membrane-bound TNF-α. The drug is believed to exert its pharmacological effect by binding to soluble TNF-α and preventing its interaction with TNFR1 and TNFR2 cell receptors [1].
Adalimumab modulates biological responses that are associated with TNF-α, including changes in the levels of adhesion molecules responsible for leucocyte migration (endothelial cell leucocyte adhesion molecule-1, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 with an IC50 of 1–2 × 10−10 M) [2].
This biological agent rapidly reduces serum concentrations of acute-phase reactants (such as C-reactive protein and erythrocyte sedimentation rate) and interleukin-6. It decreases by 50% serum levels of metalloproteinases (MMP-1 and MMP-3), involved in joint destruction [1, 2].
Pharmacokinetic profile
Adalimumab is administered subcutaneously and absorbed slowly with peak serum concentrations being reached 5 days after administration [2]. Its molecular weight is approximately 148 kDa [3], and it is therefore absorbed predominantly into lymphatic vessels [1]. Drug concentrations in the synovial fluid from RA patients range from 31 to 96% of those in serum [2].
The average absolute bioavailability estimated from three studies following a single 40-mg dose was 64%. After doses of 0.5 kg−1 (∼40 mg), clearances ranged from 11 to 15 ml h−1, the distribution volume ranged from 5 to 6 l and the mean terminal phase half-life was approximately 2 weeks. Data from over 1300 RA patients suggested higher adalimumab clearance with increased body weight. In patients with psoriasis or RA, the mean steady-state trough concentration was 5 µg ml−1 during adalimumab monotherapy treatment with 40 mg every other week [2].
Adalimumab in treatment of psoriasis
Role of TNF-α
The aetiology of psoriasis is strongly associated with the immune system [4, 5]. Efficacy studies of etanercept and infliximab have revealed that TNF-α is a key element (with T cells) in the pathogenesis of psoriasis [4] (see Figure 1).
Figure 1.
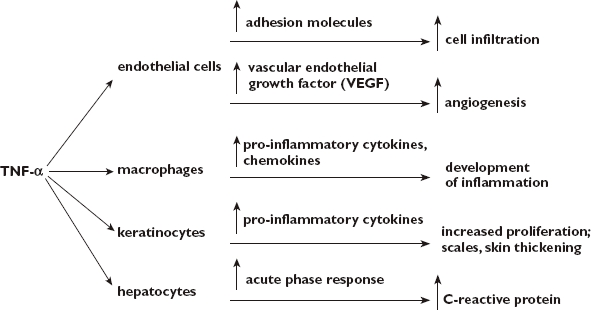
Role of tumour necrosis factor (TNF)-α in inflammatory cascade and process of psoriasis [5, 6]
Dosage and administration
Adalimumab therapy may be started in adult patients resistant to other systemic treatments. The recommended dosage of adalimumab in plaque psoriasis is 80 mg subcutaneously at week 1 followed by 40 mg every other week (eow), starting a minimum of 1 week after the first dose. Therapy should be carefully reconsidered in a patient not responding within 16 weeks of treatment [2].
Efficacy
The efficacy and safety of adalimumab have been assessed in two large randomized, controlled, multicentre studies, CHAMPION and REVEAL, the results of which followed case reports [7, 8] describing successful treatment of psoriasis with the drug.
The double-blinded comparative CHAMPION study [9] included 271 patients with moderate to severe plaque psoriasis from eight European countries and Canada. Participants received adalimumab (80 mg subcutaneously at week 1, then 40 mg eow), methotrexate (MTX) (7.5 mg orally, dose increased to 25 mg week−1, depending on individual tolerance) or placebo. The end-point was reaching 75% clinical improvement as measured by the Psoriasis Area and Sensitivity Index (PASI) after 16 weeks of treatment. Of adalimumab-treated patients, 79.6% achieved PASI 75, compared with 35.5% for MTX and 18.9% for placebo. The complete clearance of skin disease (PASI 100) after 16 weeks was observed in a much larger percentage of patients treated with adalimumab than with MTX or placebo (16.7, 7.3 and 1.9%, respectively). The significance of those results is weakened by the fact the full effect of MTX may not have been achieved in the 16-week evaluation period. Another factor which should be taken into consideration is a slow MTX dose increment, probably contributing to a less rapid response to that drug (22% improvement in mean PASI at week 4) than in the case of adalimumab (56.5%; 15.4% for placebo).
