Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
In parts of South-East Asia, Tincture of Opium (TOP) is a culturally acceptable alternative to methadone in the treatment of opioid withdrawal.
We have previously shown that TOP has a therapeutic effect when administered to opioid-dependent patients in Northern Thailand.
However, before TOP could be used clinically, an evaluation of the dosing regimen had to be undertaken.
WHAT THIS STUDY ADDS.
This study extends the previous study by evaluating the clinical effectiveness of a dosing regimen in which the TOP dose was adjusted according to prior self-reported opium use.
Adequate suppression of withdrawal can be achieved, with minimal side-effects, in opioid-dependent individuals receiving flexible dosing of TOP.
This study provides preliminary evidence for the effectiveness of TOP in the management of opioid withdrawal.
AIMS
The aim was to evaluate the clinical effectiveness, pharmacodynamics and pharmacokinetics of a range of Tincture of Opium (TOP) doses in the management of opioid withdrawal.
METHODS
Forty-five opium-dependent Thai subjects were allocated to three dosing groups (6.66, 13.3 and 20 mg morphine equivalents, twice daily) depending on their self-reported prior opium use. On day 5 of dosing subjects underwent an interdosing interval study where blood, withdrawal scores, heart rate and blood pressure (BP) were collected at 0, 1, 3 and 8 h. Plasma morphine concentrations were quantified by high-performance liquid chromatography, and plasma morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) concentrations by LCMS.
RESULTS
Thirty-two subjects completed the study. Withdrawal scores were low for all subjects (range 9–23% of maximum response). There were dose-dependent changes in both systolic and diastolic BP (P = 0.021 and P = 0.01, respectively), but these were not considered clinically significant. There were no effects of dose on respiratory rate. Plasma morphine concentrations changed significantly across the interdosing interval (P = 0.0001), rising to a maximum at 1 h after dosing. Plasma morphine concentrations also differed according to dose (P < 0.05). The mean ratios of the morphine glucuronides were found to be: M3G/M6G = 7.7, M3G/morphine = 35.6 and M6G/morphine = 4.9, values comparable to those previously reported.
CONCLUSION
The management of opioid withdrawal can be achieved, with minimal adverse effects, by using flexible dosing of TOP.
Keywords: opioid dependence, pharmacodynamics, pharmacokinetics, Thailand, tincture of opium
Introduction
A primary objective of maintenance treatment for opioid dependence is to achieve adequate suppression of withdrawal across the interdosing interval, without causing unacceptable side-effects. The failure to suppress withdrawal signs and symptoms adequately between dosing can lead to ongoing drug use and treatment drop-out [1, 2]. Globally, methadone is the principal pharmacotherapy utilized in the treatment of opioid dependence [3]. It has demonstrated efficacy in improving the physical and psychological health of those in treatment [4–8], but in some parts of South-East Asia the cost of methadone is a barrier to its widespread use [9]. Hence, there is a need to investigate alternative opioid pharmacotherapies that will be both locally acceptable and effective in the treatment of opioid dependence. One such alternative is Tincture of Opium (TOP), which is used in some Asian countries for the management of opioid withdrawal and, less commonly, as a maintenance treatment [10]. This is a preparation of opium in alcohol and water that in pharmaceutical preparation is standardized to contain 1% morphine. TOP is perceived to be a traditional medicine in some parts of South-East Asia and so appears to be culturally acceptable, and its low cost is an added advantage over methadone [9].
Few studies have investigated the effectiveness and side-effect profile of TOP in the management of opioid dependence and/or opioid withdrawal. In an early study Auriacombe and colleagues [11] demonstrated the effectiveness of TOP (10–15 g day−1 equivalent to about 100–150 mg morphine) in producing positive health outcomes in a group of six treatment-resistant heroin-dependent patients. Following >16 months of maintenance treatment, patients exhibited significant improvements in physical and psychological health, and socio-economic status [11]. Furthermore, TOP is also commonly used in the treatment of neonatal opioid withdrawal (neonatal abstinence syndrome) [12], and there have been recent reports of its relative efficacy and side-effect profile. For example, Coyle and colleagues noted the increased efficacy of treating neonatal abstinence syndrome with diluted TOP in combination with phenobarbital, rather than with dilute TOP alone [13]. In addition, Langenfield and coworkers have highlighted the problematic effects of various alkaloids and alcoholic extracts present in the TOP mixture on infants [14].
