In recent years the use of so-called fatburners such as Stacker® has gained widespread popularity in the USA. These dietary supplements contain substances such as Ephedra and caffeine. The drugs are supposed to stimulate athletic performance and reduce body weight [1]. Ephedra, also known as the Chinese ‘Ma Huang’, is a plant-derived natural source of the sympathicomimetic substance ephedrine. Caffeine (1,3,7-methylxanthine) is also a plant-derived stimulant alkaloid, which can be obtained from tea leaves, coffee beans, cacao beans and cola nuts. Its pharmacological actions are diverse, but finally result in an increasing release of endogenous catecholamines and therefore augment the effect of Ephedra alkaloids.
For years, Ephedra-containing food supplements were easily available over-the-counter herbal products in the USA and the Netherlands. When serious adverse events including stroke, heart attacks, cardiac arrhythmias, seizures and psychotic disorders were reported, the general sale of Ephedra-containing products was prohibited. However, Ephedra alkaloids can still easily be obtained through the internet or at gyms. Furthermore, an increase in the use of Ephedra-free fatburners, which are considered as ‘safe’ products, has been observed. In the following case report we describe a remarkable, potential lethal intoxication with Stacker 2®.
A 22-year-old woman with no previous medical history was admitted to our emergency room because of attempted suicide. She claimed to have ingested approximately 50 tablets of Stacker 2® ephedra, which had previously been provided by her gym. According to the label on the bottle, its main constituents were Ephedra, cola nuts and white willow bark (NVE Pharmaceuticals®, Andover, NJ, USA). The patient reported not having taken any alcohol or other medication. Furthermore, she had not been exercising on the day of admission.
On arrival, she complained of thirst, headache, abdominal pain, chest pain and she vomited several times. On examination, an anxious, hyperventilating and extremely agitated woman was observed with clammy peripheries. Vital signs included a temperature of 37.5 °C, blood pressure of 122/66 mmHg, a regular pulse of 110 bpm and a respiratory rate of 25/min. Her arterial saturation was measured at 99%. Pupils were bilaterally dilated and responsive. Apart from modest abdominal tenderness, physical examination revealed no other abnormalities. Laboratory results are shown in Table 1. Because of the strikingly low serum potassium concentration (1.6 mmol l−1), laboratory measurements were verified several times. Also remarkable was the elevated serum lactate (7.2 mmol l−1). Electrocardiography demonstrated sinustachycardia with ST-depression and U-waves in precordial leads V1–V3. Potassium chloride at a rate of 20 mmol h−1 was administered. Furthermore, sodium-potassium-phosphate was supplemented. The patient was transferred to the Intensive Care Unit for haemodynamic monitoring. Polyuria was observed with a diuresis of 250 ml h−1. The following day, the patient was less agitated, but still complained of nausea, vomiting and abdominal pain. Her respiratory rate still measured 22/min. Tachycardia had disappeared. The serum potassium concentration had increased from 1.6 to 3.8 mmol l−1. Serum lactate had decreased to 3.5 mmol l−1 (Table 1). The patient was transferred to the ward and discharged home 7 days after admittance.
Table 1.