Data on adalimumab efficacy have also been provided by the REVEAL study [10], which was carried out in order to evaluate the short- and long-term efficacy of adalimumab. Patients with moderate and severe plaque psoriasis from the USA and Canada (n = 1212) took part. In REVEAL, as in the CHAMPION study, the end-point was achieving PASI 75 after 16 weeks of treatment. Of patients treated with adalimumab (80 mg subcutaneously at week 1, followed by 40 mg subcutaneously eow), 71% met those criteria, compared with 6.5% receiving placebo. Full remission of disease was achieved in about 20% of patients in the adalimumab group, compared with 1% in the placebo group. Patients with PASI 75 at week 33 (after a 17-week open-label period in which all the patients received adalimumab 40 mg eow) were randomized to continued treatment with adalimumab or placebo. In the first group there was a much lower incidence of inadequate response (defined as <50% improvement in PASI compared with baseline and at least a 6-point worsening in PASI compared with 33 weeks) between 33 and 52 weeks than in the second one (5% vs. 28%, respectively). The meaning of ‘loss of adequate response’ has not been explained. Together with lack of data on PASI 75 at week 52, it makes assessment of the long-term efficacy more difficult. Another limitation of the REVEAL study is lack of an active comparator – only indirect comparisons (favourable to adalimumab) with observations made on other biologics [11–14] such as etanercept, infliximab, alefacept and efalizumab are possible.
A study made by Papoutsaki et al. [15] suggests adalimumab may be effective in patients not responding to conventional systemic treatment as well as other biologics. Those drugs proved unsuccessful in 30 persons with plaque psoriasis with or without PsA who were included in the trial. After 12 weeks of adalimumab treatment (40 mg subcutaneously a week), 87% of participants achieved PASI 75 compared with 83% after 24 weeks. It should be noted that the study was not randomized or placebo controlled. The study [16] of nine patients previously resistant to systemic therapies including etanercept and infliximab led to similar conclusions. PASI 75 was achieved by 62.5% of them after 20 weeks of therapy with adalimumab.
Impact on quality of life
Revicki et al. [17] have published results of a comparative analysis conducted within the CHAMPION study with participation of the same group of patients. Researchers used the Dermatology Life Quality Index (DLQI; it assesses symptoms and feelings, daily activities, leisure, work/school, personal relationships and treatment [18]), Patient's Global Assessment of Disease Severity, EuroQol (EQ-5D), the visual analogue scale for psoriasis and PsA and similar scale for itching (Psoriasis-Related Pruritus Assessment). After 16 weeks of receiving MTX, placebo or adalimumab, patients treated with the latter noticed a much greater and more dynamic improvement in quality of life than the others. It was reflected in all DLQI domains. Moreover, in direct comparison with placebo, adalimumab lowered pain and itching and gave better disease control; there was also significant improvement in itching and disease control compared with MTX.
Another study [19] was carried out with the participation of 147 patients with moderate to severe psoriasis. The following questionnaires were used: DLQI, Short-Form 36 Health Survey (SF-36) and EQ-5D. After 12 weeks of treatment with adalimumab (40 mg weekly or eow), patients displayed significant improvement vs. placebo in DLQI, EQ-5D and SF-36 Mental Component Summary scores, as well as Bodily Pain, Vitality, Social Functioning, Role-Emotional, and Mental Health domains.
Adalimumab in treatment of psoriatic arthritis
Dosage and administration
Timely treatment is vital in limiting functional limitations and deformity caused by PsA, as radiographic evidence is seen at 2 years after diagnosis [20]. Anti-TNF-α therapy (TNF plays a major role in pathogenesis [21]) is considered the most effective. Adalimumab, etanercept and infliximab have similar effects on progress of disease and quality of life [22]. Adalimumab is recommended for adults when disease-modifying anti-rheumatic treatment proves insufficient. The standard dosage of adalimumab in PsA is 40 mg subcutaneously every other week. The therapy should be carefully reconsidered in a patient not responding within 12 weeks [2].
Efficacy
The large randomized, controlled, multicentre ADEPT study [23] showed good effectiveness of adalimumab in treatment of PsA. Three hundred and thirteen adult patients with moderate to severe PsA who had had an inadequate response to previous treatment with nonsteroidal anti-inflammatory drugs took part in a 24-week trial. Adalimumab was given subcutaneously, 40 mg eow. After 12 weeks the American College of Rheumatology criteria (ACR) 20 response was achieved by 58% of adalimumab-treated patients (ACR 50/70 were 36%, 20%), compared with 14% in the placebo group (ACR 50/70 were 4%, 1%). The ACR rates did not differ between patients taking adalimumab in combination with MTX and patients taking it alone. Adalimumab proved much more effective in inhibition of joint damage as assessed by radiographic progression. At 24 weeks the mean change from baseline in modified total Sharp score in adalimumab patients was −0.2 vs. +1.0 with placebo. The therapy improved patients' condition also in aspect of skin symptoms. PASI 75 was achieved within 24 weeks by 59% of participants receiving adalimumab and by only 1% in the placebo group.
