Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
Risperidone metabolism is affected by blocking CYP2D6 and CYP3A4 (in CYP2D6 poor metabolizers) metabolizing enzymes.
Age affects risperidone disposition and renal function affects elimination of 9-hydroxy-risperidone (primary active metabolite).
WHAT THIS STUDY ADDS.
The detection of a systematic shift in estimated apparent clearance in the African-American population (it is not clear if there are biological or sociological contributors), and a shift in the clearance rate of risperidone based on concomitant administration of paroxetine, manifested as a change in assignment to a different metabolizer subpopulation group that may be primarily related to CYP2D6 metabolizer status.
The study shows an age-related decrement in 9-hydroxy-risperidone clearance across a wide range of ages.
Information on the nature of the pharmacokinetic variability with risperidone when used in a typical clinical patient population.
There are significant differences in the absolute values as well as the assignment to metabolizer status across race and concomitant paroxetine administration.
AIMS
To characterize pharmacokinetic (PK) variability of risperidone and 9-OH risperidone using sparse sampling and to evaluate the effect of covariates on PK parameters.
METHODS
PK analysis used plasma samples collected from the Clinical Antipsychotic Trials of Intervention Effectiveness. A nonlinear mixed-effects model was developed using nonmem to describe simultaneously the risperidone and 9-OH risperidone concentration–time profile. Covariate effects on risperidone and 9-OH risperidone PK parameters were assessed, including age, weight, sex, smoking status, race and concomitant medications.
RESULTS
PK samples comprised 1236 risperidone and 1236 9-OH risperidone concentrations from 490 subjects that were available for analysis. Ages ranged from 18 to 93 years. Population PK submodels for both risperidone and 9-OH risperidone with first-order absorption were selected to describe the concentration–time profile of risperidone and 9-OH risperidone. A mixture model was incorporated with risperidone clearance (CL) separately estimated for three subpopulations [poor metabolizer (PM), extensive metabolizer (EM) and intermediate metabolizer (IM)]. Age significantly affected 9-OH risperidone clearance. Population parameter estimates for CL in PM, IM and EM were 12.9, 36 and 65.4 l h−1 and parameter estimates for risperidone half-life in PM, IM and EM were 25, 8.5 and 4.7 h, respectively.
CONCLUSIONS
A one-compartment mixture model with first-order absorption adequately described the risperidone and 9-OH risperidone concentrations. Age was identified as a significant covariate on 9-OH risperidone clearance in this study.
Keywords: CATIE trial, CYP2D6, nonmem, pharmacokinetics, risperidone
Introduction
The atypical antipsychotics represent the first class of medications with significant advantages over previously developed neuroleptics. Large interindividual pharmacokinetic (PK) variability for antipsychotic drugs is commonly observed in routine therapeutic drug monitoring. This represents a significant clinical challenge in the treatment of psychiatric illness. An adequate understanding of the effects of a drug is contingent upon the characterization of PK data. Clinical studies suggest that plasma levels of risperidone correlate with adverse drug effects [1]. Thus, understanding the variability in drug exposure under typical treatment conditions is important for clinical effectiveness studies.
Risperidone is an atypical antipsychotic with selective antagonistic properties at serotonin 5-HT2 and dopamine D2 receptors [2, 3]. Some studies have suggested that risperidone is effective in the treatment of both positive and negative symptoms of schizophrenia and has fewer adverse drug effects compared with classic antipsychotics [2].
Many factors may influence risperidone plasma concentrations, such as age and renal function. Aichhorn [4] demonstrated that the concentration–dose ratio was increased by 34.8% per decade in patients >42 years old, although specific PK parameters were not assessed. Another study found that the half-life and area under the curve (AUC) of risperidone were increased in those with renal impairment compared with healthy subjects [5].
CYP2D6 polymorphisms may potentially have an impact on risperidone PK, as risperidone is primarily metabolized by CYP2D6 and to a lesser extent by CYP3A4. The formation of its major active metabolites, 9-hydroxyrisperidone (9-OH-RISP) is predominantly due to CYP2D6 [6, 7]. Drugs altering CYP2D6 or CYP3A4 activities may interact with risperidone [8]. Wang et al. [9] conducted a population PK analysis in CF1 mice to evaluate the drug–drug interactions between risperidone and CYP2D6 inhibitors (bupropion and sertraline). The results showed that AUC and elimination half-life were increased with concomitant administration of these 2D6 inhibitors. Saito et al. [10] have reported dose-dependent interaction of paroxetine with risperidone concentrations in schizophrenic patients. Spina et al. [8] have demonstrated that the levels of the active moiety (sum of the concentrations of risperidone and 9-OH-RISP) increased by 75% in schizophrenic patients taking risperidone with fluoxetine compared with risperidone alone. Moreover, de Leon et al. [11] have reported that the CYP2D6 poor metabolizer (PM) phenotype may be associated with risperidone adverse drug reactions and discontinuation, which may be due to high concentrations of risperidone resulting from the lack of CYP2D6 enzyme activity in the PM population. Other investigators have developed a mixture model for risperidone elimination in bipolar patients receiving risperidone. The subpopulations of clearance rate were described as being analogous to the unmeasured CYP2D6 metabolizer genotype/phenotype [12].
The characterization of the sources of variability in both risperidone and 9-OH risperidone using highly sparse concentration sampling has not been reported. In this study, we applied a nonlinear mixed-effect modelling approach to characterize risperidone and its metabolite PK in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trials for Alzheimer's disease (AD) and schizophrenia (SZ).
The mixed-effect population PK approach permits study of the sources and correlates of variability in plasma concentrations between individuals [13]. Compared with the traditional PK methods, population PK is more suitable for analysing large-scale clinical trials, where only a few samples are available per subject.