We recently reported on the effectiveness of TOP in comparison with oral racemic-methadone in 15 opioid-dependent patients from Northern Thailand [9]. This was an open-label, parallel-group study in which 15 heroin-dependent patients, maintained on methadone 5–20 mg day−1, were compared with 15 dependent opium smokers who received TOP (3.33–10 mg morphine equivalents) 12 hourly. At steady state they participated in an interdosing study in which blood, withdrawal scores and subjective opioid effect measures were collected predose and 1, 3 and 8 h following dosing. For the methadone group results showed a reciprocal relationship between withdrawal scores and plasma methadone concentrations. Direct subjective opioid effect (e.g. ‘drug effect’) scores were directly related to underlying plasma racemic-methadone concentrations. For the TOP group there were significant time-related changes for subjective effects (withdrawal, drug effect), but these were not related to underlying plasma morphine concentrations. Importantly, withdrawal scores were greater in patients receiving TOP than racemic-methadone, indicating less withdrawal suppression.
Although the latter data provide preliminary evidence for the effectiveness of TOP in this patient group, it appears that the dosages used, and the subsequent plasma morphine concentrations achieved, were too low to suppress withdrawal adequately. Therefore, the primary aim of this study was to investigate the clinical effectiveness of a range of dosages to determine if TOP can be used to suppress opioid withdrawal effectively, without causing unacceptable side-effects, in opioid-dependent patients. A subsidiary aim was to examine the pharmacokinetics of morphine following oral administration of different doses of TOP to patients dependent on opium. Furthermore, given recent research by Antonilli and colleagues [15] that suggested the plasma concentration ratios of morphine-3-glucuronide (M3G) to morphine-6-glucuronide (M6G) may be higher in intravenous heroin users compared with nonheroin users, and as M6G is a potent opioid agonist, we were also interested in confirming this report.
Methods
Subjects and experimental design
This open-label study was approved by the Royal Adelaide Hospital Research Ethics Committee (Adelaide, Australia) and the Thai Ministry of Public Health (Bangkok, Thailand). Subjects were recruited from the Northern Drug Dependence Treatment Centre, Chiang Mai, Thailand. Subjects provided oral informed consent, as many were not literate.
Forty-five Thai subjects dependent on opium were recruited, with no subject having to withdraw. Initially, subjects were randomly allocated to the following three treatment groups; Group I (dose 1) 10 ml of TOP mixture (6.66 mg morphine equivalents); Group II (dose 2) 20 ml of TOP mixture (13.3 mg morphine equivalents); Group III (dose 3) 30 ml of TOP mixture (20 mg morphine equivalents), twice a day for all groups. However, those subjects who reported using relatively small amounts of opium prior to starting treatment experienced sedation when given 20 and 30 ml of TOP, and so were reallocated to the lower dosage group for safety reasons. Hence, the lower dose group contained all lower level users, but also a few higher level users. Thus, a flexible dosing regimen was used, where the TOP dose was adjusted according to patients’ self-reported prior opium smoking use as amount per day.
No patient had reported taking medications known to alter the pharmacokinetics of morphine, or had significant medical illness (including positive HIV serology), and none was pregnant or breast-feeding. Each subject was studied for an 8-h period during the 12-h interdosing interval study and was required to be at steady state (unchanged dose for ≥4 days) prior to participating.
Procedures and measures
On day 5 of maintenance of TOP dosing, each subject underwent an interdosing interval study. At the commencement of testing an intravenous cannula was inserted into a suitable forearm vein. A 10-ml blood sample was collected prior to dosing and then at 1, 3 and 8 h after dosing. Plasma was separated from blood and stored at −20°C until later analysis. Pharmacodynamic measures were taken at the same time as blood sampling, and included: (i) heart rate (radial pulse palpation), blood pressure (BP) (measured manually using a sphygmomanometer) and respiration rate (measured by direct observation of the patient); and (ii) the Methadone Symptoms Checklist [16]. This lists 16 withdrawal symptoms (e.g. nervousness, feeling of coldness, aches and pains, craving, headache, stomach cramps, hot flushes, runny nose). Subjects recorded each symptom as present or absent to yield an overall measure of withdrawal (range of possible scores = 0–16).
The plasma samples (stored on dry ice) were freighted to the Discipline of Pharmacology, University of Adelaide, South Australia, for analysis.