Patients who completed the 24-week ADEPT study could decide to receive adalimumab (40 mg eow) for next 24 weeks (open-label part) [24]. At week 48, good responses to adalimumab in patients who received it during the first 24-week double-blind period were maintained (ACR20/50/70 were 56, 44 and 40%). Patients who previously received placebo and had poor responses at week 24 experienced significant improvement 24 weeks later (ACR 20/50/70 were 48, 34 and 20%). At week 48, responses of psoriasis to adalimumab treatment in both groups as measured by PASI 50/75/90/100 were 67%/58%/46%/33% and 61%/53%/44%/31%, respectively. Modified total Sharp score results indicate sustained control of radiographic progression on adalimumab for ∼1 year. For participants in the adalimumab arm of ADEPT, mean changes from baseline were −0.1 at week 24 and 0.1 at week 48. For patients who received placebo during the blinded phase, mean changes from baseline in the modified total Sharp score were 0.9 and 1.0, respectively.
Another smaller study [25] has assessed the efficacy of adalimumab in patients with moderately to severely active PsA previously unresponsive to disease-modifying antirheumatic drugs (DMARDs). One hundred patients were randomized to receive adalimumab (40 mg eow) or placebo. After 12 weeks of treatment, ACR response rates were significantly higher in the adalimumab group (ACR20/50/70 were 39, 25 and 14%) compared with placebo (ACR 20/50/70 were 16, 2 and 0%). After the next 12 weeks (open-label period) there was further improvement in patients receiving adalimumab (ACR 20/50/70 were 64, 43 and 27%), and those who started adalimumab treatment after 12 weeks of placebo improved dramatically (ACR20/50/70 were 57, 37 and 22%).
Impact on disability and quality of life
Disability and quality of life in adalimumab treatment of PsA were evaluated in the previously described ADEPT study [26]. Researchers used Health Assessment Questionnaire disability index (HAQ DI), SF36, Functional Assessment of Chronic Illness (FACIT-F) and DLQI. Disability level, measured by HAQ DI, was significantly lower in the group of patients treated with adalimumab, with many more complete resolutions of functional loss (HAQ DI = 0) compared with placebo both at 12 and 24 weeks. At week 24 adalimumab patients achieved significant improvements in Role-Physical, Bodily Pain, General Health, Vitality and Social Functioning domains of SF36. At weeks 12 and 24 there was grater improvement vs. placebo in FACIT-F scores, pain scores and disease activity measures. Significantly more patients had full recovery from dermatologically-related limitations (DLQI = 0) than in the placebo group.
Off-label use in dermatology
Adalimumab is the newest TNF-α antagonist. It explains why there are much fewer data available on its off-label use than in the case of infliximab, which has a similar mechanism of action and probably similar efficacy [27].
Hidradenitis suppurativa
Literature describes an association between hidradenitis suppurativa and Crohn's disease; the key common factor in their pathogenesis is increased activity of TNF-α [28]. It explains successful attempts to treat the disease with TNF-α antagonists, e.g. adalimumab. There is a case report [29] of a 67-year-old man with a history of inflammatory bowel disease and colectomy. During the last 20 years patient had undergone many unsuccessful therapies for painful purulent draining sinuses, cysts, and nodules involving his ears, axillae, buttocks and groin; methods included oral antibiotics, surgical resection and radiotherapy. The solution was adalimumab at a dose of 40 mg subcutaneously eow. His condition improved significantly after the first injection and further improved throughout treatment.
Neutrophilic dermatoses
Another condition in which adalimumab demonstrates good efficacy is pyoderma gangrenosum (PG). There are publications describing cases of successful treatment in patients with therapy-resistant PG. The doses varied: 80 mg for the first 2 weeks, then 40 mg weekly [30], 40 mg subcutaneously eow [31], 40 mg a week [32] or 80 mg subcutaneously eow [33]. In the latter case report adalimumab brought rapid improvement in a patient previously unresponsive to infliximab.
PG sometimes requires differentiation from another neutrophil dermatosis – Sweet's syndrome (acute neutrophil dermatosis with fever). A case report [34] of a patient with severe Sweet's syndrome and autoimmune inflammatory process in lungs suggests adalimumab can provide longstanding improvement in this disease.