The purpose of this study was (i) to apply a nonlinear mixed-effect modelling approach to describe simultaneously risperidone and 9-OH risperidone PK parameters using limited sampling in a large number of subjects from the CATIE clinical trials, and (ii) to evaluate the impact of covariates including age, weight, sex, race, concomitant medications and smoking status on risperidone and 9-OH risperidone PK parameters.
Subjects and methods
CATIE comprises two separate trials investigating the comparative effectiveness of new atypical antipsychotics in patients suffering from either AD or SZ [14–16]. Approximately 2250 patients were enrolled in these studies (450 in CATIE-AD Trial and 1800 in CATIE-SZ Trial) and a fraction of these individuals were randomized to receive risperidone. One to six random plasma samples per subject were obtained. The methods for the CATIE trial have been published in detail previously [14–16]. Brief descriptions of both studies are given below.
CATIE-AD study
The AD trial was a randomized, parallel group, double-blind study comparing olanzapine, quetiapine, risperidone and placebo in AD outpatients with delusions or hallucinations and/or clinically significant aggression or agitation. It used a treatment algorithm approach consisting of three phases, each of which may last up to 12 weeks. Medication changes were allowed after 2 weeks for patients who had suboptimal responses or experience side-effects. Three plasma levels were designated to be taken at weeks 2, 4 and 12 or when a medication switch was made.
CATIE-SZ study
The CATIE-Schizophrenia Trial was a randomized, double-blind study that evaluated the effectiveness of atypical and conventional antipsychotics over an 18-month period in patients with SZ. Subjects (aged 18–65 years) were included if they met the Diagnostic and Statistical Manual of Mental Disorders-IV diagnosis of SZ. Patients were initially randomized to perphenazine, olanzapine, quetiapine, risperidone or ziprasidone for up to 18 months of treatment. This study consisted of three phases. In the event of treatment failure, patients had the option of being re-randomized to a new treatment in phase II or were offered an open-label treatment not previously received in phase I. Patients who entered phase III of the study were offered an open-label treatment not previously received in the study (in either phases I or II). Treatment options for phase II included one of the new atypicals (not received in phase I) or clozapine. Phase III treatment options included one of the new atypicals (not received in phases I or II), clozapine, fluphenazine decanoate, or dual (risperidone or perphenazine augmentation) antipsychotic therapy. The CATIE-SZ protocol included obtaining random blood samples for antipsychotic levels every 3 months for a total of up to six samples per patient.
Analytical procedures
Risperidone and 9-OH-RISP plasma concentrations were determined using a highly sensitive and specific LC-MS-MS method with a detection limit of 0.1 ng ml−1[14]. After a simple one-step extraction, samples were injected onto a Perkin-Elmer LC-MS-MS using a phenyl-hexyl high-performance liquid chromatogrphy column, 50 × 4.6 mm. Quantitative analysis was carried out by multiple reaction monitoring mode using ion transitions for risperidone m/z 411→191, for 9-OH-RISP m/z 427→207 and for the internal standard m/z 421→201. The assay was linear for both risperidone and 9-OH-RISP over the range of 0.1–100 ng ml−1 when 0.5 ml of plasma was used in the extraction. The overall intra- (within-day) and interassay (between-day) variations were <11%. The variations in the concentrations of two long-term quality control plasmas analysed over a period of 6 months were ∼10% [17].
Population pharmacokinetic analysis
The population PK analysis includes the base model and final (covariate) model development. The base model defines the PK parameters and describes the plasma concentration–time profile. The final model describes the influence of fixed effects (i.e. demographic factors) on the PK parameters. Analysis platform, minimization methods and model building criteria are described below.
Analysis platform
Nonlinear mixed effects modelling was used for the population PK analysis using the nonmem computer program (Version 5, level 1.1; GloboMax, Hanover, MD, USA) [18, 19]. The models consisted of a structural model that described the disposition of the drug following oral administration, and a pharmacostatistical model that described the inter- and intra-individual variability. Diagnostic graphics, exploratory analyses and post-processing of nonmem outputs were performed using s-plus (Version 6.2; Insightful, Seattle, WA, USA).
Minimization methods and model building criteria
The first order estimation method (FO) was used for model building. The adequacy of the developed structural models was evaluated using both statistical and graphical methods. The likelihood ratio test was used to discriminate between alternative models. The likelihood ratio test was based on the property that the ratio of the nonmem objective function values (OFV) (-2 log-likelihood) was asymptotically χ2 distributed. An objective function decrease of 7.88 units was considered significant (χ2P < 0.005, d.f. = 1). Standard errors for all parameters were obtained using the covariance option in nonmem. The modelling approach is to determine the most parsimonious model that adequately describes the data.
Base model development
The population PK model for risperidone and its 9-OH risperidone was developed to describe simultaneously risperidone and its 9-OH metabolite concentrations.
Structural PK model
The structural PK model represents the best description of the data without considering the effect of subject-specific covariates. The population PK analysis was performed using nonmem[18, 19]. In order to describe the PK for both risperidone and its 9-OH risperidone, three PK disposition models were tested: (i) a standard one-comparment model with subroutine ADVAN2 TRANS2 (risperidone parent only); (ii) a standard two-comparment model with subroutine ADVAN4 TRANS4 (risperidone parent only); and (iii) a mixture model to distinguish subpopulations related to CYP2D6 polymorphisms with subroutine ADVAN5 (both risperidone and 9-OH risperidone).
In the mixture model, risperidone clearance (CL) and fraction of risperidone to 9-OH risperidone (KF) were separately estimated for CYP2D6 subpopulations [PM, intermediate metabolizer (IM) and extensive metabolizer (EM)].
