Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
Neovascular glaucoma (NVG) is a form of secondary glaucoma in which fibrovascular tissue grows, leading to progressive angle closure with elevation of intraocular pressure.
NVG is poorly responsive to the conventional treatment and has a poor visual prognosis.
WHAT THIS STUDY ADDS.
The present study, performed in 23 patients (26 eyes), shows that three intravitreal injections of bevacizumab scheduled at 4-week intervals are able to result in significant regression of neovascularization, with best response achieved after the third injection.
Our study suggests that bevacizumab is effective in rapidly controlling neovascularization and in preventing angle closure.
Improvements are demonstrated to last at 12 months, and additional interest comes from the lack of any significant adverse effects.
AIMS
Neovascular glaucoma (NVG) represents one of the most severe forms of secondary glaucoma, caused by a number of ocular and systemic conditions, which share the common element of retinal ischaemia/hypoxia that initiates the subsequent release of angiogenesis factors, with consequent development of new abnormal vessels through the ciliary body. The aim was to examine the potential efficacy and safety of intravitreal injection of bevacizumab (IVB) (Avastin®) in the treatment of NVG in patients who had already undergone the standard retinal ablative procedure.
METHODS
This was a prospective pilot trial. Clinical data of 26 eyes from 23 patients, including diagnosis, visual acuity, iris fluorescein angiography stage and intraocular pressure (IOP), were collected. Three injections of bevacizumab were scheduled for each recruited eye at 4-week intervals from the start. All investigations were repeated the day before the IVB (1.25 mg/0.05 ml) and at the 1-, 3-, 6-, 9- and 12-month follow-up.
RESULTS
Regression of corneal oedema together with significant pain reduction was achieved in all eyes already after the first IVB, without any noteworthy improvement of visual acuity. At the end of the scheduled protocol (three IVB), regression of iris neovascularization was documented in all patients, together with significant improvement of visual acuity. The IOP reduction from baseline ranged from 30 to 0 mmHg (12.1 ± 8 mmHg).
CONCLUSIONS
Intravitreal bevacizumab, as adjunctive treatment to the standard retinal ablative procedure, seems promising for the management of conditions responsible of retinal ischaemia/hypoxia associated with NVG.
Keywords: angle neovascularization, bevacizumab (Avastin®), iris neovascularization, neovascular glaucoma, vascular endothelial growth factor
Introduction
Neovascular glaucoma (NVG) is classified as a secondary glaucoma. First documented in 1871 [1], it has been referred to as haemorrhagic glaucoma, thrombotic glaucoma, congestive glaucoma, rubeotic glaucoma and diabetic haemorrhagic glaucoma. Numerous secondary ocular and systemic diseases that share a common element, retinal ischaemia/hypoxia, may induce NVG. Hypoxia is responsible of the release, from ischaemic tissue, of vascular endothelial growth factor (VEGF), a vasoproliferative substance that acts upon healthy endothelial cells of viable capillaries to stimulate the formation of a fragile new plexus of vessels (neovascularization). A close temporal correlation between aqueous VEGF levels and the degree of iris NVG has been recorded [2]. NVG occurrence depends from the development, throughout the ciliary body, of these new abnormal vessels [2–4]. Continuous growth of this fibrovascular membrane causes the formation of anterior synechiae and angle closure, which mechanically block the aqueous humour outflow through the trabecular meshwork. The consequent intraocular pressure (IOP) increase impairs the perfusion pressure, even decreasing blood flow in the retina, choroid and optic nerve head.
NVG is a potentially devastating disease; delayed diagnosis or poor management can result in complete loss of vision or, possibly, loss of the globe itself. Thus, early diagnosis followed by immediate and aggressive treatment is crucial. In managing NVG, it is essential to treat both elevated IOP and the underlying cause of the disease. Thus, both the IOP decrease and ocular blood flow improvement must be considered to ensure the correct amount of oxygen and nutrients to ganglion cells. There is evidence that specific inhibition of VEGF can inhibit neovascularization in the iris, choroid, cornea and retina [2, 5, 6].