Sarcoidosis
Adalimumab therapy is reported to be successful in treating cutaneous sarcoidosis. Cutaneous involvement is seen in 25% of patients with sarcoidosis. There is a report [35] of a 46-year-old black woman with extensive skin changes to her face and legs. She responded to adalimumab 40 mg once a week (added to prior regimen of pentoxifylline and hydroxychloroquine). The complete healing of numerous lesions was observed after 5 weeks of therapy, with further improvement within the next 5 weeks. Another case report [36] featured a woman with ulcerative cutaneous sarcoidosis responding to adalimumab, 40 mg weekly (added to on-going prednisone and hydroxychloroquine therapy). After 9 weeks the patient experienced complete resolution of the disease.
The drug at a dose of 40 mg eow proved effective in a patient with multiorgan sarcoidosis (knee and pulmonary involvement) not responding to different therapies, including MTX, cyclophosphamide and corticosteroids [37].
Systemic vasculitis
Adalimumab (like infliximab) is thought to be effective in sarcoidosis thanks to its ability to bind to TNF-α on all cells and activate complement. This leads to destabilization of granulomas. A similar mechanism is suspected in the treatment of Wegener's granulomatosis [6].
Cases of patients with a different systemic vasculitis – Behçet's disease treated with adalimumab – have been quoted in the literature. In a case series [38] of three patients with sight-threatening panuveitis resistant to immunosuppressants, adalimumab (40 mg eow) proved as effective as infliximab in maintaining disease remission and preventing relapse. A study [39] featuring six patients not responding to immunosuppressive therapy has also illustrated the efficacy of adalimumab treatment in Behçet's disease.
Pemphigus
Adalimumab may become an important drug in managing pemphigus. This thesis is proved by a case report [40] of a 41-year-old man with aggressive IgA pemphigus treated with adalimumab (40 mg subcutaneously eow) and mycophenolate mophetil (1 g daily). After the third dose of TNF-α antagonist his skin completely cleared.
Multicentric reticulohistiocytosis
There is a case report [41] describing a patient with multicentric reticulohistiocytosis with erosive arthritis despite immunosuppressive therapy. A 37-year-old woman had symmetrical erosion in distal intrapharyngeal and first intrapharyngeal joints. After 8 weeks of adalimumab therapy (40 mg eow; added to her prior regimen of mycophenolate mofetil, ciclosporin, prednisone, simvastatin and tramadol), the patient improved significantly.
Stomatitis
Further evidence of the versatility of possible indications of adalimumab is a report [42] of an 18-year-old man with a 7-year history of aphthous stomatitis refractory to multiple standard therapies. The patient responded completely to adalimumab.
Safety
Available large analyses of adalimumab safety are mainly based on trials of patients with RA [43]. Data they provide generally converge with observations made during studies on treating psoriasis or PsA. However, a cautious approach is needed, especially in cutaneous psoriasis therapy, which noticeably differs in number and severity of adverse events from RA, other inflammatory arthritis or inflammatory bowel disease treatment. The main reasons are different comorbidities in the dermatological diseases and the fact that adalimumab in dermatology is usually given in monotherapy [44]. It seems possible that extrapolations regarding the safety of TNF-α inhibitors derived from combination therapy data may overestimate the potential risk of this agent when used as monotherapy in psoriasis [45]. Patients with PsA, compared with patients with skin involvement only, have a higher likelihood of being treated with combined TNF-α inhibitor and a DMARD (usually MTX) [46].
Tolerability
Adalimumab, like other TNF-α inhibitors, is usually well tolerated. In studies of RA patients that included >10 050 cases of RA, representing 12 506 patient-years, in <10% of cases was therapy discontinued because of adverse events [47].
In the CHAMPION study [9], adverse events were of similar frequency in adalimumab, MTX and placebo groups (73.8, 80.9 and 79.2%, respectively) and were mainly mild to moderate. Percentages of serious adverse events were 1.9, 0.9 and 1.9%, respectively. The first 16-week period of the REVEAL study [10] was completed by 96% of adalimumab patients vs. 89% of placebo; 0.6% of patients experienced serious adverse events vs. 1.0% in the placebo group.
During the 24-week ADEPT [23] study there was a similar occurrence of adverse events in the adalimumab and placebo groups. Serious adverse events occurred in 3.3 and 4.4%, respectively. Only one of five adalimumab treatment discontinuations was due to a serious adverse event (viral meningitis). Results from the open-label extension period of ADEPT [24] are consistent with previous observations.
Injection site reactions
Studies of RA patients [48] have shown that the most common adverse events after adalimumab exposure are injection site reactions. They are usually mild and transient with findings of local erythema, urticarial plaques and pruritus [6]. These reactions occurred in 20.3% of adalimumab-treated patients vs. 13.8% of placebo-treated patients [43].
Infections
In clinical studies, 52.7% of adalimumab patients with RA developed infections compared with 46.7% of placebo-treated patients. The incidence of serious infections was 1.7 and 1.4%, respectively [49].