Interindividual variability
The interindividual variability (IIV) model describes the unexplained random variability in individual values of structural model parameters. It was assumed that the IIV of the PK parameters was log-normally distributed. The relationship between a PK parameter (P) and its variance could therefore be expressed as shown below:
where Pj is the value of PK parameter for the jth individual, PTV is the typical value of P for the population, and ηP denotes the difference between Pj and PTV, independently, which was identically distributed with a mean of zero and variance of ωP2.
Intra-individual variability
The residual variability, which was comprised of, but not limited to, intra-individual variability, experimental errors, process noise and/or model misspecifications, was modelled using additive, proportional and combined error structures as described below:
Additive error: yij = ŷij + εij
Proportional error: yij = ŷij(1 + εij)
Combined additive and proportional error: yij = ŷij(1 + εij) + εij′
where yij is the jth observation in the ith individual, ŷij is the corresponding model prediction, and εij (or εij′) is a normally distributed random error with a mean of zero and a variance of σ2. A unique residual error model was tested for both the parent and metabolite in this model.
Final model development
The final model was developed by testing the effect of subject-specific covariates, including age, weight, sex, smoking status, race and concomitant medications, on PK parameter estimates. The two types of covariate, including continuous covariates (e.g. age and weight) and discrete covariates (e.g. sex, and race), were introduced into each parameter in a stepwise fashion.
The relationship between the typical value of a parameter (PTV) and a continuous valued covariate (R) was tested using the following relationship
where P1 and P2 are estimated fixed-effect parameters, and Rref is a reference value of the covariate. The reference value for a covariate was specified to be a nominal value that approximates the median for the covariate.
The relationship between the population average value of a parameter and categorical covariate (R) was tested using the following relationship:
where Pi (i = 1, …, M) are fixed-effect parameters, and Im are indicator variables, where Im = 1 for the mth category and 0 otherwise.
The graphical assessment of POSTHOC parameter estimates vs. covariates was evaluated to help identify possible covariate relationships using s-plus 6.2. In addition, goodness of fit plots were utilized to assess model robustness [20]. The covariate was retained in the model if it decreased the OFV by 7.88 (χ2P < 0.005, d.f. = 1). No model was accepted solely based on the reduction of the OFV value due to the inherent statistical inaccuracy of this change using the FO method [21]. Goodness of fit plots, reductions in IIV of structure model parameters and residual error, robust model parameter estimation and model stability served as additional model selection criteria.
Results
Patient characteristics
The CATIE-AD clinical trial included 110 subjects that received risperidone (52 men), providing 168 risperidone and 9-OH risperidone concentrations. The CATIE-SZ clinical trial included 380 subjects (279 men) that received risperidone, providing 1068 risperidone concentrations and 1068 9-OH risperidone concentrations. A total of 313 subjects had a once-daily dose of risperidone and 177 subjects had a twice-daily dose of risperidone. Subjects had an average (mean ± SD) age and weight of 49.1 ± 18.8 years and 84.1 ± 22.5 kg, respectively (Table 1, Figure 1).
Table 1.
Patient characteristics for the two studies (CATIE-AD and CATIE-SZ)
| Demographics | CATIE-AD mean ± SD (range) | CATIE-SZ mean ± SD (range) | Combine mean ± SD (range) |
|---|---|---|---|
| Sample size | 110 | 380 | 490 |
| Dose range (mg) | 0.5–3.5 | 0.75–6.0 | 0.5–6.0 |
| No. of observations | 168 | 1068 | 1236 |
| Risperidone concentration (ng ml−1) | 2.25 ± 3.13 | 8.89 ± 11.66 | 8.02 ± 11.13 |
| (0.05–20.49) | (0.02–99.05) | (0.02–99.05) | |
| 9-OH risperidone concentration (ng ml−1) | 20.93 ± 15.29 | 10.15 ± 8.49 | 19.46 ± 15.02 |
| (0.15–111.24) | (0.5–51.1) | (0.15–111.24) | |
| Age (years) | 78.3 ± 6.7 | 40.6 ± 11.2 | 49.1 ± 18.8 |
| (57–93) | (18–67) | (18–93) | |
| Weight (kg) | 68.8 ± 14.7 | 88.6 ± 22.4 | 84.1 ± 22.5 |
| (42.7–102.3) | (45.9–187.7) | (42.7–187.7) | |
| Gender (male/female) | Male: 52 | Male: 279 | Male: 331 |
| Female: 58 | Female: 101 | Female: 159 | |
| Race | American Indian: 1 | American Indian: 4 | American Indian: 5 |
| Asian: 2 | Asian: 10 | Asian: 12 | |
| Black/AA: 16 | Black/AA: 124 | Black/AA: 140 | |
| Hawaiian: 0 | Hawaiian: 1 | Hawaiian: 1 | |
| Two or more races: 1 | Two or more races: 3 | Two or more races: 4 | |
| White: 90 | White: 238 | White: 328 |
CATIE, Clinical Antipsychotic Trials of Intervention Effectiveness; AA, African-American.
Figure 1.
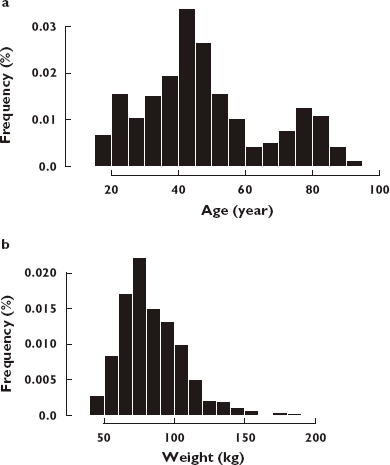
Frequency histogram showing the age (a) and weight (b) distribution for all subjects in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)-SZ and CATIE-AD studies
The majority of the subjects were White (n = 328) and African-Americans (n = 140). The rest of the patients comprised one Native Hawaiian, 12 Asian and five American-Indian individuals. The distribution of the risperidone sampling times (time after most recent dose) is shown in Figure 2.