Laboratory results at admittance and day 2
| Parameters | Reference | At admission | Day 2 |
|---|---|---|---|
| C-reactive protein | 0–10 mg l−1 | 5 | 42 |
| Haemoglobin | 7.8–10.0 mmol l−1 | 8.7 | 8.4 |
| Leucocytes | 4.0–10.0 × 109 l−1 | 13.9 | 18.9 |
| Sodium | 135–146 mmol l−1 | 135 | 134 |
| Potassium | 3.8–5.0 mmol l−1 | 1.6 | 3.8 |
| Chloride | 97–108 mmol l−1 | 102 | 106 |
| Magnesium | 0.70–1.05 mmol l−1 | 0.88 | 0.95 |
| Calcium | 2.15–2.60 mmol l−1 | 2.3 | – |
| Phosphate | 0.80–1.45 mmol l−1 | 0.57 | 0.80 |
| Creatinin | 65–100 µmol l−1 | 81 | 65 |
| Glucose | 4.0–7.8 mmol l−1 | 11.7 | 10.8 |
| Creatine kinase | 25–170 IU l−1 | 57 | – |
| Lactate | 0.5–2.2 mmol l−1 | 7.2 | 3.5 |
| Ethanol | ‰ | <0.05 | – |
| Arterial blood gas analysis | |||
| pH | 7.35–7.45 | 7.41 | 7.49 |
| pO2 | 9.3–13.3 kPa | 10.4 | 11.8 |
| pCO2 | 4.7–6.0 kPa | 3.0 | 3.4 |
| Bicarbonate | 23–28 mmol l−1 | 14 | 19 |
| Base excess | −3–3 mmol l−1 | –8 | –3 |
| Urine | |||
| Glucose | – | +++ | – |
| Ketons | – | +++ | – |
| Potassium | > 20 mmol l−1 | 36 | 33 |
Subsequently, toxicological analysis for caffeine was performed by means of high-pressure liquid chromatography UV detection: platinum enhanced polar selectivity C18 column, particles; 3 µm, size 10 × 4.6 cm; Alltech®, photodiode array detector (Waters®, Milford, MA, USA). The mobile phase consisted of a solution of water, phosphor acid, nonilamine (67%) and acetonitril (33%). Ephedrine analysis was performed with gas chromatography combined with mass spectrometry [2]. Serum collected 8 h post ingestion showed a toxic caffeine level of 110 mg l−1 (Figure 1). In total, seven serum samples at different time points were obtained for analysis. After 2 days, caffeine concentration had diminished to 36 mg l−1. No ephedrine could be traced in serum samples or in urine. Further analysis of the Stacker 2® tablets demonstrated caffeine levels of approximately 400 mg in each tablet without any trace of ephedrine. Considering the severe hypokalaemia, the patient's urine was also tested for the presence of laxatives and diuretics, which could not be demonstrated. Hyperthyroidism was also excluded.
Figure 1.
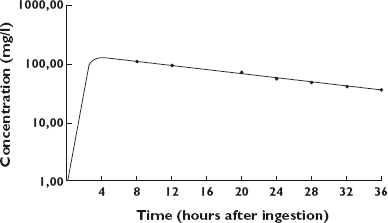
The concentration–time profile of caffeine in the serum of the patient; the vertical axis reflects the caffeine concentration (in mg l−1), the horizontal axis reflects the time after ingestion (in hours)
This intoxication with Stacker 2® resulted in severe life-threatening hypokalaemia in combination with lactate accumulation. Strikingly, the ingested food supplement contained only caffeine. Caffeine is a xanthine derivative (1,3,7-trimethylxanthine) and closely resembles theophylline. After oral administration, caffeine is fully absorbed and peak plasma concentration occurs within 20–75 min. The volume of distribution is 0.6 l kg−1[3, 4]. Caffeine undergoes hepatic metabolism and its main active metabolites are paraxanthine, theobromine and theophylline [3–5]. These metabolites are then further broken down by the liver and about a dozen metabolites can be traced in urine such as 1-methyluric acid and 1-methylxantine [4, 5]. The elimination half-life of caffeine has been reported as 3–6 h in healthy adults with modest intake [4]. However, the metabolism of caffeine has been shown to be dose dependent with clearance decreasing as the dose is increased, suggesting saturable metabolism [3]. In our patient, a half-life of approximately 16 h was found (Figure 1).
After normal ingestion, caffeine has mild stimulant properties on the central nervous system. The pharmacological mechanism of action has not been fully elucidated, but is thought to rely on three mechanisms: (i) antagonism of central and peripheral adenosine receptors, (ii) inhibition of phosphodiesterase, and (iii) release of calcium from intracellular stores [4–7]. Binding of caffeine to the adenosine receptor in the brain results in a central stimulatory effect leading to an increased sympathetic tone. Due to blockade of presynaptic adenosine receptors, the systemic release of catecholamines by sympathetic neurons is increased [5, 6, 8]. This release of catecholamines leads, in addition to α-adrenergic vasoconstriction, to a β2-adrenergic response resulting in a shift of K+-ions to the intracellular fluid compartment. Furthermore, blockade of adenosine receptors inhibits K+-efflux through adenosine triphosphate (ATP)-dependent potassium channels across the cell membrane [5]. Accordingly, hypokalaemia is observed. The second action of caffeine is thought to be related to inhibition of phosphodiesterase, leading to increased concentrations of cyclic-AMP augmenting the effect of catecholamines [4, 5]. However, this mechanism is presumed to require higher levels of caffeine than those found in ordinary consumption. The third mechanism of action of caffeine involves calcium release from intracellular stores, resulting in an increase of intracellular calcium concentration, which stimulates various cell functions [9].