Drugs
TOP was administered to patients 12 hourly. This was formulated as Tincture Gential Co 6 ml, Berberies Extract 6 ml, Tincture of Opium 6 ml (containing 1% anhydrous morphine), peppermint spirit 1.8 ml and purified water to 90 ml. This mixture contained 10 mg of anhydrous morphine in 15 ml. The formulation was produced by the Quality Assurance Department of the Government Pharmaceutical Organization, Bangkok, Thailand. Routine high-performance liquid chromatography (HPLC) analysis was carried out to verify that the concentration of morphine in the TOP used was within 5% of the designated dosages.
Analytical methods
Morphine concentrations were quantified in plasma using a HPLC-electrochemical detection method described previously [17]. This assay was accurate and reproducible. High (20 ng ml−1) and low (2 ng ml−1) quality control samples were assayed with each subject's set of plasma samples. Inter- and intraday inaccuracy and imprecision of the quality control samples were <16%. Using this method the lower limit of quantification was 1 ng ml−1.
Plasma M3G and M6G concentrations were subsequently assayed by a modified [18] Liquid Chromatographic-Mass Spectrometry (LCMS) method. The clean up of the plasma samples was via the use of Sep-Pak cartridges as reported by Milne et al. [18] using dihydromorphine as the internal standard. The samples were separated using a Luna 5-µm C18 column and a mobile phase of methanol:50 mm ammonium formate : formic acid (25:475:2.75). The MS detector (Shimadzu LCMS-2010A) was set to the electrospray ionization probe and monitored in secondary ion mass-positive ion mode at 462.15 atomic mass units (amu) (M6G and M3G), 286.15 amu (morphine) and 288.15 amu (dihydromorphine internal standard). Retention times were 4.6 min (M3G), 7.7 min (M6G), 8.8 min (morphine) and 8.0 min (dihydromorphine). The calibration curve ranged between 10 and 1000 ng ml−1 with low- and high-quality controls of 40 and 400 ng ml−1, respectively. For M3G the intra-assay imprecision and accuracy were (n = 6) <7 and >95%, respectively, whereas the interassay imprecision and accuracy (n = 3) were 19 and >93%, respectively. For M6G the intra-assay imprecision and accuracy for M6G were <13 and >94%, respectively, and the interassay imprecision and accuracy were <13 and >91% at 40 and 400 ng ml−1.
Analyses
Pharmacokinetic calculations
Pharmacokinetic parameters were derived using noncompartmental methods. The area under the plasma morphine concentration–time curve from 0 to 8 h (AUC) (ng ml−1 h−1) was determined using the linear trapezoidal method (GraphPad Prism 4.01; GraphPad Software Inc., San Diego, CA, USA). The plasma morphine concentration predose (C0) and at maximum measured concentration (Cmax) for each dose were obtained by visual inspection of individual data. M3G and M6G concentrations were determined in plasma at 0, 1 and/or 3 h to increase the likelihood that the analytes could be quantified.
Statistical and other analyses
One-way analysis of variance (anova) was used to determine differences between groups for demographic data (age, body weight and self-reported prior daily opium use), pharmacokinetic parameters derived from plasma morphine concentrations (C0, Cmax and AUC), and for plasma M3G and M6G concentrations. Two-way anova was used to analyse changes in plasma morphine concentrations with time (0, 1, 3, 8 h) as the within-subjects factor and dose (10, 20, 30 ml) as the between-subjects factor.
Pharmacodynamic data (diastolic and systolic BP, heart rate) were analysed using a repeated measure mixed model of covariance (ancova), with time as the repeated measure, prior self-reported daily opium use as a covariate, and plasma morphine concentration as a time varying covariate. Log transformations of the withdrawal and respiration data were required to meet underlying assumptions of normality and equal variance for the mixed model anova. The latter analysis was carried out using SAS version 8.2 (SAS Institute Inc., Cary, NC, USA). Bonferonni post hoc comparisons were made between levels of significant effects. Statistical significance was set at P < 0.05 and data are presented as mean ± SD. Where appropriate, 95% confidence intervals (CIs) of difference between means were also presented. Unless otherwise stated, all statistical analyses were performed using GraphPad Prism.