Figure 2.
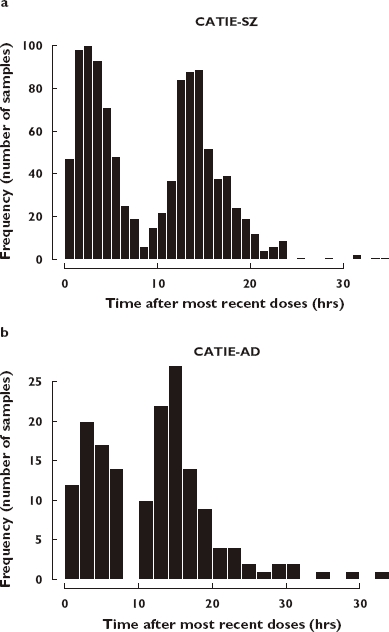
Frequency histogram showing the sampling distribution for risperidone sampling measurements in Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE)-SZ (a) and CATIE-AD (b) studies. The abscissa is broken into 1-h bins. The ordinate is the proportion of samples taken in each interval
Population PK modelling
Base model
The base risperidone and 9-OH risperidone model is a one-compartment model with first-order absorption and first-order elimination, parameterized in terms of apparent CL, volume of distribution for risperidone (V), first-order absorption rate constant (Ka), fraction of risperidone to 9-OH risperidone (KF), apparent clearance for 9-OH risperidone (CLM) and volume of distribution for 9-OH risperidone (VM). The model structure is shown schematically in Figure 3. Due to the identifiability problem associated with KF and VM, VM was set to the same value as V.
Figure 3.
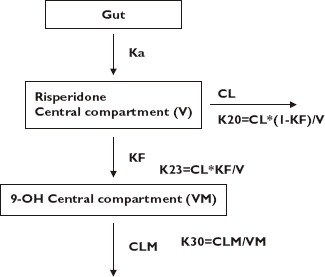
Base population pharmacokinetic model structure for risperidone and 9-OH risperidone, KF is the fraction of risperidone to 9-OH risperidone
Among the three PK disposition models described in Subjects and methods, the one-compartment model with mixture structure to distinguish CYP2D6 polymorphism-related subpopulations provided the best description of risperidone and 9-OH risperidone concentration data, based on the Akaike information criterion (AIC) [22] and diagnostic plots. The IIV on CL, V and CLM were described by a log-normal IIV model. Residual errors were separately estimated for parent and metabolite. The best residual error model was a combined additive and proportional model. The fraction of the risperidone oral clearance leading to formation of 9-OH risperidone (KF) in PM, IM and EM subpopulations were estimated. The model with estimation of all three parameters was unstable, including utilizing the literature published values (0.05, 0.2, 0.3 for PM, IM and EM, respectively) for the three groups, thus KF for subjects in the IM group was fixed to stabilize the model estimation of KF in the PM and EM populations.
The mixture model with two subpopulations (PM and EM) was also evaluated. The OFV was 186.6 points worse than the three-subpopulation mixture (AIC, P < 0.005). This suggested that the mixture model with three subpopulations (PM, EM and IM) significantly improved the goodness of fit.
Both FO and FOCE with interaction methods were tested during model building and confirmation. The models evaluated using the FOCEI method were unstable and significantly more computationally intensive. This may relate to the sparse nature of the data collected for this analysis. In addition, the FOCE Interaction models that did run relatively stably provided biased results with respect to the population and individual predictions. Thus, the FO method was selected for the analysis.
Final model
The model with a mixture structure was selected as the final base model and was used in the subsequent covariate model development. Covariates were screened one at a time, by assessing the strength of relationship between covariate and PK parameters for risperidone and 9-OH risperidone. Only age was identified as a statistically significant covariate on 9-OH risperidone clearance (CLM). The inclusion of age on CLM resulted in significant reduction in OFV at the 0.5% level (ΔOFV = −68.1; P < 0.005, d.f. = 1). The details of covariates selection during model development for risperidone and 9-OH risperidone is shown in Table 2.
Table 2.