The most frequent infections were upper respiratory tract infections, rhinitis (both were most common in psoriasis [9, 10] and PsA studies [23]), bronchitis and urinary tract infections [42]. Adalimumab caused a twofold increased incidence of rare infections such as fungal pneumonia, septic arthritis or pyelonephritis [50].
Tuberculosis
Adalimumab therapy increases the risk of tuberculosis (TB). It is believed that TNF-α plays an essential role in host immunity against TB, which probably explains this phenomenon [51]. TB occurred in seven cases during the first 534 patient-years of adalimumab exposure in clinical trials; the rate of TB decreased by 75% in European clinical trials after the introduction of routine TB screening [47]. In most cases, TB is reactivation of a latent form and occurs within the first 8 months of treatment [49].
Skin adverse events
In a prospective cohort study [52] of 289 RA patients treated with TNF-α antagonists, the frequency of dermatological adverse events during or after adalimumab treatment (0.12 per patient-year) was similar to infliximab, etanercept and lenercept. They were skin infections (most cases), eczema, drug-related eruptions, tumours, actinic keratosis, vasculitis, ulcers and psoriasis (or psoriasiform eruptions). The phenomenon of the latter dermatological condition during anti-TNF-α treatment (with infliximab or adalimumab) has also been described in a case series of 12 RA patients [53]. Two cases were of adalimumab treatment-related plaque psoriasis and plantar pustulosis.
There is a case report [54] of urticaria and angio-oedema-like skin reactions after adalimumab in an RA patient.
Lymphoma
In trials of patients with RA treated with adalimumab [47], the standardized incidence rate of lymphomas was 3.19, but reports quoted in the literature show that RA itself causes a twofold increased occurrence of lymphomas over that in the general population, which is probably even more increased in highly actively disease [43]. So the precise answer to the question of the role of adalimumab in lymphomas will have to await future analyses.
Demyelinating disease
Patients treated with adalimumab tend to have a higher incidence of demyelinating conditions, especially multiple sclerosis (MS). This subject must be further investigated, while both RA and psoriasis are thought to increase the risk of MS [43].
Congestive heart failure
Some reports have suggested aggravation of congestive heart failure (CHF) by adalimumab. During trials of 10 006 patients with RA, 44 participants reported a medical history of CHF, three (7%) of whom reported CHF events in a period of trials. There were 32 cases of CHF (0.3%) in patients who did not report a medical history of CHF [43].
Drug-induced lupus
Approximately 3–12% of patients treated with adalimumab develop autoantibodies to antinuclear and double-stranded (ds) DNA. However, in 12 506 patient-years of adalimumab exposure only 13 cases of systemic lupus erythematosus and lupus-like syndromes have been reported [43]. It seems that the clinical implications of the increase of antinuclear antibodies and ds-DNA antibodies caused by adalimumab require further analysis.
Hepatotoxicity
There are data suggesting possible hepatotoxicity (but not leading into liver failure and death, as in some infliximab treatment reports) and risk of hepatitis B reactivation in adalimumab patients. On the other hand, many reports have stated that anti-TNF-α treatment in hepatitis C is safe and well tolerated by patients [6].
Precautions and use in particular conditions
Active TB (appropriate screening tests should be performed in all patients), serious infections, reactivation of hepatitis B and moderate to severe heart insufficiency [New York Heart Association (NYHA) III, IV] are considered contraindications [2]. Adalimumab should be used cautiously when latent TB, malignancies, mild heart insufficiency (NYHA I/II) or haematological disorders coexist [2] (cases of aplastic anaemia and pancytopenia have occurred during treatment with TNF-α inhibitors [6]).
Use of adalimumab in combination with anakinra is not recommended. Serious infections have been seen in clinical studies with concurrent use of the latter drug and etanercept [2].
Patients on adalimumab may receive concurrent vaccinations, except for live vaccines; no data are available on the secondary transmission of infection by live vaccines in patients receiving that drug [2].
The safety and efficacy of adalimumab in patients with immunosuppression have not been evaluated [3]. However, in a study of 64 RA patients there was no evidence of depression of delayed-type hypersensitivity, depression of immunoglobulin levels or change in enumeration of effector T and B cells, natural killer cells, monocytes/macrophages or neutrophils [2, 3].
No conclusive data on the use of adalimumab in HIV/AIDS patients, especially in dermatological conditions, are available; there are no specific recommendations in official drug profile [2, 3] and current guidelines (from the American Academy of Dermatology) [45, 46]. A retrospective study [55] of eight patients with rheumatic diseases (treated with etanercept, followed by adalimumab or infliximab) seems to suggest the drug may be safe and effective in HIV-infected persons without advanced retroviral disease.