Ranibizumab is a 48-kDa humanized Fab fragment of a murine monoclonal anti-VEGF antibody that acts nonspecifically by binding to all VEGF isoforms. Pharmacokinetic studies in the rabbit have indicated a half-life in the vitreous cavity of 3 days after intravitreal injection [6]. Recently, several authors have demonstrated that inhibition of VEGF activity may play a role in the treatment of NVG, but these were retrospective studies, with short-term follow-up, and performed on a small number of patients [7–22]. The aim of this study was to explore the potential efficacy and safety of intravitreal injection of bevacizumab (IVB) (Avastin®; Genentech Inc., S. San Francisco, CA, USA) in a case series of 26 eyes throughout a prospective clinical trial with 1 year's follow-up.
Methods
The clinical trial included 26 eyes of 23 consecutive patients (age 55 ± 14 years; 13:10 male:female ratio) affected by NVG. Nineteen eyes were diagnosed as proliferative diabetic retinopathy (PDR), seven as central retinal vein occlusion (CRVO) and all presented at first examination with IOP ranging between 25 and 51 mmHg (36 ± 8 mmHg) (Tables 1 and 2). All patients were under maximum topical IOP-lowering therapy and seven of them were also on dorzolamide tablets (patients 7, 11 12, 13, 15, 17 and 21) Inclusion criteria consisted of presence of dilated capillary or neovascular vessels at pupillary margin, aqueous flare, elevated IOP, corneal oedema, hyphaema, severe rubeosis. Patients <18 years old, those with severe systemic disease, pregnancy as well as any uncontrolled ocular disease were excluded.
Table 1.
Baseline patient characteristics
| Patient | Eye | Gender | Age | Lens status | Visual acuity | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | Right | M | 42 | Phakic | CF | PDR |
| 1 | Left | M | 42 | Phakic | 6/120 | PDR |
| 2 | Right | F | 36 | Phakic | 6/60 | PDR |
| 3 | Right | M | 45 | Phakic | 6/120 | PDR |
| 3 | Left | M | 45 | Pseudophakic | 6/60 | CRVO |
| 4 | Right | M | 62 | Phakic | 6/120 | CRVO |
| 5 | Right | F | 61 | Pseudophakic | 6/120 | PDR |
| 6 | Right | M | 66 | Pseudophakic | 6/12 | CRVO |
| 7 | Right | F | 72 | Pseudophakic | HM | PDR |
| 8 | Right | M | 51 | Phakic | 6/120 | PDR |
| 9 | Left | F | 37 | Aphakic | HM | PDR |
| 10 | Right | M | 45 | Phakic | 6/24 | PDR |
| 11 | Right | F | 32 | Pseudophakic | CF | PDR |
| 12 | Right | M | 41 | Phakic | HM | PDR |
| 12 | Left | M | 41 | Pseudophakic | 6/60 | PDR |
| 13 | Left | F | 35 | Pseudophakic | 6/60 | PDR |
| 14 | Left | M | 54 | Phakic | 6/15 | PDR |
| 15 | Left | F | 56 | Phakic | 6/60 | PDR |
| 16 | Left | M | 47 | Aphakic | 6/120 | CRVO |
| 17 | Left | F | 72 | Pseudophakic | HM | PDR |
| 18 | Right | M | 58 | Pseudophakic | 6/60 | PDR |
| 19 | Left | M | 80 | Pseudophakic | 6/120 | PDR |
| 20 | Left | F | 72 | Pseudophakic | HM | CRVO |
| 21 | Left | F | 69 | Aphakic | HM | CRVO |
| 22 | Right | M | 76 | Phakic | 6/120 | CRVO |
| 23 | Left | M | 59 | Phakic | CF | PDR |
CF, counter finger; CRVO, central retinal vein occlusion; HM, hand motion; PDR, proliferative diabetic retinopathy.
Table 2.