Population pharmacokinetic model development (one-compartment linear model)
| Covariate | Model | -2LL | Δ-2LL | P-value |
|---|---|---|---|---|
| 1 | Base model (No mixture) | 6 111.639 | ||
| 2-1 | ||||
| CL | ||||
| Race | M1 | 6 009.777 | −101.862 | <0.005 |
| Sex | M2 | 6 101.284 | −10.355 | <0.005 |
| Age | M3 | 6 108.711 | −2.928 | >0.05 |
| Weight | M4 | 6 111.215 | −0.424 | >0.05 |
| Smoking status | M5 | 6 101.284 | −10.355 | <0.005 |
| CM-fluoxetine | M6 | 6 084.481 | −27.158 | <0.005 |
| CM-paroxetine | M7 | 6 101.568 | −10.071 | <0.005 |
| 2-2 | ||||
| V | ||||
| Race | M8 | 6 094.077 | −17.562 | <0.005 |
| Sex | M9 | 6 100.865 | −10.774 | <0.005 |
| Age | M10 | 6 107.901 | −3.738 | >0.05 |
| Weight | M11 | 6 104.669 | −6.97 | >0.005 |
| Smoking status | M12 | 6 111.103 | −0.536 | >0.05 |
| 3-1 (mixture model on CL M13) | ||||
| CL (mixture on CL two-component) | M13 | 5 289.626 | −822.013 | <0.005 |
| CL (mixture on CL three-component) | M14 | 5 103.056 | −1008.582 | <0.005 |
| CL(RACE) | M15 | 5 128.500 | 25.444 | >0.05 |
| CL(SEX) | M16 | 5 121.475 | 18.419 | >0.05 |
| CL(AGE) | M17 | 5 146.085 | 43.029 | >0.05 |
| CL(SMOK) | M18 | 5 145.838 | 42.782 | >0.05 |
| CL(CM-PAROXETINE) | M19 | 5 145.996 | 42.94 | >0.05 |
| CL(CM-FLUOXETINE) | M20 | 5 117.367 | 14.311 | >0.05 |
| 3-2 (Mixture on CL three-component with covariates on V) | ||||
| V (Race) | M21 | 5 145.845 | 42.789 | >0.05 |
| V (Sex) | M22 | 5 146.080 | 43.024 | >0.05 |
| V (Age) | M23 | 5 143.338 | 40.282 | >0.05 |
| V (Weight) | M24 | 5 101.473 | −1.583 | >0.05 |
| 3-3 base model parent and metabolite with mixture elimination for parent (as above) | ||||
| Base model – three-component mixture on CL of parent. | M25 | 12 469.226 | ||
| CLM (age) | M26 | 12 397.024 | −72.202 | <0.005 |
CM, concomitant medication; V, volume of distribution; Δ-2LL, objective function value (OFV) from covariate model minus base model; -2LL values in 2-1 and 2-2 were compared with base model; -2LL values of M13 and M14 in 3-1 were compared with model M1; whereas values of Model 15 to Model 24 in 3-1 and 3-2 were compared with 3-1 M14; values in 3-3 was compared with 3-2 M25. The incorporation of covariates is described in Subjects and methods.
The final PK parameter estimates for risperidone and 9-OH risperidone are shown in Table 3. Diagnostic plots are shown in Figure 4, including observed concentrations vs. population predicted risperidone and 9-OH risperidone concentrations (Figure 4a,e); observed concentrations vs. individual predicted risperidone and 9-OH risperidone concentrations (Figure 4b,f); weighted residual error (WRES) vs. population predicted risperidone and 9-OH risperidone concentrations (Figure 4c,g); and WRES vs. time after the most recent doses (Figure 4d,h).
Table 3.
Pharmacokinetic parameter estimates for the one-compartment mixture model
| Parameters | Parameter estimates | SE% |
|---|---|---|
| CL in PM (l h−1) | 12.9 | 6.5 |
| CL in EM (l h−1) | 65.4 | 9.9 |
| CL in IM (l h−1) (fixed) | 36 | NA |
| V, VM (l) | 444 | 17.8 |
| Ka (1 h−1) (fixed) | 1.7 | NA |
| CLM (l h−1) | 8.83 | 42.6 |
| Age on CLM | −0.378 | 34.7 |
| KF_PM | 0.96 | 42.8 |
| KF_EM | 0.595 | 40.0 |
| KF_IM (fixed) | 1 | NA |
| P1 | 41.2 | 8.1 |
| P2 | 52.4 | 6.2 |
| ωCL_PM% | 95.9 | 39.5 |
| ωCL_EM% | 56.6 | 16.8 |
| ωV,VM% | 36.1 | 24.4 |
| ωka% | 53.7 | 89.3 |
| σ1 for risperidone % | 63.9 | 12.5 |
| σ2 for 9-OH risperidone % | 37.9 | 35.4 |
| σ3 for risperidone (µg/l) | 4.29 | 104.9 |
| σ4 for 9-OH risperidone (µg/l) | 0.88 | 38.7 |
CL, risperidone clearance; V, risperidone volume of distribution of central compartment; CLM, 9-OH risperidone clearance; VM, 9-OH risperidone volume of distribution of central compartment; KF, fraction of risperidone to 9-OHrisperidone; P1, percentage of subjects who were PM; P2, percentage of subjects who were EM; SE, standard error; PM, poor metabolizer; IM, intermediate metabolizer; EM, extensive metabolizer; ω, coefficient of variation of interindividual variability; σ, coefficient of variation of residual error; N/A: not available.
Figure 4.
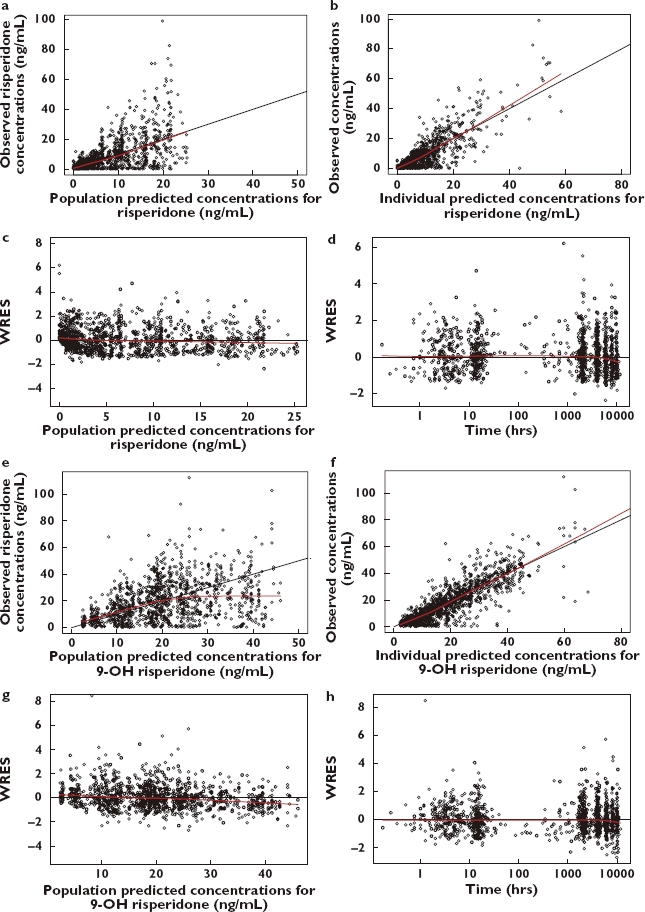
Diagnostic plots of final pharmacokinetic model. (a,e) Plot of observed concentrations vs. population predicted risperidone (a) and 9-OH risperidone (e) concentrations. Dots represent individual data points; solid line represents the unity line and dashed line represents the smooth line. (b,f) Plot of observed concentrations vs. individual population predicted risperidone (b) and 9-OH risperidone (f) concentrations. Dots represent individual data points; solid line represents the unity line and dashed line represents the smooth line. (c,g) Plot of weighted residual error (WRES) vs. population predicted risperidone (c) and 9-OH risperidone (g) concentrations. Dashed line represents the smooth line. (d,h) Plot of WRES vs. time after most recent dose for risperidone (d) and 9-OH risperidone (h). Dashed line represents the smooth line
Compared with the base model, the IIV on CLM decreased 6.3% in the final model. The population parameter estimates for CLM is 8.8 l h−1. The magnitude of age effect on CLM was >20%, suggesting age effect may be clinically relevant (covariate effect on CLM exceeded 20%) [23]. CLM decreases as age increases; the average CLM estimate in subject at 45 years old is 6.1 l h−1, decreased to 4.9 l h−1 in subject at 70 years old. The association between age and CLM is presented in Figure 5.