Ingestion of caffeine at a toxic dose can result in a variety of symptoms, listed in Table 2. These symptoms can be observed at serum caffeine levels >60 mg l−1. Lethal intoxication has been reported with caffeine concentrations of 80–350 mg l−1[10]. In this patient, a level of 110 mg l−1 was measured 8 h post ingestion. Application of a mathematical model (MWPharm, version 3.30) on the concentration–time curve calculated a maximum caffeine concentration of approximately 150 mg l−1 at 3–4 h (Figure 1). This concentration correlates with the ingestion of a minimum of 5 g of caffeine (maximum caffeine concentration is 150 mg l−1; distribution volume is 0.6 l kg−1; body weight is 55 kg: 0.6 l kg−1 × 55 kg × 150 mg l−1 = 4.95 g caffeine), assuming no loss due to incomplete and/or prolonged absorption. The content of caffeine in coffee, tea and the soft drink coke is respectively 100, 40 and 45 mg [2–5].
Table 2.
| Organ (system) | Symptoms |
|---|---|
| Central nervous system | Fear, hallucinations, restlessness, insomnia, coma, tremor, seizures, headache, photophobia |
| Cardiovascular system | Arrhythmias (most frequently atrial or ventricular tachycardia), hypertension (or in severe cases hypotension), myocardial infarction, ischaemic stroke, collapse |
| Respiratory system | Hyperventilation, acute lung injury |
| Gastrointestinal tract | Nausea, vomiting |
| Kidneys | Polyuric state, acute renal failure |
| Muscles | Paralysis, rhabdomyolysis |
| Haematology | Leucocytosis |
| Metabolism | Hyperglycaemia, lipolysis, ketogenesis |
| Acid-base balance | Lactic acidosis caused by hyperadrenergic state or by seizures or hypotension; respiratory alkalosis |
| Electrolytes | Hypokalaemia, hyponatraemia |
In this case, severe, life-threatening hypokalaemia was observed with a paradoxal accumulation of lactate. Severe hypokalaemia has previously been described after the use of caffeine, but never this extreme [5, 11, 12]. Hypokalaemia is usually caused by the loss of electrolytes through kidneys or the gastrointestinal tract, but in this case potassium transport to the intracellular compartment seems to be the major mechanism. When hypokalaemia occurs, the expected compensatory response is decreased potassium excretion by the kidneys. However, activation of Na+/K+-ATPase in the renal tubuli and increasing levels of renine result in persistent potassium excretion in the urine [13, 14]. Moreover, potassium homeostasis is influenced by acid-base balance. Persistent vomiting, as illustrated in this case, causes loss of H+-ions resulting in metabolic alkalosis. In addition, the stimulating effect of caffeine on the respiratory centre enhances (respiratory) alkalosis. Despite these mechanisms, arterial blood gas analyses revealed a neutral pH with lowered bicarbonate (Table 1), which can be attributed to accumulation of lactate. Elevated circulating catecholamines, inducing α-adrenergic vasoconstriction, probably caused tissue ischaemia resulting in anaerobic metabolism with production of lactate.
The management of caffeine intoxication is threefold: prevention of further absorption, enhancing elimination and supportive care. Gastric lavage is recommended 1–2 h post ingestion. This also accounts for the administration of activated charcoal and laxatives. Elimination is enhanced by haemodialysis [8, 10]. In the present case, gastric lavage was not performed because of persistent vomiting. Haemodialysis was not applied either, because of the assumed ephedrine intoxication (short half-life and large volume of distribution and therefore no useful intervention). Supportive care concentrates on circulation, respiration and the central nervous system. Furthermore, electrolytes and acid-base balance should be adjusted. Hypokalaemia, metabolic acidosis, hyperglycaemia and ketonuria can occur (Table 2) [8, 10]. Cardiac rhythm should also be monitored. Sinustachycardia is frequently observed and if haemodynamic instability occurs, a β-blocker (e.g. propanolol or the short-acting esmolol) is indicated. As a consequence, slight correction of hypokalaemia can be observed [5]. Benzodiazepines are administered when extreme agitation or seizures occur. Rhabdomyolysis requires saline infusion to enhance excretion of myoglobin and to prevent renal failure. Sodium bicarbonate can be applied when hypokalaemia is corrected.