Results
The patient's demographic details are presented in Table 1. In total, 32 subjects (31 male and one female) completed the requirements of the interdosing study, with transcription errors and incomplete blood samples occurring in 13 of the original 45 subjects. Thirteen subjects received 10 ml of TOP (Group I), eight received 20 ml (Group II) and 11 subjects 30 ml (Group III), all twice daily. Patients’ ages ranged between 18 and 53 years. There were differences between groups for prior daily opium use (F2,29 = 20.41, P < 0.0001), so that subjects in Groups 2 and 3 self-reported significantly greater daily opium use than those in Group 1 (P < 0.001). There were no other between group differences for any other demographic variable (P > 0.05).
Table 1.
Demographic details for subjects participating in the interdosing interval study while maintained on oral Tincture of Opium according to the dose (10, 20 or 30 ml) of Tincture of Opium prescribed
| Group I | Group II | Group III | |
|---|---|---|---|
| Group | 10 ml Tincture of Opium | 20 ml Tincture of Opium | 30 ml Tincture of Opium |
| n in group | 13 | 8 | 11† |
| Age, years (range) | 40 ± 11 (23–53) | 38 ± 6 (33–50) | 37 ± 8 (33–45) |
| Body weight, kg (range) | 50 ± 5 (42–62) | 52 ± 7 (44–62) | 52 ± 4 (47–58) |
| Prior self-reported daily opium use, g day−1 (range) | 25 ± 24 (4–80) | 73 ± 15 (40–80)* | 78 ± 25 (30–120)* |
P < 0.001 significant differences between Group II and III vs. Group I. Bonferonni post hoc test used to compare self-reported daily opium use with Group 1. Data are mean ± SD.
Group III included one female subject.
Plasma morphine concentrations
Figure 1 shows the mean plasma morphine concentrations for all patients participating in the interdosing interval study. The plasma morphine concentration at baseline for five patients in Group 1, one in Group 2 and one in Group 3 was below the lower limit of quantification. Plasma morphine concentrations showed significant changes over time (P < 0.0001), rising to a maximum at 1 h (range 5–47 ng ml−1 for Group 1; 9–28 ng ml−1 for Group 2; and 9–37 ng ml−1 for Group 3) post dosing and then declining rapidly to a minimum at 8 h following the ingestion of all three doses. There were significant differences between the plasma concentration–time profiles produced by the three doses of TOP (P = 0.024). Specifically, post hoc comparisons showed that the plasma morphine concentration was significantly greater at 3 h post dosing for subjects taking 30 ml of TOP compared with that obtained after taking 10 ml TOP (P < 0.05).
Figure 1.
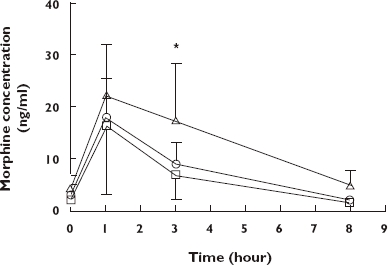
Mean plasma morphine concentration–time profiles for 32 patients maintained on twice-daily Tincture of Opium (TOP). Thirteen patients received 10 ml (dose 1), eight patients 20 ml (dose 2) and 11 patients 30 ml (dose 3) of TOP twice daily. TOP was administered at time zero. Values are mean ± SD. *P < 0.05 significant difference between dose 1 and dose 3. Dose 1 ( ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( )
)
The mean pharmacokinetic data for all patients are provided in Table 2. There was a large range of interindividual variation (see % CV) for all parameters presented. The time to maximum plasma morphine concentration (Cmax) was 1 h for all three groups. AUC for plasma morphine concentration differed significantly between groups (P = 0.0085), with the AUC for those receiving 30 ml TOP (Group 3) being higher than for those receiving 10 ml TOP (Group I) (P < 0.01). There were no other differences in pharmacokinetic parameters.
Table 2.
Mean ± SD pharmacokinetic values for morphine in patients following dosing with 10, 20 and 30 ml Tincture of Opium
| Group I | Group II | Group III | |
|---|---|---|---|
| Parameter | 10 ml Tincture of Opium | 20 ml Tincture of Opium | 30 ml Tincture of Opium |
| Co (ng ml−1) | 2.2 ± 2.3 (104) | 3.0 ± 2.2 (73) | 4.3 ± 2.6 (60) |
| Cmax(ng ml−1) | 16.5 ± 13 (79) | 18.9 ± 7.7 (41) | 23.3 ± 9.7 (42) |
| AUC (ng ml−1 h−1) | 3237 ± 1976 (61) | 3822 ± 1643 (43) | 6727 ± 3278 (49)* |
Co, plasma morphine concentration predose; Cmax, maximum plasma morphine concentration; AUC, area under the plasma concentration–time curve. For all groups Tmax was 1 h after dosing.