Figure 5.
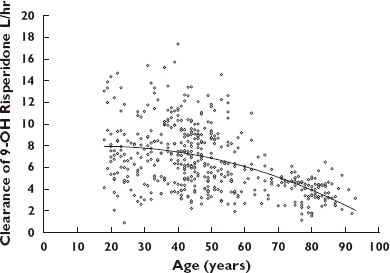
9-OH risperidone clearance (CLM) vs. age. Line represents the smooth line
The estimated risperidone CL in PM, EM and IM subjects was 12.9 l h−1, 65.4 l h−1 and 36 l h−1, the corresponding half-life estimates in these subpopulations were 25 h, 4.7 h and 8.5 h, respectively. The percentage of subjects in the PM and EM groups was estimated to be 41.2% and 52.4%. The distribution of clearance values is shown in Figure 6.
Figure 6.
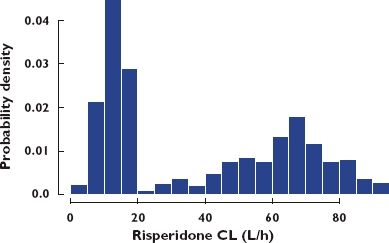
Histogram of risperidone clearance estimates (CL)
Discussion
Many variables are associated with the IIV of a drug's disposition. In this study, we successfully implemented a population PK analysis in the spirit of Krecic-Shepard et al. [24] and Kang et al. [25] using a small number of samples per subject in a large number of subjects to characterize the sources of variability in risperidone PK from the CATIE study. The risperidone and 9-OH risperidone concentrations appear to be adequately described by a one-compartment model with inclusion of a mixture model structure to estimate risperidone elimination separately in CYP2D6 polymorphism-related subpopulations.
Age was identified as a statistically significant and clinically relevant covariate (with a >20% effect) on 9-OH risperidone elimination (CLM). Elderly subjects taking oral risperidone had higher plasma concentrations than younger subjects [4]. Previously, we identified age as a significant covariate on the PK of citalopram [26]. Age was not a significant covariate on risperidone PK in this study; however, age had a significant effect on the elimination of the major active metabolite, 9-OH risperidone. The average CLM in subjects at age 45 is 6.1 l h−1. This decreases to 4.9 l h−1 in a typical 70-year-old subject. Creatinine clearance in this same age range for an individual weighing 70 kg and having a serum creatinine measurement of 1 mg dl−1 shows a reduction from 92 ml min−1 to 68 ml min−1, a decrease of approximately 25%. This is similar to the 20% decrease observed in the elimination of 9-OH risperidone across this age range. The result of the age effect on 9-OH risperidone elimination is consistent with previous reports [5, 27]. Thus, older individuals may have a higher exposure to the active metabolite of risperidone. This may be a significant contributor to adverse drug reactions in this population. An additional concern is that these higher exposures may potentially affect the safety of risperidone in elderly individuals, where a higher risk of death has been suggested in elders treated with risperidone based on a meta-analysis in this patient population [28].
Risperidone is mainly metabolized by CYP2D6. More than 80 allelic variants have been identified for the CYP2D6 gene among different ethnic populations [29]. These polymorphisms result in variable enzymatic activity [30, 31]. It has been reported that CYP2D6 polymorphisms influence paroxetine (CYP2D6 substrate and inhibitor) PK [32]. In this analysis, the standard one-compartment model without mixture model structure was first used as base model to describe concentrations. Race was a significant covariate on risperidone CL, when a mixture approach for clearance was not incorporated in the model [32, 33]. Race was no longer a significant covariate in the model with mixture structure, where risperidone CL was estimated separately in three different clearance subpopulations (possibly reflecting at least partially the CYP 2D6 metabolizer phenotype). These results suggest that, due to the differential expression of CYP2D6 phenotype across race, the use of the mixture analysis approach has accounted for the differences in CYP2D6 activity. In addition, these differences are not present when tested within each of the subpopulations.