We have described a potential lethal intoxication with caffeine. In particular, the observed hypokalaemia is a medical emergency with potential paralysis and fatal cardiac arrhythmias. The presented hypokalaemia should be attributed to a potassium shift towards the intracellular fluid compartment in combination with persistent kaliuresis. Its main mechanisms of action are the blockade of the cellular adenosine receptors by caffeine in combination with an increasing release of catecholamines and β2-adrenergic stimulation of Na+/K+-ATPase. The observed sympathicomimetic syndrome in combination with elevated serum lactate is a manifestation of a hyperadrenergic state.
This intoxication was caused by excessive intake of a dietary supplement. It is alarming that potentially dangerous substances such as these are easily available through the internet or at gyms. Incorrect labelling, as described in the present case, further increases health risks. Potentially fatal caffeine intoxications have also been described after excessive intake of the soft drink coke [14]. Consequently, in a patient presenting with severe hypokalaemia, intoxication with caffeine should be considered.
REFERENCES
- 1.van Riel AJHP, de Vries I, Meulenbelt J. Gezondheidsrisico's door Ephedra in voedingssupplementen. Ned Tijdschr Geneeskd. 2003;147:2017–9. [PubMed] [Google Scholar]
- 2.Marchei E, Pellegrini M, Pacifici R, Zuccaro P, Pichini S. A rapid and simple procedure for the determination of ephedrine alkaloids in dietary supplements by gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2006;41:1633–41. doi: 10.1016/j.jpba.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 3.Cheng WS, Murphy TL, Smith MT, Cooksley WG, Halliday JW, Powell LW. Dose-dependent pharmacokinetics of caffeine in humans: relevance as a test of quantitative liver function. Clin Pharmacol Ther. 1990;47:516–24. doi: 10.1038/clpt.1990.66. [DOI] [PubMed] [Google Scholar]
- 4.Mandel HG. Update on caffeine consumption, disposition and action. Food Chem Toxicol. 2002;40:1231–4. doi: 10.1016/s0278-6915(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 5.Alazami M, Lin SH, Cheng CJ, Davids MR, Halperin ML. Unusual causes of hypokalaemia and paralysis. Q J Med. 2006;99:181–92. doi: 10.1093/qjmed/hcl011. [DOI] [PubMed] [Google Scholar]
- 6.Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–72. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undem BJ. Pharmacotherapy of asthma. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 11th edn. New York: McGraw-Hill; 2005. pp. 727–30. [Google Scholar]
- 8.Benowitz NL. Clinical pharmacology of caffeine. Annu Rev Med. 1990;41:277–88. doi: 10.1146/annurev.me.41.020190.001425. [DOI] [PubMed] [Google Scholar]
- 9.Shi D, Padgett WL, Daly JW. Caffeine analogs: effects on ryanodine-sensitive calcium-release channels and GABAA receptors. Cell Mol Neurobiol. 2003;23:331–47. doi: 10.1023/A:1023688604792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerrigan S, Lindsey T. Fatal caffeine overdose: two case reports. Forensic Sci Int. 2005;153:67–9. doi: 10.1016/j.forsciint.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Passmore AP, Kondowe GB, Johnston GD. Caffeine and hypokalemia. Ann Intern Med. 1986;105:468. doi: 10.7326/0003-4819-105-3-468_1. [DOI] [PubMed] [Google Scholar]
- 12.Appel CC, Myles TD. Caffeine-induced hypokalemic paralysis in pregnancy. Obstet Gynecol. 2001;97:805–7. doi: 10.1016/s0029-7844(00)01210-2. [DOI] [PubMed] [Google Scholar]
- 13.Gennari FJ. Hypokalemia. N Engl Med J. 1998;339:451–8. doi: 10.1056/NEJM199808133390707. [DOI] [PubMed] [Google Scholar]
- 14.Mudge DW, Johnson DW. Coca-Cola and kangaroos. Lancet. 2004;364:1190–1. doi: 10.1016/S0140-6736(04)17111-0. [DOI] [PubMed] [Google Scholar]