There is limited safety experience of surgical procedures in patients treated with adalimumab. The long half-life of the drug should be taken into consideration, and a patient requiring surgery should be closely monitored for infections [2].
Because of the possible impact on newborn's immune responses, adalimumab treatment in women of reproductive age requires contraception during treatment and at least 5 months after taking the last dose [2]. It is a pregnancy category B drug [3].
Women must not breast feed for at least 5 months after the last adalimumab treatment. It has not been proved that adalimumab is secreted in human milk, but it is known that human autoantibodies are [2].
Conclusion
Adalimumab is an increasingly important drug in dermatology. Phase III trials have shown its excellent efficacy in treating psoriasis and PsA. Moreover, CHAMPION, REVEAL and ADEPT studies have proved this biological agent to be generally safe and well tolerated by patients. There have been many reports describing off-label use that show the range of possible indications. Unfortunately, the relatively high price of the drug is a serious barrier to its wide use, especially in less developed countries.
Competing interests
None to declare.
REFERENCES
- 1.Shukla R, Vender RB. Pharmacology of TNF inhibitors. In: Weinberg JM, Buholtz R, editors. TNF-alpha Inhibitors. Basel: Birkhäuser Verlag; 2006. pp. 23–43. [Google Scholar]
- 2.European Medicines Agency. Summary of product characteristics. [last accessed: 3 April 2008]. Available at http://www.emea.europa.eu/humandocs/PDFs/EPAR/humira/H-481-PI-en.pdf.
- 3.Abbott. Full prescribing information. 2008. [last accessed: 3 April 2008]. February. Available at http://www.fda.gov/cder/foi/label/2008/125057s114lbl.pdf.
- 4.Griffiths CEM, Barker J. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 5.Malerich P, Elston DM. Introduction of TNF/Pathophysiology of TNF. In: Weinberg JM, Buholtz R, editors. TNF-alpha Inhibitors. Basel: Birkhäuser Verlag; 2006. pp. 1–44. [Google Scholar]
- 6.Jackson JM. TNF-α inhibitors. Derm Ther. 2007;20:251–64. doi: 10.1111/j.1529-8019.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- 7.Chew AL, Bennet A, Smith A, Barker J, Kirkham B. Successful treatment of severe psoriasis and psoriatic arthritis with adalimumab. Br J Dermatol. 2004;151:492–6. doi: 10.1111/j.1365-2133.2004.06105.x. [DOI] [PubMed] [Google Scholar]
- 8.Sladden MJ, Mortimer NJ, Hutchinson PE. Extensive plaque psoriasis successfully treated with adalimumab (Humira) Br J Dermatol. 2005;152:1091–2. doi: 10.1111/j.1365-2133.2005.06582.x. [DOI] [PubMed] [Google Scholar]
- 9.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, Unnebrink K, Maul M. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–66. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 10.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Yihua G, Okun M, Papp K. Adalimumab for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–15. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, Gaspari AA, Ling M, Weinstein GD, Nayak A, Gordon KB, Zitnik R. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139:1627–32. doi: 10.1001/archderm.139.12.1627. [DOI] [PubMed] [Google Scholar]
- 12.Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719–27. doi: 10.1001/archderm.139.6.719. [DOI] [PubMed] [Google Scholar]
- 13.Menter A, Gordon K, Carey W, Ortonne JP, Roberts J, Griffiths CE. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol. 2005;141:31–8. doi: 10.1001/archderm.141.1.31. [DOI] [PubMed] [Google Scholar]
- 14.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicenter, double-blind trial. Lancet. 2005;366:1367–74. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 15.Papoutsaki M, Costanzo A, Chimenti MS, Talamonti M, Zangrilli A, Giunta A, Bianchi L, Chimenti S. Adalimumab for severe psoriasis and psoriatic arthritis: an open-label study in 30 patients previously treated with other biologics. J Am Acad Dermatol. 2007;57:269–75. doi: 10.1016/j.jaad.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Pitarch G, Sanchez-Carazo JL, Mahiques L, Perez-Ferriols MA, Fortea JM. Treatment of psoriasis with adalimumab. Clin Exp Dermatol. 2007;32:18–22. doi: 10.1111/j.1365-2230.2006.02288.x. [DOI] [PubMed] [Google Scholar]