P < 0.001 significant difference between Group II and III and Group I. Bonferonni post hoc test used to compare self-reported daily opium use with Group 1. Data are mean ± SD (CV%).
Plasma M3G and M6G concentrations
Plasma M3G concentrations ranged from 78 to 285 ng ml−1 (Group 1), 89 to 525 ng ml−1 (Group 2) and 69 to 710 ng ml−1 (Group 3); for plasma M6G concentrations ranges were 11–31 ng ml−1 (Group 1), 11–47 ng ml−1 (Group 2) and 13–57 ng ml−1 (Group 3). The mean ± SD plasma M3G to morphine concentration ratio was 35.6 ± 19.2, M6G to morphine 4.9 ± 2.0 and M3G to M6G 7.7 ± 3.4. The mean ± SD M6G/M3G ratio was 0.16 ± 0.07.
Physiological measures
The effect of different doses of TOP in patients is shown for BP and respiration in Figures 2 and 3, respectively. There were few observable changes in either diastolic or systolic BP for each dose of TOP (Figure 2). However, following adjustment for prior daily opioid use and plasma morphine concentrations (ancova), there were significant effects of dose for both diastolic (F2,86 = 4.06, P = 0.021) and systolic BP (F2,86 = 4.86, P = 0.01), but not for time (P > 0.05). Post hoc analyses showed that the mean systolic BP for Group 1 (10 ml) was significantly lower than for Group 2 (20 ml) (95% CI −23.18, −5.04; P = 0.003). Mean diastolic BP was significantly lower for Group 1 than for Group 2 (95% CI −20.06, −1.16; P = 0.03), and also for Group 3 than for Group 2 (95% CI 1.96, 16.93; P = 0.014). In contrast, there were no significant effects of either time (F3,86 = 2.35, P = 0.08) or dose (F2,86 = 0.91, P = 0.41) on heart rate.
Figure 2.
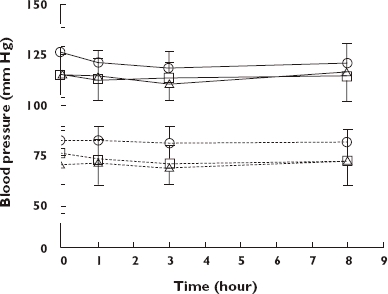
Mean ± SD systolic blood pressure (SBP) and diastolic blood pressure (DBP) (mmHg) in patients maintained on either 10 ml (dose 1), 20 ml (dose 2) or 30 ml (dose 3) of Tincture of Opium twice daily. SBP (Dose 1) ( ); SBP (Dose 2) (
); SBP (Dose 2) ( ); SBP (Dose 3) (
); SBP (Dose 3) ( ); DBP (Dose 1) (
); DBP (Dose 1) ( ); DBP (Dose 2) (
); DBP (Dose 2) ( ); DBP (Dose 3) (
); DBP (Dose 3) ( )
)
Figure 3.
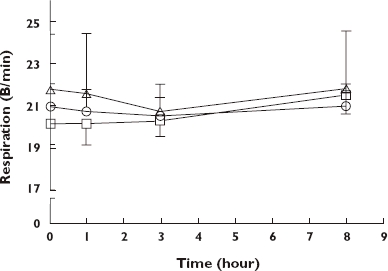
Mean ± SD respiratory rate (breaths per minute) for patients maintained on either 10 ml (dose 1), 20 ml (dose 2) or 30 ml (dose 3) of Tincture of Opium twice daily. Dose 1 ( ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( )
)
There were few observable changes across the interdosing interval for respiration, with most values ranging between 20 and 22 per minute (Figure 3). Repeated measures ancova showed that the effect of time on respiratory rate was significant (F3,86 = 3.67, P = 0.0014), with mean respiratory rates at 1 h and at 3 h post dosing significantly lower than those at 8 h post dosing (95% CI −0.082, −0.002; P = 0.04; and −0.08, −0.02; P = 0.0014, respectively).