Sex and concomitant medications (paroxetine and fluoxetine) were found to be significant covariates affecting risperidone oral clearance when the one-compartment PK model without the mixture model structure was used as base model for covariate screening. The first of these covariates can have two possible roles in contributing to clearance variability: sociological and biological. All three of these covariates are associated with differences reported in the literature of CYP2D6 activity, which might contribute to covariates effect on clearance. The race covariate effect on clearance here should be tempered with the other possible contributory factors. These include the CYP2D6 differences, but could also be due to systematic differences in adherence patterns in this group, thus resulting in a different clearance based on different dose-taking patterns. Snoeck et al. [5] have reported that renal function may influence risperidone and 9-OH risperidone concentrations, thus creatinine clearance (CrCL) could also be a potential covariate. CrCL was unavailable in this study and its effect on the PK parameters could therefore not be assessed.
Paroxetine and fluoxetine are potent CYP2D6 inhibitors. Saito et al. [10] have reported that risperidone concentrations during co-administration of paroxetine 10, 20 and 40 mg day−1 were 3.8-fold, 7.1-fold and 9.7-fold higher than before paroxetine co-administration in SZ patients. Spina et al. [8] have demonstrated that the levels of the active moiety (sum of the concentrations of risperidone and 9-OH-RISP) increased by 75% in SZ patients taking both risperidone and fluoxetine compared with baseline where patients did not receive fluoxetine. In this study, when the standard one-compartment model without mixture model structure was applied for covariate screening, both paroxetine and fluoxetine were significant covariates affecting risperidone CL. However, paroxetine and fluoxetine were not significant covariates in the mixture model, where risperidone CL was separately estimated in CYP2D6 polymorphism-related subpopulations. The POSTHOC estimates showed that subjects taking paroxetine or fluoxetine appeared to have lower risperidone clearance compared with those not taking co-medications. Moreover, the χ2 test was used to assess the difference between the proportion of individuals with a particular characteristic assigned to the PM, IM or EM groups. Significant differences in the proportion of individuals assigned to a particular metabolizer subpopulation were observed between the African-American and White populations (P < 0.001) and subjects with and without concomitant paroxetine (P < 0.002). The differences across sex and individuals taking fluoxetine in assignment to a metabolizer subpopulation were not significant (P = 0.055 for both conditions).
In the CATIE trials adherence was assessed in the AD arm of this study using a clinical impression metric. A better measure of adherence in these clinical trials would have improved estimation and probably decreased the residual error in the development of the population PK models. As shown in our results, potentially important differences in exposure between populations are present and may be due to variable adherence. In this study, the percentage of subjects in PM was estimated to be 41.2%. This is higher than the reported proportion of the population with this CYP 2D6 metabolizer genotype. If one considers that taking either paroxetine or fluoxetine changes an individual from an EM or IM elimination status to a PM status, then this group would be enriched by approximately 41 individuals. This is substantial (approximately 10% of the total number of individuals in the study), but still not sufficient to increase the PM group to the calculated proportion. This difference may be attributable to variable adherence or unreported concomitant interacting medications resulting in increased numbers of individuals assigned a PM elimination status.
The relative clearances reflecting the conversion of parent risperidone to the metabolite were 39 l h−1 (EM), 36 l h−1 (IM) and 12.3 l h−1 (PM). The fraction of the total clearance of the parent that corresponded to these clearance values was 0.6, 1 and 0.96, respectively. If the fraction of the overall clearances was fixed to values closer to the typically observed proportion of administered dose converted to the 9-OH metabolite of 0.3 (EM), 0.2 (IM) and 0.05–0.1 (PM), substantial worsening in model fitness was observed (>450 point increase in objective function). This worsening in the model included a substantial increase in the clearance rate associated with a KF of 0.05–0.1, making this group the extensive metabolizers.
In conclusion, a one-compartment model with mixture structure to estimate risperidone CL separately in three subpopulations comprising a PM, EM and IM group adequately described the concentrations data for risperidone and its active metabolite 9-OH risperidone. Age was identified as a significant covariate on 9-OH risperidone clearance, with elderly subjects having higher 9-OH risperidone levels.
Competing interests
R.R.B. has received funding from the National Institute of Mental Health, National Cancer Institute and the National Institute for Bioimaging and Bioengineering. B.G.P. has received grant/research support from the National Institute of Mental Health, Janssen Pharmaceutica, Forest Pharmaceuticals and GlaxoSmithKline. He is a consultant for Forest Pharmaceuticals, Janssen Pharmaceuticals, and GlaxoSmithKline. D.M. has received grant/research support from the National Institute of Mental Health, Forest Pharmaceuticals, Pfizer and Eli Lilly & Co. He is a consultant for Pfizer and Janssen Pharmaceutica.
Acknowledgments
This work has been supported by NIMH nos N01MH90001, MH64173, MH65416, MH52247; Sandra A. Rotman program in neuropsychiatry. The authors are extremely grateful to the investigators of the CATIE studies and the CATIE Executive Committee of Drs Lieberman, Stroup, Tariot, and Schneider for their essential contributions and support that resulted in the success of this study. We would also like to thank Ms Denise Sorisio for her careful analysis of the plasma drug concentrations for this project.