Clinical data of our patient series over the follow-up period
| Visual acuity‡ | IFA§ | IOP¶ | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Baseline | 1 month | 3 months | 6 months | 9 months | 12 months | Baseline | 1 month | 3 months | 6 months | 9 months | 12 months | Baseline | 1 month | 3 months | 6 months | 9 months | 12 months |
| 1 | CF | 6/120 | 6/60 | 6/24 | 6/18 | 6/18 | 4 | 3 | 3 | 1 | 1 | 1 | 40 | 36* | 32* | 30* | 28* | 28* |
| 1 | 6/120 | 6/60 | 6/60 | 6/24 | 6/18 | 6/18 | 3 | 3 | 2 | 1 | 1 | 1 | 28 | 25 | 22 | 22 | 24 | 22 |
| 2 | 6/60 | 6/60 | 6/24 | 6/15 | 6/15 | 6/12 | 1 | 1 | 0 | 0 | 1 | 1 | 32 | 28 | 28 | 26 | 24 | 24 |
| 3 | 6/120 | 6/120 | 6/60 | 6/24 | 6/18 | 6/24 | 2 | 1 | 1 | 0 | 0 | 0 | 30 | 26 | 24 | 24 | 22 | 22 |
| 3 | 6/60 | 6/60 | 6/30 | 6/24 | 6/18 | 6/18 | 1 | 1 | 0 | 0 | 0 | 0 | 28 | 22 | 20 | 20 | 22 | 20 |
| 4 | 6/120 | 6/120 | 6/24 | 6/15 | 6/15 | 6/18 | 1 | 0 | 0 | 1 | 0 | 1 | 36 | 24 | 24 | 22 | 24 | 22 |
| 5 | 6/120 | 6/60 | 6/60 | 6/24 | 6/36 | 6/30 | 3 | 3 | 1 | 0 | 1 | 0 | 32 | 30* | 24* | 24* | 26* | 24* |
| 6 | 6/12 | 6/12 | 6/12 | 6/15 | 6/15 | 6/18 | 2 | 1 | 2 | 0 | 0 | 0 | 30 | 26 | 26 | 26* | 24* | 24* |
| 7 | HM | 6/60 | 6/36 | 6/60 | 6/30 | 6/30 | 3 | 1 | 1 | 1 | 1 | 1 | 45 | 30 | 28* | 28* | 26* | 26* |
| 8 | 6/120 | 6/120 | 6/24 | 6/24 | 6/18 | 6/24 | 2 | 1 | 1 | 0 | 0 | 1 | 25 | 19 | 20 | 20 | 22 | 22 |
| 9 | HM | 6/120 | 6/120 | 6/30 | 6/36 | 6/30 | 4 | 2 | 2 | 1 | 1 | 1 | 35 | 30* | 30* | 30* | 26* | 26* |
| 10 | 6/24 | 6/24 | 6/24 | 6/15 | 6/15 | 6/24 | 2 | 1 | 0 | 0 | 0 | 0 | 30 | 18 | 18 | 18 | 20 | 18 |
| 11 | CF | CF | CF | 6/60 | 6/30 | 6/36 | 4 | 3 | 3 | 1 | 0 | 0 | 50 | 38* | 32* | 22* | 20 | 20* |
| 12 | HM | CF | 6/60 | 6/60 | 6/30 | 6/36 | 4 | 2 | 2 | 1 | 1 | 1 | 45 | 18* | 20* | 20* | 22 | 20* |
| 12 | 6/60 | 6/24 | 6/24 | 6/24 | 6/18 | 6/24 | 2 | 1 | 0 | 0 | 0 | 0 | 30 | 18* | 18* | 18* | 20 | 20* |
| 13 | 6/60 | 6/60 | 6/18 | 6/18 | 6/18 | 6/18 | 3 | 1 | 2 | 1 | 0 | 0 | 51 | 45 | 30† | 21 | 22 | 21 |
| 14 | 6/15 | 6/12 | 6/15 | 6/12 | 6/15 | 6/18 | 2 | 2 | 0 | 0 | 1 | 1 | 18 | 16 | 19 | 18 | 20 | 18 |
| 15 | 6/60 | 6/36 | 6/24 | 6/24 | 6/18 | 6/18 | 3 | 3 | 1 | 0 | 0 | 0 | 50 | 32* | 20* | 22* | 20* | 20* |
| 16 | 6/120 | 6/60 | 6/60 | 6/30 | 6/36 | 6/30 | 2 | 1 | 1 | 1 | 1 | 1 | 50 | 41† | 18 | 20 | 22 | 22 |
| 17 | HM | 6/36 | 6/36 | 6/36 | 6/30 | 6/30 | 3 | 2 | 1 | 2 | 2 | 2 | 44 | 40* | 36* | 32* | 28* | 28* |
| 18 | 6/60 | 6/24 | 6/24 | 6/36 | 6/30 | 6/36 | 3 | 1 | 0 | 0 | 0 | 0 | 32 | 32 | 28* | 30* | 28* | 30* |
| 19 | 6/120 | 6/36 | 6/60 | 6/60 | 6/30 | 6/36 | 4 | 2 | 1 | 1 | 1 | 0 | 30 | 24* | 24* | 22* | 24* | 22* |