- 17.Revicki D, Willian MK, Saurat JH, Papp KA, Ortonne JP, Sexton C, Camez A. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008;158:549–67. doi: 10.1111/j.1365-2133.2007.08236.x. [DOI] [PubMed] [Google Scholar]
- 18.Katugampola RP, Lewis VJ, Finlay AY. The Dermatology Life Quality Index: assessing the efficacy of biological therapies for psoriasis. Br J Dermatol. 2007;156:945–50. doi: 10.1111/j.1365-2133.2007.07817.x. [DOI] [PubMed] [Google Scholar]
- 19.Shikiar R, Heffernan MP, Langley RG, Willian MK, Okun MM, Revicki DA. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a Phase II randomized controlled trial. J Dermatol Treat. 2007;18:25–31. doi: 10.1080/09546630601121060. [DOI] [PubMed] [Google Scholar]
- 20.Simpson D, Scott LJ. Adalimumab in psoriatic arthritis. Drugs. 2006;66:1487–98. doi: 10.2165/00003495-200666110-00008. [DOI] [PubMed] [Google Scholar]
- 21.Gladman DD. Psoriatic arthritis. Derm Ther. 2004;17:350–63. doi: 10.1111/j.1396-0296.2004.04038.x. [DOI] [PubMed] [Google Scholar]
- 22.Mease P. Management of psoriatic arthritis: the therapeutic interference between rheumatology and dermatology. Curr Rheum Rep. 2006;8:348–54. doi: 10.1007/s11926-006-0064-9. [DOI] [PubMed] [Google Scholar]
- 23.Mease P, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EHS, Sharp JT, Ory PA, Weinberg M. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis. Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 24.Gladman DD, Mease P, Ritchlin CT, Choy EHS, Sharp JT, Ory PA, Pardok RJ, Sasso EH. Adalimumab for long-term treatment of psoriatic arthritis. Forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007;56:476–88. doi: 10.1002/art.22379. [DOI] [PubMed] [Google Scholar]
- 25.Genovese MC, Mease PJ, Thomson GTD, Kivitz AJ, Perdok RJ, Weinberg MA. Safety and efficacy of adalimumab treatment in patients with psoriatic arthritis who had failed disease modifying therapy. J Rheumatol. 2007;34:1040–50. [PubMed] [Google Scholar]
- 26.Gladman DD, Mease P. Adalimumab improves joint- and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the Adalimumab Effectiveness in Psoriatic Arthritis Trial. Ann Rheum Dis. 2007;66:163–8. doi: 10.1136/ard.2006.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graves JE, Nunley K, Heffernan MP. Off-label uses of biologics in dermatology: rituximab, omalizumab, infliximab, etanercept, adalimumab, efalizumab, and alefacept (Part 2 of 2) J Am Acad Dermatol. 2007;56:e55–79. doi: 10.1016/j.jaad.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Jacob S, Kerdel F. Biologics for hidradenitis suppurativa (Verneuil's disease in the era of biologics) In: Jemec GBE, Revuz J, Leyden JJ, editors. Hidradenitis Suppurativa. Heidelberg: Springer; 2006. pp. 145–9. [Google Scholar]
- 29.Moul DK, Korman NJ. Severe hidradenitis suppurativa treated with adalimumab. Arch Dermatol. 2006;142:1110–2. doi: 10.1001/archderm.142.9.1110. [DOI] [PubMed] [Google Scholar]
- 30.Heffernan MP, Anadkat MJ, Smith DJ. Adalimumab treatment for pyoderma gangrenosum. Arch Dermatol. 2007;143:306–8. doi: 10.1001/archderm.143.3.306. [DOI] [PubMed] [Google Scholar]
- 31.Pomerantz RG, Husni ME, Mody E, Qureshi AA. Adalimumab for treatment of pyoderma gangrenosum. Br J Dermatol. 2007;157:1267–304. doi: 10.1111/j.1365-2133.2007.08212.x. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard VG, Friedman AC, Goldsmith P. Systemic pyoderma responding to infliximab and adalimumab. Br J Dermatol. 152:1059–61. doi: 10.1111/j.1365-2133.2005.06467.x. [DOI] [PubMed] [Google Scholar]
- 33.Margaret AF, Cummins DL, Ehst BD. Adalimumab therapy for recalcitrant pyoderma gangrenosum. J Burns Wounds. 2006;5:e8. [PMC free article] [PubMed] [Google Scholar]
- 34.Karamlou K, Gorn AH. Refractory Sweet syndrome with autoimmune organizing pneumonia treated with monoclonal antibodies to tumor necrosis factor. J Clin Reumatol. 2004;10:331–5. doi: 10.1097/01.rhu.0000147053.60795.46. [DOI] [PubMed] [Google Scholar]
- 35.Heffernan MP, Smith DJ. Adalimumab for treatment of cutaneous sarcoidosis. Arch Dermatol. 2006;142:17–9. doi: 10.1001/archderm.142.1.17. [DOI] [PubMed] [Google Scholar]