Withdrawal
Withdrawal scores for all patients, according to dose of TOP administered, are shown in Figure 4a. Repeated measures ancova, controlling for prior opioid use and plasma morphine concentrations, revealed significant effects of time (F3,86 = 2.89, P = 0.0398), but not dose (F2,86 = 1.81, P = 0.1696). Log withdrawal scores adjusted for prior daily use and plasma morphine concentration at each time point for all subjects are shown in Figure 4b. Log withdrawal scores were greater at 1 h after dosing than 3 h (95% CI 0.18, 1.46; P = 0.013).
Figure 4.
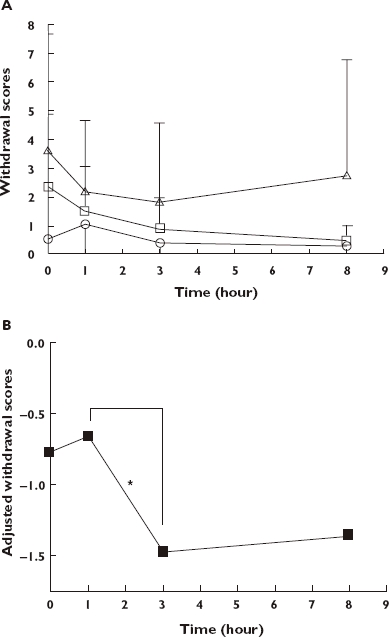
(A,B) Withdrawal scores for 32 patients maintained on twice-daily Tincture of Opium. Thirteen patients received 10 ml (dose 1), eight patients 20 ml (dose 2) and 11 patients 30 ml (dose 3) of Tincture of Opium twice daily. (A) Mean ± SD withdrawal scores (maximum score 16). Dose 1 ( ); Dose 2 (
); Dose 2 ( ); Dose 3 (
); Dose 3 ( ) (B) Log withdrawal scores adjusted for prior daily opium use and plasma morphine concentration at each time point for all subjects. *P < 0.05; time point 1 h vs. 3 h
) (B) Log withdrawal scores adjusted for prior daily opium use and plasma morphine concentration at each time point for all subjects. *P < 0.05; time point 1 h vs. 3 h
Discussion
In many developed nations, the substitution drug of choice for the maintenance treatment of opioid dependence is methadone. However, in some parts of South-East Asia the cost of methadone precludes its use and TOP is more culturally acceptable. We have previously shown that TOP has a therapeutic effect, but that the dosing regimen had to be further investigated before it could be used clinically [9]. Therefore, the current study evaluated the efficacy of a range of TOP doses (6.66, 13.3 and 20 mg morphine equivalents) in suppressing opioid withdrawal in patients who were dependent on opium. The main finding was that self-reported opioid withdrawal scores were low (6–23% of maximum) for all patients in the study. It is noteworthy that maximum withdrawal scores for patients in this study were substantially lower than for patients who participated in our previous study (mean ± SD 9.1 ± 3.0) and who received smaller doses of TOP (3.33–10 mg morphine equivalents; [9]). Therefore, these data suggest that patients in the current study were experiencing adequate withdrawal suppression with the TOP doses administered.
Although patients were initially allocated to the three different dosage groups based on their self-reported prior opium use, some patients experienced sedation when they received the higher dosages and so had to be re-allocated to the lower dosage group. Thus, a flexible approach to dosing had to be adopted. Although this might be considered a limitation of the study, it does make the results more valid and reflects clinical practice.
An important consideration when evaluating the suitability of pharmacotherapy for the treatment of opioid dependence is that it effectively suppresses withdrawal across its interdosing interval without causing significant adverse effects. In the current study there were significant, although small dose-related differences in systolic and diastolic BP at the TOP dosages used. Furthermore, after controlling for prior opioid use and plasma morphine concentrations, there were significant fluctuations in respiratory rate across the interdosing interval. However, given the small magnitude of these changes it is unlikely that they would be considered clinically significant. Furthermore, the excessive sedation reported by patients who had relatively low prior opium use, and who were allocated to higher dose groups, soon resolved when these patients were administered the lowest dose. Consistent with these findings, there were no reports of adverse effects in subjects taking TOP in our previous study [9].