| 20 | HM | 6/36 | CF | 6/36 | 6/30 | 6/30 | 3 | 3 | 2 | 0 | 0 | 1 | 32 | 22* | 24* | 22* | 22* | 22* |
| 21 | HM | CF | 6/60 | 6/36 | 6/36 | 6/36 | 3 | 2 | 1 | 0 | 0 | 1 | 42 | 33† | 24 | 22 | 22 | 22 |
| 22 | 6/120 | 6/36 | 6/60 | 6/24 | 6/24 | 6/30 | 4 | 2 | 1 | 1 | 1 | 0 | 38 | 28* | 30* | 28* | 24* | 26* |
| 23 | CF | 6/60 | 6/36 | 6/24 | 6/18 | 6/24 | 4 | 2 | 2 | 1 | 1 | 1 | 34 | 28* | 32* | 28* | 26* | 28* |
On treatment with topical IOP-lowering drops.
Ahmed glaucoma valve implant.
Paired t-test (baseline vs. 12 months; t = −7.912, P = 0.0001; 95% CI −0.22, −0.13).
Spearman's rank correlation (P < 0.01).
Paired t-test (baseline vs. 12 months; t = 7.867, P = 0.0001; 95% CI 9.91, 16.94). CRVO, central retinal vein occlusion; IFA, iris fluorescein angiography (graded according to the Ehrenberg's system); IOP, intraocular pressure; PDR, proliferative diabetic retinopathy.
All patients underwent a complete ophthalmic examination including slit lamp biomicroscopy, retinal and iris fluorescein angiography (IFA). All patients had already undergone the standard retinal ablative procedure in the previous 3 months, without significant improvement. After discussion of both the natural course of the disease and the results of the alternative approach for NVG, together with the potential risks and benefits of off-label bevacizumab intravitreally administered, all patients gave written consent to receive a pars plana injection of 1.25 mg of bevacizumab (Avastin®; 1.25 mg/0.05 ml). The four local Human Subjects Review Committees (Campobasso, Larino, Brescia and Napoli) approved the project.
Data collected from each recruited patient included diagnosis, visual acuity, IFA stage, IOP and number of IVB injections. The angiograms were interpreted and graded according to the Ehrenberg and associates system [23].
All investigations were repeated the day before the IVB injection (baseline) and at 1, 3, 6, 9 and 12 months’ follow-up. Three injections of bevacizumab were scheduled for each patient every 4 weeks after baseline observation.
Statistical comparisons (Systat statistical packages, Evanston, IL, USA) were made using the Spearman's ρ rank correlation (IFA stage) and paired t-test (visual acuity and IOP). A probability of randomness <0.05 was considered statistically significant.
Results
Twenty-six eyes of 23 patients (12 phakic, 11 pseudophakic and three aphakic), were included in the study. At the time of the first injection, visual acuity ranged from 20/50 to counter finger (CF) and the fundus was not visible on indirect examination in any subject.