- 36.Philips MA, Lynch J, Azmi FH. Ulcerative cutaneous sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917. doi: 10.1016/j.jaad.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Callejas-Rubio JL, Ortego-Centeno N, Lopez-Perez L, Benticuaga MN. Treatment of therapy-resistant sarcoidosis with adalimumab. Clin Rheumatol. 2006;25:596–7. doi: 10.1007/s10067-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 38.Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behçet disease. Eye. 2007;21:824–5. doi: 10.1038/sj.eye.6702352. [DOI] [PubMed] [Google Scholar]
- 39.van Laar JA, Missoten T, van Daele PL. Adalimumab: a new modality of Behçet disease? Ann Rheum Dis. 2007;66:565–6. doi: 10.1136/ard.2006.064279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howell SM, Bessinger GT, Altman CE, Belnap C. Rapid response of IgA pemphigus of the subcorneal pustular dermatosis subtype to treatment with adalimumab and mycophenolate mofetil. J Am Acad Dermatol. 2005;53:541–3. doi: 10.1016/j.jaad.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 41.Shannon SE, Schumacher HR, Self S, Brown AN. Multicentric reticulohistiocytosis responding to tumor necrosis factor-alpha inhibition in a renal transplant patient. J Rheumatol. 2005;32:565–7. [PubMed] [Google Scholar]
- 42.Vujevich J, Zirwas M. Treatment of severe, recalcitrant major aphthous stomatitis with adalimumab. Cutis. 2005;76:129–32. [PubMed] [Google Scholar]
- 43.Salfeld J, Kupper H. Adalimumab. In: Boehncke W-H, Radeke H, editors. Biologics in General Medicine. Heidelberg: Springer; 2007. pp. 14–31. [Google Scholar]
- 44.Menter A, Griffiths CEM. Current and future management of psoriasis. Lancet. 2007;370:272–84. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- 45.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JYM, Elmets CA, Korman NJ, Beutner KR, Bhushan R. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Psoriasis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58:826–50. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 46.Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JYM, Van Voorhees AS, Elmets CA, Beutner KR, Bhushan R, Menter A. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58:851–64. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 47.Schiff MH, Burmester GR, Kent J, Pangan AL, Kupper H, Fitzpatrick SB, Donovan C. Safety analyses of adalimumab (Humira) in global clinical trials and US postmarketing 850 surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:889–94. doi: 10.1136/ard.2005.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wells AF, Kupper H, Fischkoff S, Chartash E. Injection site reactions in adalimumab rheumatoid arthritis (RA) pivotal clinical trials. Ann Rheum Dis. 2003;62(Suppl I):411. abstract AB0166. [Google Scholar]
- 49.Weinberg JM. A review of the safety of the tumor necrosis factor inhibitors infliximab, etanercept, and adalimumab. In: Weinberg JM, Buholtz R, editors. TNF-alpha Inhibitors. Basel: Birkhäuser Verlag; 2006. pp. 115–27. [Google Scholar]
- 50.Scheinfeld N. Adalimumab: a review of side effects. Expert Opin Drug Saf. 2005;4:637–41. doi: 10.1517/14740338.4.4.637. [DOI] [PubMed] [Google Scholar]
- 51.Bakleh M, Tleyjech I, Matesson EL, Osmon DR, Berbari EF. Infectious complications of tumour necrosis factor-alfa-antagonists. Int J Dermatol. 2005;44:443–8. doi: 10.1111/j.1365-4632.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- 52.Flendrie M, Vissers WHPM, Creemers MCJ, de Jong EMGJ, van de Kerkhof PCM, van Riel PLCM. Dermatological conditions during TNF-a-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005;7:R666, 76. doi: 10.1186/ar1724. Epub 4 Apr 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aslanidis S, Pyrpasopoulou A, Dourma S, Triantafyllou A. Tumor necrosis factor-α antagonist-induced psoriasis: yet another paradox in medicine. Clin Rheumatol. 2008;27:377–80. doi: 10.1007/s10067-007-0789-5. [DOI] [PubMed] [Google Scholar]
- 54.Nikas SN, Voulgari PV, Drosos AA. Urticaria and angiedema-like skin reactions in a patient treated with adalimumab. Clin Rheumatol. 2007;26:787–88. doi: 10.1007/s10067-005-0197-7. [DOI] [PubMed] [Google Scholar]
- 55.Cepeda EJ, Williams FM, Ishimori ML, Weisman MH, Reveille JD. Use of anti-tumour necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710–2. doi: 10.1136/ard.2007.081513. [DOI] [PubMed] [Google Scholar]