REFERENCES
- 1.Spina E, Avenoso A, Facciola G, Salemi M, Scordo MG, Ancione M, Madia AG, Perucca E. Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl) 2001;153:238–43. doi: 10.1007/s002130000576. [DOI] [PubMed] [Google Scholar]
- 2.Chouinard G, Arnott W. Clinical review of risperidone. Can J Psychiatry. 1993;38(Suppl 3):S89–95. [PubMed] [Google Scholar]
- 3.Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1988;247:661–70. [PubMed] [Google Scholar]
- 4.Aichhorn W, Weiss U, Marksteiner J, Kemmler G, Walch T, Zernig G, Stelzig-Schoeler R, Stuppaeck C, Geretsegger C. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19:395–401. doi: 10.1177/0269881105053306. [DOI] [PubMed] [Google Scholar]
- 5.Snoeck E, Van Peer A, Sack M, Horton M, Mannens G, Woestenborghs R, Meibach R, Heykants J. Influence of age, renal and liver impairment on the pharmacokinetics of risperidone in man. Psychopharmacology (Berl) 1995;122:223–9. doi: 10.1007/BF02246543. [DOI] [PubMed] [Google Scholar]
- 6.Fang J, Bourin M, Baker GB. Metabolism of risperidone to 9-hydroxyrisperidone by human cytochromes P450 2D6 and 3A4. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:147–51. doi: 10.1007/pl00005334. [DOI] [PubMed] [Google Scholar]
- 7.He H, Richardson JS. A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5-HT2 and dopamine D2 receptors. Int Clin Psychopharmacol. 1995;10:19–30. doi: 10.1097/00004850-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Spina E, Avenoso A, Scordo MG, Ancione M, Madia A, Gatti G, Perucca E. Inhibition of risperidone metabolism by fluoxetine in patients with schizophrenia: a clinically relevant pharmacokinetic drug interaction. J Clin Psychopharmacol. 2002;22:419–23. doi: 10.1097/00004714-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Wang JS, DeVane CL, Gibson BB, Donovan JL, Markowitz JS, Zhu HJ. Population pharmacokinetic analysis of drug–drug interactions among risperidone, bupropion, and sertraline in CF1 mice. Psychopharmacology (Berl) 2006;183:490–9. doi: 10.1007/s00213-005-0209-y. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Yasui-Furukori N, Nakagami T, Furukori H, Kaneko S. Dose-dependent interaction of paroxetine with risperidone in schizophrenic patients. J Clin Psychopharmacol. 2005;25:527–32. doi: 10.1097/01.jcp.0000185428.02430.c7. [DOI] [PubMed] [Google Scholar]
- 11.de Leon J, Susce MT, Pan RM, Fairchild M, Koch WH, Wedlund PJ. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66:15–27. doi: 10.4088/jcp.v66n0103. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A, Piotrovsky V, Ludwig E. Population pharmacokinetics of risperidone and 9-hydroxyrisperidone in patients with acute episodes associated with bipolar I disorder. J Pharmacokinet Pharmacodyn. 2007;34:183–206. doi: 10.1007/s10928-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 13.Aarons L. Population pharmacokinetics: theory and practice. Br J Clin Pharmacol. 1991;32:669–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Swartz MS, Perkins DO, Stroup TS, McEvoy JP, Nieri JM, Haak DC. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull. 2003;29:33–43. doi: 10.1093/oxfordjournals.schbul.a006989. [DOI] [PubMed] [Google Scholar]
- 15.Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29:15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- 16.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Leiberman JA Catie-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355:1525–38. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 17.Aravagiri M, Marder SR, Van Putten T, Midha KK. Determination of risperidone in plasma by high-performance liquid chromatography with electrochemical detection: application to therapeutic drug monitoring in schizophrenic patients. J Pharm Sci. 1993;82:447–9. doi: 10.1002/jps.2600820503. [DOI] [PubMed] [Google Scholar]
- 18.Sheiner LB, Rosenberg B, Marathe VV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5:445–79. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 19.Beal B, Sheiner L. (1989–1998) NONMEM User's Guide, Part I. San Francisco, CA: University of California San Francisco; 1992. [Google Scholar]
- 20.Ette EI, Ludden TM. Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res. 1995;12:1845–55. doi: 10.1023/a:1016215116835. [DOI] [PubMed] [Google Scholar]
- 21.Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–52. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 22.Akaike H. A new look at the statistical model indentification. IEEE Trans Automat Control. 1974;AC-19:716–23. [Google Scholar]
- 23.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;56:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krecic-Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, Schwartz JB. Race and sex influence clearance of nifedipine: results of a population study. Clin Pharmacol Ther. 2000;68:130–42. doi: 10.1067/mcp.2000.108678. [DOI] [PubMed] [Google Scholar]
- 25.Kang D, Verotta D, Krecic-Shepard M-E, Modi NB, Gupta SK, Schwartz JB. Population analysis of sustained-release verapamil in patients: effects of sex, race and smoking. Clin Pharmacol Ther. 2003;73:31–40. doi: 10.1067/mcp.2003.21. [DOI] [PubMed] [Google Scholar]
- 26.Bies RR, Feng Y, Lotrich FE, Kirshner MA, Roose S, Kupfer DJ, Pollock BG. Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. J Clin Pharmacol. 2004;44:1352–9. doi: 10.1177/0091270004269647. [DOI] [PubMed] [Google Scholar]
- 27.Mannens G, Huang ML, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos. 1993;21:1134–41. [PubMed] [Google Scholar]
- 28.Schneider L, Dagerman K, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 29.Human Cytochrome P450 (CYP) Allele Nomenclature Committee. [last updated: 21 May 2008]. Available at http://www.cypalleles.ki.se.
- 30.Kagimoto M, Heim M, Kagimoto K, Zeugin T, Meyer UA. Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem. 1990;265:17209–14. [PubMed] [Google Scholar]
- 31.Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002;3:229–43. doi: 10.1517/14622416.3.2.229. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Pollock BG, Ferrell RE, Kimak MA, Reynolds CF, III, Bies RR. Paroxetine: population pharmacokinetic analysis in late-life depression using sparse concentration sampling. Br J Clin Pharmacol. 2006;61:558–69. doi: 10.1111/j.1365-2125.2006.02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Pollock B, Reynolds C, Bies R. Paroxetine pharmacokinetics in geriatric patients. [last accessed: 5 August 2008];AAPS PharmSci. 2004 6(S1) Abstract M1116. Available at http://www.aapsj.org/abstracts/AM_2004/AAPS2004-003172.pdf. [Google Scholar]