After the first IVB the cornea had cleared and significant pain reduction together with partial regression of leakage from the iris vessels was achieved in all patients, without any significant improvement in visual acuity. At 1 month's follow-up the mean IOP reduction from baseline ranged from 18 to 0 mmHg (8 ± 5 mmHg), and only in one patient was a highly significant IOP reduction recorded (−27 mmHg, patient 12, Right eye) (Table 2).
After the third IVB, complete regression of iris neovascularization (Figure 1, Table 3) in most cases, together with visual acuity recovery (from CF to 20/50), was achieved. The mean IOP decrease was approximately 13 mmHg.
Figure 1.

Massive iris neovascularization in two patients with neovascular glaucoma secondary to proliferative diabetic retinopathy. Iris fluorescein angiography shows complete regression of fluorescence leakage at 12 months' follow-up after three intravitreal injections of bevacizumab
Table 3.
Iris fluorescein angiography
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| T 0 | 0 | 4 | 7 | 8 | 7 |
| T 12 | 13 | 12 | 1 | 0 | 0 |
Spearman's rank correlations.
At 12 months’ follow-up all patients showed further improvement in visual acuity to 20/200 or better [paired t-test: baseline vs. 12 months, P = 0.0001; 95% confidence interval (CI) −0.22, −0.13] (Figure 2). The fundus was clearly visible with detail already after the second injection, thus we were able to complete the panretinal photocoagulation (PRP). The IOP reduction ranged from 30 to 0 mmHg (12.1 ± 8 mmHg) (paired t-test baseline vs. 12 months, P = 0.0001; 95% CI 9.91, 16.94) (Figure 3). Three eyes of three patients (patients 13, 16 and 21) required surgery with Ahmed glaucoma valve implant and 15 eyes of 14 patients (patients 1, 5, 6, 7, 9, 11, 12, 15, 17, 18, 19, 20, 22 and 23) were treated with topical IOP-lowering drops alone (11 out of 15 on timolol + dorzolamide and four out of 15 on timolol + dorzolamide + prostaglandin) (Table 2). Neither systemic nor ocular adverse events due to the IVB were recorded either immediately after the IVB injections or over the follow-up period.
Figure 2.

Scatter plot of visual acuity at 12 months' follow-up vs. baseline
Figure 3.
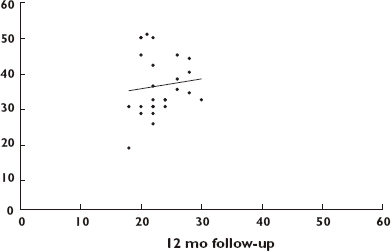
Scatter plot of intraocular pressure at 12 months' follow-up vs. baseline
Discussion
Neovascular glaucoma is one of the most severe complications in patients affected by PDR or CRVO and it remains one of the most important causes of blindness in these eyes [4]. The presence of a clear visible fundus is necessary for appropriate monitoring of the disease and also to try to minimize the growth of neovascular tissue.
Currently, there is no satisfactory treatment of NVG, thus the first goal should be to prevent its development by appropriate management of causative disease. If NVG develops, early diagnosis together with aggressive control of IOP is crucial to minimize visual field loss. Potential success has been achieved with the combination of medical management, including the use of topical eye-drop medications and steroids, and PRP [24]. The aim of PRP is to destroy ischaemic tissue, decreasing the VEGF production that leads to irreversible development of an abnormal vascular network through the ciliary body [2]. Aqueous VEGF levels represent the severity of anterior segment ischaemia and could be used as an indicator of the extent of ischaemia [25]. Some controversy still exists about the pathogenesis, prognosis and management of NVG. In fact, laser ablation of ischaemic areas is only occasionally effective and is often used as a temporary measure that helps to keep the angle open. The beneficial effect of PRP on neovascularization is perhaps directly related to the concomitant intravitreal treatment of angiogenic factors. It has been postulated that the IVB may play a role in the treatment of ischaemic areas by inhibiting VEGF activity and, consequently, the growth of tissue through the angle structure [2]. In fact, bevacizumab is a full-length humanized monoclonal antibody with some advantages in pharmacokinetics and tissue distribution. In a recent study by Mordenti et al.[26] on an experimental animal model, it has been demonstrated that the half-life of bevacizumab within the vitreous body is longer than the Fab antibody (ranibizumab), due to its smaller size and its significant diffusion through the retinal layers. Using microautoradiography assays, these authors have pointed out that bevacizumab did not penetrate the inner limiting membrane, whereas ranibizumab distributed through the neural retina to the retinal pigment epithelial layer and persisted in this location for up to 7 days [26].