There are relatively few published data on the plasma morphine concentrations that result from the dosages of oral morphine used in this study (i.e. 20 mg of oral morphine or less). Sawe and colleagues [19] have examined the disposition of 20–30 mg oral morphine in chronic cancer sufferers and reported Cmaxvalues of 37.8–112 ng ml−1at 10–120 min post dosing. Patients in the current study, who received 20 mg morphine equivalent (30 ml TOP), achieved Cmax values of 9.30–36.6 ng ml−1. Importantly, patients in the former study were suffering chronic cancer and therefore the effect of malignant disease and catabolism may have altered the disposition of morphine to produce greater plasma morphine concentrations than would be expected.
Consistent with previous literature that has examined the pharmacokinetics of orally administered morphine, we found considerable interindividual variability in plasma morphine concentrations in subjects receiving the same dose of TOP [19, 20]. Coefficients of variation ranged from 40 to >100% for reported pharmacokinetic values. This variability is likely to affect therapeutic efficacy. Furthermore, using LCMS, plasma morphine-3- and morphine-6-glucuronides were quantifiable from all but one patient. The mean M3G/M6G ratio and ratios of each glucuronide to morphine obtained in this study are comparable to those previously reported in the literature [21–24]. Furthermore, the mean ratio of M6G/M3G for patients in this study was lower than that found in the intravenous heroin users, but comparable to nonheroin users, studied by Antonilli and colleagues [15]. The latter authors suggested that intravenous heroin users are exposed to an array of contaminants, such as heroin itself, and heavy metals in cigarette smoke (such as cadmium), that are likely to inhibit M3G glucuronidation and that may result in relatively greater plasma M6G concentration [15]. We did not collect details of drug use, other than opium, for patients participating in this study, and so are unable to speculate on their likely effect on the rate of glucuronidation. Furthermore, the co-variation between plasma M6G concentrations and withdrawal scores could not be explored in this study, as plasma glucuronide concentrations were determined only in a limited number of plasma samples.
In conclusion, our main finding is that adequate suppression of withdrawal can be achieved, with minimal side-effects, in opioid-dependent individuals receiving flexible dosing of TOP. Thus, this study has provided preliminary evidence for the effectiveness of TOP in the management of opioid withdrawal. Given that TOP is a culturally acceptable alternative to methadone, which is likely to attract opioid-dependent individuals into treatment, it provides an important alternative medication for opioid withdrawal.
Acknowledgments
We thank staff at the Northern Drug Dependence Centre, Chiang Mai, Northern Thailand for data collection. We also thank Andrew Menelaou and David Foster for technical assistance in drug and metabolite assays; Justin Lokhorst at the Department of Public Health, University of Adelaide, for statistical advice; and David Newcombe, Discipline of Pharmacology, University of Adelaide, for finalizing the manuscript. The study was financially supported by the investigators’ institutions.
REFERENCES
- 1.Holmstrand J, Anggård E, Gunne LM. Methadone maintenance: plasma levels and therapeutic outcome. Clin Pharmacol Ther. 1978;23:175–80. doi: 10.1002/cpt1978232175. [DOI] [PubMed] [Google Scholar]
- 2.Schall U, Pries E, Katta T, Kloppel A, Gastpar M. Pharmacokinetic and pharmacodynamic interactions in an outpatient maintenance therapy of intravenous heroin users with levomethadone. Addict Biol. 1996;1:105–13. doi: 10.1080/1355621961000124736. [DOI] [PubMed] [Google Scholar]
- 3.Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–6. doi: 10.1016/S0140-6736(98)05356-2. [DOI] [PubMed] [Google Scholar]
- 4.Bell J, Mattick R, Hay A, Chan J, Hall W. Methadone maintenance and drug-related crime. J Subst Abuse. 1997;9:15–25. doi: 10.1016/s0899-3289(97)90003-1. [DOI] [PubMed] [Google Scholar]
- 5.Grøndbladh L, Ohlund LS, Gunne LM. Mortality in heroin addiction: impact of methadone treatment. Acta Psychiatr Scand. 1990;82:223–7. doi: 10.1111/j.1600-0447.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 6.Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann NY Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 7.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behaviour and criminality: a meta-analysis. Addiction. 1998;93:515–32. doi: 10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- 8.Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18 month prospective follow-up. J Acquir Immune Defic Syndr. 1993;6:1049–56. [PubMed] [Google Scholar]
- 9.Jittiwutikarn J, Ali R, White J, Bochner F, Somogyi AA, Foster DJ. Comparison of tincture of opium and methadone to control opioid withdrawal in a Thai treatment centre. Br J Clin Pharmacol. 2004;58:536–41. doi: 10.1111/j.1365-2125.2004.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO/UNODC/UNAIDS. Substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention: position paper. 2004. [last accessed 27 March 2008]. Available at http://www.who.int/substance_abuse/publications/en/PositionPaper_English.pdf.