In light of these findings, bevacizumab seem to possess a better therapeutic profile for attacking vessel growth in the preretinal space in PDR, CRVO and many other conditions that result in ischaemia of the retina or ciliary body [26]. In addition, IVB is able to bind all isoforms of VEGF [27]. Recently, Spitzer and co-workers, in a comparative study in vitro of the antiproliferative and cytotoxic properties of bevacizumab, pegaptanib and ranibizumab, have demonstrated that all tested compounds significantly suppress choroidal endothelial cell proliferation. Thus, when used at the currently established doses, none of the drugs was superior to others in respect of endothelial cell growth inhibition [28]. The biocompatibility of these compounds seems to be excellent when used at the currently recommended intravitreal dose. Bevacizumab is a full-length anti-VEGF antibody that was originally approved for use in metastatic colonic cancer and is under investigation as a low-cost off-label alternative for patients with age-related macular degeneration. There is growing evidence that this drug may be an effective and safe alternative to the more expensive ranibizumab and pegaptanib, although prospective trials are required to investigate this issue fully, and its continued unlicensed use raises ethical, legal and policy questions [29, 30].
In this small case series the potential efficacy and safety of IVB as adjunctive treatment in patients suffering from NVG has been confirmed. The best response, in terms of regression of neovascularization, was achieved after the third injection. Baseline IFA showed pathological leakage from the vessels in all quadrants of the iris, with the same pattern of reaction persisting after the first injection. At the 12-month check only a slight leakage in the late phases was recorded. IOP did not show any significant reduction, in fact patients needed adjunctive medical or surgical treatment.
In conclusion, intravitreal bevacizumab represents a worthwhile option in association with photocoagulation treatment. The combined therapy, despite limitations related to both the small number of patients and short-term follow-up, has revealed promising results in the management of PDR and CRVO associated with NVG.
Competing interests
None to declare.
REFERENCES
- 1.Pagenstecher H. Beitrage zur Lehre vom hamorrhagischen Glaukom. Graefes Arch Ophthalmol. 1871;17:98–130. [Google Scholar]
- 2.Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007;26:470–85. doi: 10.1016/j.preteyeres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manschot WA. Retinal neovascularization after retinal vaso-obliteration. Frequency-origin-morphology. Doc Ophthalmol. 1983;55:117–20. doi: 10.1007/BF00140469. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998;105:232–7. doi: 10.1016/s0161-6420(98)92782-8. [DOI] [PubMed] [Google Scholar]
- 5.Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40:352–68. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- 6.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 7.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144–6. [PubMed] [Google Scholar]
- 9.Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU Tuebingen Bevacizumab Study Group. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158–60. doi: 10.1016/j.ajo.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Silva Paula J, Jorge R, Alves Costa R, Rodrigues Mde L, Scott IU. Short-term results of intravitreal bevacizumab (Avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmol Scand. 2006;84:556–7. doi: 10.1111/j.1600-0420.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 11.Mason JO, III, Albert MA, Jr, Mays A, Vail R. Regression of neovascular iris vessels by intravitreal injection of bevacizumab. Retina. 2006;26:839–41. doi: 10.1097/01.iae.0000230425.31296.3b. [DOI] [PubMed] [Google Scholar]
- 12.Geitzenauer W, Michels S, Prager F, Kornek G, Vormittag L, Rosenfeld P, Schmidt-Erfurth U. Early effects of systemic and intravitreal bevacizumab (avastin) therapy for neovascular age-related macular degeneration. Klin Monatsbl Augenheilkd. 2006;223:822–7. doi: 10.1055/s-2006-926875. [DOI] [PubMed] [Google Scholar]
- 13.Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–6. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 14.Jonas JB, Spandau UH, Schlichtenbrede F. Intravitreal bevacizumab for filtering surgery. Ophthalmic Res. 2007;39:121–2. doi: 10.1159/000099248. [DOI] [PubMed] [Google Scholar]
- 15.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41:614–25. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 16.Vatavuk Z, Bencic G, Mandic Z. Intravitreal bevacizumab for neovascular glaucoma following central retinal artery occlusion. Eur J Ophthalmol. 2007;17:269–71. doi: 10.1177/112067210701700220. [DOI] [PubMed] [Google Scholar]
- 17.Batioğlu F, Astam N, Ozmert E. Rapid improvement of retinal and iris neovascularization after a single intravitreal bevacizumab injection in a patient with central retinal vein occlusion and neovascular glaucoma. Int Ophthalmol. 2008;28:59–61. doi: 10.1007/s10792-007-9105-2. [DOI] [PubMed] [Google Scholar]
- 18.Chilov MN, Grigg JR, Playfair TJ. Bevacizumab (Avastin) for the treatment of neovascular glaucoma. Clin Experiment Ophthalmol. 2007;35:494–6. doi: 10.1111/j.1442-9071.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 19.Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007;16:437–9. doi: 10.1097/IJG.0b013e3180457c47. [DOI] [PubMed] [Google Scholar]
- 20.Kelkar AS, Kelkar SB, Kelkar JA, Nagpal M, Patil SP. The use of intravitreal bevacizumab in neovascular glaucoma: a case report. Bull Soc Belge Ophtalmol. 2007;303:43–5. [PubMed] [Google Scholar]
- 21.Gheith ME, Siam GA, de Barros DS, Garg SJ, Moster MR. Role of intravitreal bevacizumab in neovascular glaucoma. J Ocul Pharmacol Ther. 2007;23:487–91. doi: 10.1089/jop.2007.0036. [DOI] [PubMed] [Google Scholar]
- 22.Ichhpujani P, Ramasubramanian A, Kaushik S, Pandav SS. Bevacizumab in glaucoma: a review. Can J Ophthalmol. 2007;42:812–5. doi: 10.3129/i07-160. [DOI] [PubMed] [Google Scholar]
- 23.Ehrenberg M, McCuen BW, II, Schindler RH, Machemer R. Rubeosis iridis: preoperative iris fluorescein angiography and periocular steroids. Ophthalmology. 1984;91:321–5. doi: 10.1016/s0161-6420(84)34292-0. [DOI] [PubMed] [Google Scholar]
- 24.Laatikainen L. A prospective follow-up study of panretinal photocoagulation in preventing neovascular glaucoma following ischaemic central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 1983;220:236–9. doi: 10.1007/BF02308081. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Matsuo T, Ohtsuki H. Aqueous vascular endothelial growth factor increases in anterior segment ischemia in rabbits. Jpn J Ophthalmol. 1998;42:85–9. doi: 10.1016/s0021-5155(97)00116-0. [DOI] [PubMed] [Google Scholar]
- 26.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM, et al. Comparisons of the intraocular tissue distribution pharmacokinetics and safety of 125 I-labeled Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–44. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N. Vascular endothelial growth factor: basic science in clinical progress. Endocrinol Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer MS, Yoeruek E, Sierra A, Wallenfels-Thilo B, Schraermeyer U, Spitzer B, Bartz-Schmidt KU, Szurman P, et al. Comparative antiproliferative and cytotoxic profile of bevacizumab (Avastin), pegaptanib (Macugen) and ranibizumab (Lucentis) on different ocular cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:1837–42. doi: 10.1007/s00417-007-0568-7. [DOI] [PubMed] [Google Scholar]
- 29.Waisbourd M, Loewenstein A, Goldstein M, Leibovitch I. Targeting vascular endothelial growth factor: a promising strategy for treating age-related macular degeneration. Drugs Aging. 2007;24:643–62. doi: 10.2165/00002512-200724080-00003. [DOI] [PubMed] [Google Scholar]
- 30.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]