- 11.Auriacombe M, Grabot D, Daulouede J-P, Vergnolle J-P, O'Brien C, Tignol JA. Naturalistic follow-up study of French-speaking opiate-maintained heroin-addicted patients: effect of biopsychosocial status. J Subst Abuse Treat. 1994;11:565–8. doi: 10.1016/0740-5472(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 12.Sarka S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J. Perinatol. 2006;26:15–7. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 13.Coyle MG, Ferguson A, Lagasse L, Oh W, Lester B. Diluted tincture of opium (DTO) and phenobarbitol versus DTO alone for neonatal opiate withdrawal in term infants. J Pediatr. 2002;140:561–4. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 14.Langenfield S, Birkenfeld L, Herkenrath P, Muller C, Hellmich M, Theisohn M. Therapy of neonatal abstinence syndrome with tincture of opium or morphine drops. Drug Alcohol Depend. 2005;77:31–6. doi: 10.1016/j.drugalcdep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Antonilli L, Semeraro F, Suriano C, Signore L, Nencini P. High levels of morphine-6-glucuronide in street heroin addicts. Psychopharmacology. 2003;170:200–4. doi: 10.1007/s00213-003-1531-x. [DOI] [PubMed] [Google Scholar]
- 16.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: comparison of those who do and do not experience withdrawal and concentration–effect relationships. Clin Pharmacol Ther. 1999;65:685–94. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 17.Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–63. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 18.Milne RW, Nation RL, Reynolds GD, Somogyi AA, van Crugten JT. High performance liquid chromatographic determination of morphine and its 3- and 6-glucuronide metabolites: improvements to the method and application to stability studies. J Chromatogr Biomed Appl. 1991;565:457–64. doi: 10.1016/0378-4347(91)80410-e. [DOI] [PubMed] [Google Scholar]
- 19.Säwe J, Svensson JO, Rane A. Morphine metabolism in cancer patients on increasing oral doses – no evidence for autoinduction or dose dependence. Br J Clin Pharmacol. 1983;16:85–93. doi: 10.1111/j.1365-2125.1983.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemann PB, Henriksen H, Grosman N, Broen Christensen C. Plasma morphine concentrations during chronic oral administration in patients with cancer pain. Pain. 1982;13:247–52. doi: 10.1016/0304-3959(82)90014-8. [DOI] [PubMed] [Google Scholar]
- 21.Ashby M, Fleming B, Wood M, Somogyi A. Plasma morphine and glucuronide (M3G and M6G) concentrations in hospice inpatients. J Pain Symptom Manage. 1997;14:157–67. doi: 10.1016/S0885-3924(97)00020-1. [DOI] [PubMed] [Google Scholar]
- 22.Somogyi AA, Nation RL, Olweny C, Tsirgiotis P, van Crugten J, Milne RW, Cleary JF, Danz C, Bochner F. Plasma concentrations and renal clearance of morphine, morphine-3-glucuronide and morphine-6-glucuronide in cancer patients receiving morphine. Clin Pharmacokinet. 1993;24:413–20. doi: 10.2165/00003088-199324050-00005. [DOI] [PubMed] [Google Scholar]
- 23.Wolff T, Samuelsonn H, Hedner T. Morphine and morphine metabolite concentrations in cerebrospinal fluid and plasma in cancer pain patients after slow-release oral morphine administration. Pain. 1995;62:147–54. doi: 10.1016/0304-3959(94)00268-J. [DOI] [PubMed] [Google Scholar]
- 24.Westerling D, Personn C, Höglund P. Plasma concentration of morphine, morphine-3-glucuronide, and morphine-6-glucuronide after intravenous and oral administration to healthy volunteers: relationship to nonanalgesic actions. Ther Drug Monit. 1995;17:287–301. doi: 10.1097/00007691-199506000-00013. [DOI] [PubMed] [Google Scholar]


